Plant traits mediate foliar uptake of deposited nitrogen by mature woody plants
Abstract
Increased atmospheric nitrogen (N) deposition significantly disturbs ecosystem N cycle. Although foliar interception and uptake of N deposition can provide an important alternative N supply to forest ecosystems, the mechanisms regulating foliar N uptake from wet deposition are not fully understood. Here, we selected 19 woody species with a wide range of plant traits from different functional groups and conducted a 15N isotope labelling experiment through brushing 15NH4+ and 15NO3− solution on canopy leaves. Our findings demonstrate that leaves can directly absorb N from wet deposition within a few hours. The average leaf 15N recoveries were 10% and 28% under 15NH4+ and 15NO3− treatments across species, respectively, while twig N recoveries were only 1%–7% of leaf N recoveries. Differences in foliar N uptake efficiency among species were closely associated with leaf traits but were little influenced by meteorological conditions or soil nutrient status. Specifically, plants with higher leaf N concentration, larger specific leaf area and lower wax concentration exhibited higher leaf N recovery. Our results indicated that tree canopies could directly absorb N from atmospheric deposition. We highlight the critical role of leaf traits in determining canopy foliar N uptake, which may consequently influence plant competition under elevated N deposition.
1 INTRODUCTION
Atmospheric nitrogen (N) deposition has increased rapidly due to human activities and has reached high levels currently (Galloway et al., 2004; Yu et al., 2019). This increased atmospheric N deposition induces profound impacts on carbon (C) and N cycles of forest ecosystems (de Vries & Posch, 2011; Liu & Greaver, 2009; Sutton et al., 2008; Xia & Wan, 2008). In the absence of anthropogenic disturbances, plants acquire most of their nutrients from the soil through their roots. However, growing evidence indicates that canopy interception and uptake of atmospheric N deposition can provide an important alternative N supply for forests. Extensive research has demonstrated that forest canopies can intercept 20%–80% atmospheric N deposition (Bryan Dail et al., 2009; Ferraretto et al., 2022; Gaige et al., 2007; Sievering et al., 2007; Wortman et al., 2012), and canopy N uptake could supply 2%–15% of the annual N demand for plant growth (Boyce et al., 1996; Nave et al., 2009).
Unlike N uptake via roots, N uptake through foliage could be directly incorporated into plant metabolism without competition with soil microbes (Bourgeois et al., 2019; Sparks, 2009). Therefore, foliar N uptake may have a more rapid impact on plant growth, and potentially alter N allocation patterns (Nair et al., 2016; Wang et al., 2021). Similar to root uptake, foliar N uptake capacity showed large variations across species (Adriaenssens et al., 2012; Garten et al., 1998; Wuyts et al., 2015). These species-level differences might alter interspecific competition, and consequently, community composition and ecosystem functions. However, the underlying mechanisms regulating foliar N uptake are not fully understood (Bortolazzi et al., 2021; Fernández et al., 2021; Sparks, 2009).
Leaves can absorb inorganic N from the atmosphere in various forms, including dissolved, particulate, and gaseous N. Gaseous N compounds, such as NH3 and NOx, enter the leaves primarily through the stomata (Krupa, 2003; Sparks et al., 2001). Dissolved and particulate forms of N mainly deposit on the leaf surface, and subsequently enter leaf cells through cuticular diffusion or stomatal pathway (Burkhardt, 2010; Harrison et al., 2000; Peuke et al., 1998; Sparks, 2009). Dissolved N compounds in precipitation, including ammonium (NH4+) and nitrate (NO3−), constitute significant components of atmospheric N deposition. Several studies suggested that foliar uptake of NH4+ was one to five times higher than that of NO3− (Boyce et al., 1996; Wuyts et al., 2015). However, other studies found that canopies had a higher NO3− retention than NH4+ (Bryan Dail et al., 2009; Fenn et al., 2013; Ferraretto et al., 2022). The discrepancy might be due to the differences in plant N preference, soil N status and meteorological condition (Fernández & Eichert, 2009; Harrison et al., 2000; Wuyts et al., 2015). Previous studies have indicated that soil N availability and meteorological conditions (e.g., light, relative air humidity and air temperature) could affect foliar N uptake by altering plant nutrient requirements, physiological activities and the residence time of deposited N (Eichert & Fernández, 2012; Vallano & Sparks, 2008). Given that atmospheric N deposition and its composition have large spatial and temporal variations (Li et al., 2016; Yu et al., 2019), understanding the factors that control foliar N uptake preferences across species could help to better predict how ecosystems will respond to changing N deposition in the future.
Previous studies suggested that foliar N uptake capacity varied among different tree species and functional groups (Adriaenssens et al., 2011; Garten et al., 1998). This variation in foliar N uptake could be greatly influenced by the retention of surface water on leaves. Leaf surface characteristics, such as wax content and crystal forms, as well as the number and distribution of trichomes, affect leaf wettability and permeability, thereby influencing foliar absorption of deposited N (Berry et al., 2019; Fernández et al., 2017, 2021; Ishfaq et al., 2022). For example, studies have shown that leaves with lower wax concentration typically exhibit higher wettability, facilitating the cuticular diffusion of deposited N (Holloway, 1969; Koch et al., 2006; Sase et al., 2008; Wang et al., 2015). However, the precise mechanisms by which wax composition and the presence of trichomes affect foliar N uptake are not yet fully understood (Fernández et al., 2021).
In addition to leaf wettability, variations of foliar N uptake among species could also be associated with plant traits related to plant resource-use strategies. According to the framework of leaf economics spectrum, species characterized by high leaf N concentration and specific leaf area (SLA) have fast-growing rates, high physiological activities and efficient resource acquisition (Díaz et al., 2016; Freschet et al., 2010; Reich, 2014; Wright et al., 2004). Previous studies showed that foliar N uptake is positively correlated with leaf N concentration (Adriaenssens et al., 2011; Wuyts et al., 2015). This correlation may arise because plants with high leaf N concertation typically have a greater N demand and higher physiological activities (Wright et al., 2004), facilitating the assimilation of N absorbed through foliar uptake (Vallano & Sparks, 2008; Wuyts et al., 2015). Consequently, it is reasonable to speculate that resource-acquisitive species might exhibit greater foliar N uptake compared with species employing more conservative strategies (Adriaenssens et al., 2011; Garten et al., 1998). However, due to the limited number of studies that have included multiple species comparisons of foliar N uptake capacity, there remains insufficient evidence to fully support this hypothesis.
In this study, we selected 19 woody species in North China in a common garden and conducted a 15N labelling experiment. We applied 15N-NH4+ and 15N-NO3− solutions to leaves located in the upper canopy of mature individuals. We aimed to quantitatively compare the foliar uptake capacity to 15N-NH4+ and 15N-NO3− among species and functional groups, and investigate how plant traits regulate these processes. We hypothesized that: (1) leaf recovery of 15N-NH4+ would be higher than that of 15N-NO3− for mature woody individuals; (2) leaf N recovery would be influenced by leaf traits and phylogeny; Species with higher N concentration and SLA should have higher foliar N uptake; (3) soil and meteorological conditions could affect foliar N uptake efficiency by altering leaf physiological activities.
2 MATERIALS AND METHODS
2.1 Study site and experiment treatment
This experiment was conducted at the China National Botanical Garden, Beijing (39.98° N, 116.20° E), which is one of the largest sites for ex situ conservation of biodiversity and plant germplasm in China. The mean annual air temperature is 13°C, and the mean annual precipitation is 538 mm. The ambient total wet inorganic N deposition in 2018 was 20 kg N ha−1 yr−1, with nitrate-N deposition at 5.8 kg N ha−1 yr−1 and ammonium-N deposition at 14.2 kg N ha−1 yr−1. Furthermore, the deposition during July and August accounted for 25% of the annual deposition.
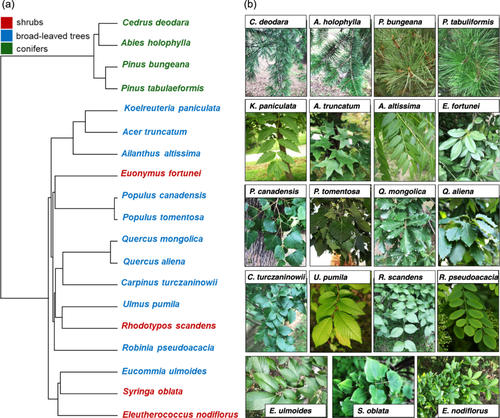
Nineteen woody species commonly distributed in North China were selected, including four shrubs, 11 broad-leaved trees and four conifers. The 19 woody species covered 14 families with a wide range of plant traits (Figure 1 and Supporting Information S1: Table S1). Ten of them are associated with arbuscular mycorrhizae (AM) and nine of them are associated with ectomycorrhizae (ECM). All individuals were grown in full sun conditions and received regular maintenance, including watering and pest control, but no fertilization.
For each species, we selected three mature healthy individuals with similar breast or basal diameters. All trees are older than 50 years and shrubs are over 25 years old. In total, there were 57 individuals. As we intended to sample three times (3 h, 5 days after 15N application and at the end of growing season), each treatment were assigned to three branches of each individual: (1) control: applied as deionized water, (2) 15N-NH4+ application: applied as 15NH4Cl solution (10 mg N/l, 30% 15N fraction), and (3) 15N-NO3− application: applied as Na15NO3 solution (10 mg N/l, 30% 15N fraction). We used a mobile hydraulic scissor lifting platform to reach the upper canopy (Supporting Information S1: Figure S1) and conducted leaf 15N labelling experiments. For each individual tree, we applicated water, 15N-NH4+ and 15N-NO3− to the branches at the same time.
The prerequisite for calculating the N recovery rate lies in accurately quantifying the amount of 15N applied to the leaf surface. Spraying and brushing are common application methods in previous manipulation studies investigating canopy uptake of atmospheric wet N deposition (Bryan Dail et al., 2009; Ferraretto et al., 2022; Nair et al., 2016; Putz et al., 2011). Although spraying treatment more closely mimics the natural rain event, accurately quantifying the amount of N applied to the leaf surface is challenging due to potential losses through volatilization or deposition on twigs during spraying. Therefore, we applied the N solution directly to the leaf surface with a brush in this study. To ensure good contact of the applied solution with the leaf surface, we added a trace amount of wetting agent (0.025%, V/V) to the N solution, following Putz et al. (2011). We recognized that brushing and using of wetting agent might introduce some potential bias in estimating the foliar nitrogen uptake capability under natural conditions. Through using the same experimental protocol, our study still can provide valuable insights into how trait variation among species could affect the process of foliar nitrogen uptake.
The treatment solutions were prepared and stored in numbered tubes. Before application, we presoaked a clean and dry brush with treatment solution, then squeezed out the excess solution. We used the soaked brush to dip the solution in a numbered tube, gently applied it to the top leaf and continued on all other leaves on the same branch. Each branch corresponded to a uniquely numbered tube. The amount of applied N on each branch was estimated by the weight difference of the tube between before and after application. The amount of N retained on the soaked brush after application can be slightly different than before application, which may introduce errors in estimating the amount of solution applied. However, as we labelled a whole branch instead of a few leaves, this error was negligible compared with the amount of N applied to the branch. The total amount of N applied to the branch varied between species due to different branch sizes and leaf surface properties (Supporting Information S1: Table S1). For broad-leaved species, the 15N solution was applied only on the upside of the leaves, as N deposition was primarily intercepted by the upside of the leaves. After the applied solution had dried, the labelled branches were wrapped with nylon mesh with a mesh size of 2 cm to ensure all leaves could be completely recovered.
The 15N applications were conducted between 8:30 and 11:30 AM on clear and windless days. Given that it was not feasible to label all 57 individuals in 1 day, the application dates ranged from late July to early August 2018 (Supporting Information S1: Table S1). Photosynthetic active radiation (PAR), air temperature and relative humidity (RH) were continuously monitored at 30-min intervals using an in situ meteorological monitoring system (Decagon Devices Inc.). Vapour pressure deficit (VPD) was calculated based on the measured air temperature and RH. The average values of meteorological variables recorded from the time of 15N labelling to the sample collection (3 h after 15N labelling or 5 days after 15N labelling) were used for further statistical analysis. Each plant individual experienced slightly different meteorological conditions during the sampling period due to the varied application dates.
2.2 Plant and soil sampling and measurements
Three plant samplings were conducted 3 h after the 15N application, 5 days after the 15N application and at the end of the growing season. The timing of the samplings conducted 3 h and 5 days after the 15N applications depended on the application time for each individual (Supporting Information S1:Table S1). The samplings at the end of the growing season were conducted in early November, when most leaves of the deciduous species had senesced and started to fall. For each sampling, we randomly collected one of the labelled branches per treatment of each individual and transported the samples to the laboratory immediately. In the first two samplings, we collected branches from all 57 individuals under each treatment. However, in the third sampling, due to damage to several branches and labels, we were only able to collect branches from 43 individuals, of which 32 were deciduous species. Leaves and twigs were separated from each branch. To simulate rain washing that occurs under natural conditions, the samples were washed and rinsed with distilled water to remove any residue of the solution left on the surface. We noted that washing leaf samples with deionized water could remove the remaining N physically attached to the leaf surface, but it might not remove all N retained in the cuticle with the wax layer or by epiphytic phyllosphere microbes (Ferraretto et al., 2022; Kembel et al., 2014). However, nitrogen uptake by these processes is also an important pathway for canopy interception of N deposition.
After drying the surface with paper towels, the fresh leaves were stored at 4°C for further measurements of leaf morphological and biochemical traits. Detailed measurements of 12 plant traits (diameter at breast height, plant height, SLA, leaf water content, total chlorophyll concentration, nitrogen concentration, soluble phenols concentration, soluble sugars concentration, condensed tannins concentration, lignin concentration, hemicellulose concentration and wax concentration) were provided in Supporting Information S1: Methods S1. The N stable isotope (15N fractional abundance, 15N/(14N + 15N)) and N concentration of leaf and twig samples were determined using a Delta Plus XP isotope ratio mass spectrometer (Thermo Finnigan).
Given the inherent heterogeneity of soil physicochemical properties, even in a common garden, we also evaluated how variations in soil conditions could affect leaf N uptake. Three soil cores (5 cm in diameter and 10 cm in depth) were collected at a distance of 0.5 m from the trunk of each individual and then mixed to form one composite sample. The processes of soil samples and measurements of 11 soil properties (dissolved inorganic nitrogen concentration [DIN], including ammonium and nitrate, total N concentration, total C concentration, available phosphorus concentration, total phosphorus concentration, water content, water holding capacity, bulk density, clay, silt and sand concentration) were detailed in Supporting Information S1: Methods S1.
2.3 The calculation of 15N recovery
2.4 Statistical analysis
A phylogenetic tree, including all 19 woody species, was constructed using the ‘V. PhyloMaker’ package in R (Figure 1) (Jin & Qian, 2019). Blomberg's K and Pagel's λ, calculated using the package ‘phytools’, was used to evaluate the strength of the phylogenetic signal for each plant trait as well as N recoveries of leaves and twigs (Supporting Information S1: Table S2) (Blomberg et al., 2003; Pagel, 1999; Revell, 2012).
To assess the differences in leaf N recoveries and twig N recoveries, we conducted linear mixed-effects models (LMEs) using lmer function in the ‘lme4’ package (Bates et al., 2015). When assessing the effects of N form and species, we considered N form, species and their interactions as fixed effects, and date of application as random factor. The significance of each fixed factor was tested by the ‘lmerTest’ package (Kuznetsova et al., 2017). When comparing functional groups, we fit LMEs with N form, functional group and their interaction as fixed factors, and species identity and application date as random factors. The differences among functional groups were estimated by Post hoc analysis of the Tukey method using the ‘emmeans’ package. For mycorrhizal types, we considered N form, mycorrhizal type and their interaction as fixed factors and species identity and application date as random factors. Furthermore, we evaluated the effects of N form and N application duration (3 h after 15N labelling, 5 days after 15N labelling and the end of growing season) on leaf N recovery as well as twig N recovery using LMEs with N form, N application duration and their interaction as fixed effects, and species identity and application date as random factors. Additionally, we assessed the impact of each plant trait, soil property and meteorological condition on leaf N recovery separately. We fitted LMEs with each plant trait, soil property and meteorological condition as a fixed factor, and species identity and date of application were considered as random factors.
Principal components analyses (PCA) were separately performed for the 12 plant traits and for the 11 soil characteristics at the individual level (57 observations for each variable). Horn's Parallel Analysis using the ‘paran’ package was conducted to determine the component retention in PCA analysis. Here, the first two principal components (PC) of plant traits and soil conditions should be retained for further analysis (Supporting Information S1: Figure S2). LMEs were used to examine the relations between each PC of plant traits/soil conditions and leaf/twig N recovery and to compare whether the slopes of these relationships were different between the two N applications separately. In these models, we considered each PC as a fixed factor, and species identity and date of application as random factors. Marginal R2 representing the variance explained by fixed factors of the linear mixed-effects models were estimated using the ‘MuMIn’ Package (Nakagawa & Schielzeth, 2013).
Statistical significance was defined as p < 0.05. All statistical analyses were performed in R version 4.3.0.
3 RESULTS
3.1 Leaf N recovery at three sampling times after 15N labelling
Leaf recoveries of different N forms exhibited significant variation among the 19 woody species (Figure 2) but did not show significant phylogenetic signals (Supporting Information S1: Table S2). For samples collected 3 h and 5 days after 15N labelling, the leaf 15N recovery under 15N-NO3− treatment was significantly higher than that of 15N-NH4+ treatment for shrubs and broad-leaved trees, but not for conifers (Figure 2a,b). Conifer trees exhibited lower leaf 15NO3− recovery than broad-leaved trees at the 3rd h and 5th day collections (Figure 2a,b). Additionally, for samples collected 5 days after 15N labelling, the average leaf 15N-NH4+ recovery decreased, while the leaf 15N-NO3− recovery increased compared with the 3rd h collection (Figure 4a). Mycorrhizal types had no significant effects on leaf N recovery for all species or for broad-trees only (Supporting Information S1: Figure S3).
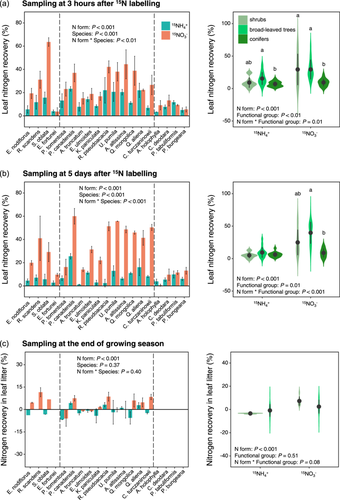
At the end of the growing season, the 15N recoveries in senescent leaves did not show significant differences between functional groups or mycorrhizal types (Figure 2c, Supporting Information S1: Figure S3). The 15N recoveries in senescent leaves were significantly lower than the two previous collections (Figure 4a). Furthermore, the N concentrations of leaf litter at the end of the growing season were significantly lower than that of green leaves in the growing season (Supporting Information S1: Table S3).
3.2 Twig nitrogen recovery at three sampling times after 15N labelling
The twig N recoveries were much smaller than the leaf N recoveries and showed variation among the 19 woody species (Figure 3). The twig 15N recovery under 15NO3− treatment was significantly higher than that under 15NH4+ recovery for all three functional groups at the 3rd h collection (Figure 3a,b). The twig 15N-NO3− recovery of conifers was higher than that of shrubs and broad-leaved trees (Figure 3a,b). At the end of the growing season, N forms and functional groups had no significant effects on twig 15N recovery (Figure 3c). Furthermore, the average twig 15N-NH4+ recovery at the end of the growing season was higher than that of the 3rd h and the 5th day collections (Figure 4b). However, it is worth to note that these values reflect the amount of 15N incorporated by the twigs and do not account for the amount of 15N transported from the leaves to other parts of the plants.

3.3 Effects of soil and meteorological conditions on leaf N recovery
Soil physiochemical characteristics varied among individuals (Supporting Information S1: Figure S4). However, the leaf N recoveries under 15NH4+ and 15NO3− treatments did not correlate with most of the 11 soil characteristics (Supporting Information S1: Table S4). The results of PCA showed that the first two PCA axes represented a soil fertility gradient and a soil texture gradient, respectively (Supporting Information S1: Figure S4). However, neither soil fertility nor soil texture was significantly related with leaf N recovery (Table 1, Supporting Information S1: Figure S5).
| Sampling at 3 h after 15N labelling | Sampling at 5 days after 15N labelling | |||||||
|---|---|---|---|---|---|---|---|---|
| 15NH4+ | 15NO3− | 15NH4+ | 15NO3− | |||||
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| Soil conditions | ||||||||
| Soil fertility | −0.25 | 0.73 | 0.71 | 0.55 | 0.11 | 0.84 | 0.90 | 0.46 |
| Soil texture | 0.82 | 0.33 | 0.05 | 0.97 | 0.89 | 0.12 | −0.17 | 0.89 |
| Meteorological conditions | ||||||||
| PAR | 0.01 | 0.08 | 0.02 | 0.03 | −0.01 | 0.37 | 0.06 | 0.08 |
| Air temperature | 1.71 | 0.12 | 0.19 | 0.92 | −0.90 | 0.58 | 7.59 | 0.13 |
| RH | −0.51 | 0.08 | −0.62 | 0.20 | 0.51 | 0.54 | −3.67 | 0.16 |
| VPD | 6.64 | 0.10 | 5.57 | 0.40 | −8.72 | 0.43 | 38.36 | 0.27 |
- Note: Environmental conditions include soil properties (soil fertility: loadings on the first PCA axis for the 11 soil characteristics; soil texture: loadings on the second PCA axis for the 11 soil characteristics) and meteorological conditions (PAR, air temperature, RH, VPD). The bold values indicate significant relationships (p < 0.05).
- Abbreviations: PAR, photosynthetically active radiation; PCA, principal components analyses; RH, relative humidity; VPD, vapour pressure deficit.
For sampling at 5 days after 15N labelling, leaf N recovery under 15NO3− treatment was positively related to PAR but had nonsignificant relationships with air temperature, RH and VPD across all 19 species (Table 1). Furthermore, the leaf 15N-NH4+ recovery across all 19 species was not related to any meteorological factor at the two collections (Table 1).
3.4 The relationships between plant traits and nitrogen recovery of leaves and twigs
In general, leaves with high chlorophyll, high leaf N and low wax concentrations have higher 15N recoveries. Compared with 15N-NH4+, the recovery of 15N-NO3− was more correlated by plant traits (Table 2).
| Sampling at 3 h after 15N labelling | Sampling at 5 days after 15N labelling | Sampling at the end of the growing season | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15NH4+ | 15NO3− | 15NH4+ | 15NO3− | 15NH4+ | 15NO3− | |||||||
| Estimate | p | Estimate | p | Estimate | p | Estimate | p | Estimate | p | Estimate | P | |
| DBH | 0.07 | 0.60 | 0.02 | 0.95 | 0.16 | 0.17 | −0.16 | 0.63 | 0.01 | 0.93 | −0.33 | 0.03 |
| H | 0.37 | 0.26 | 0.20 | 0.76 | 0.46 | 0.09 | −1.62E-04 | 1.00 | 0.12 | 0.59 | −0.61 | 0.02 |
| ChlT | 1.36 | 0.06 | 3.13 | 0.01 | 0.74 | 0.18 | 3.94 | <0.01 | 0.98 | 0.11 | −0.06 | 0.94 |
| SLA | 0.02 | 0.56 | 0.09 | 0.14 | −0.01 | 0.59 | 0.13 | 0.04 | 0.02 | 0.54 | 0.06 | 0.06 |
| LWC | 0.17 | 0.56 | 0.27 | 0.62 | 0.22 | 0.35 | −0.09 | 0.88 | 0.07 | 0.73 | −0.16 | 0.54 |
| N | 10.19 | <0.01 | 18.97 | <0.01 | 7.65 | 0.01 | 24.15 | <0.001 | 3.66 | 0.23 | −2.42 | 0.49 |
| Phenols | 0.02 | 0.15 | 0.05 | 0.09 | −4.54E-03 | 0.74 | 0.05 | 0.17 | 0.01 | 0.41 | −0.01 | 0.40 |
| Sugar | 0.02 | 0.43 | 0.03 | 0.47 | 0.01 | 0.52 | 0.07 | 0.15 | −3.65E-03 | 0.88 | −0.04 | 0.08 |
| Tannin | 0.03 | 0.27 | 0.04 | 0.42 | −4.91E-03 | 0.81 | −9.99E-04 | 0.99 | 0.02 | 0.44 | 6.49E-04 | 0.98 |
| Lignin | −0.09 | 0.36 | 0.14 | 0.46 | 1.25E-03 | 0.99 | −0.17 | 0.37 | −0.01 | 0.85 | 0.09 | 0.28 |
| Hemicellulose | −4.08E-03 | 0.23 | −9.55E-04 | 0.85 | −2.49E-03 | 0.27 | 1.79E-04 | 0.97 | 0.01 | 0.19 | −1.86E-04 | 0.98 |
| Wax | −4.99 | 0.09 | −11.72 | 0.04 | −2.01 | 0.43 | −19.62 | <0.01 | −3.36 | 0.74 | −19.58 | 0.12 |
- Note: Plant traits include diameter at breast height (1.3 m, for trees) or basal height (0.2 m, for shrubs) (DBH), height (H), total leaf chlorophyll concentration (ChlT), specific leaf area (SLA), leaf water content (LWC), leaf nitrogen concentration (N), leaf soluble phenols concentration (Phenols), leaf soluble sugar concentration (Sugar), leaf condensed tannins concentration (Tannin), leaf lignin concentration (Lignin), leaf hemicellulose concentration (Hemicellulose) and leaf wax concentration (Wax). The bold values indicate significant relationships (p < 0.05).
The results of PCA showed that the first two axes for the 12 plant traits accounted for 51.2% of the overall variation (Figure 5a). The values of the first PCA axis (PC1) could be used to represent the leaf economic spectrum of plants, with the positive PC1 represented individuals with high SLA, LWC, total chlorophyll and leaf N, while the negative values represented individuals with high lignin, wax and hemicellulose concentration (Figure 5a). The values of the second PCA axis represented plant size, and the positive PC2 indicated the larger plant individuals with greater height and DBH (Figure 5a).
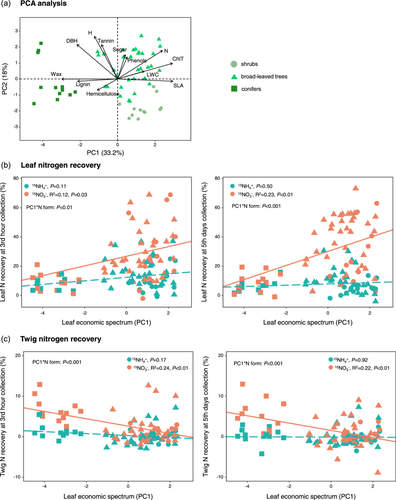
Plant individuals with higher PC1 values of leaf economic spectrum showed higher leaf N recoveries under 15NO3− treatments at the 3rd h and the 5th day collection (Figure 5b), but lower twig 15N-NO3− recovery (Figure 5c). N recovery in the senescent leaves and twigs did not correlate with leaf economic spectrum at the end of the growing season (Supporting Information S1: Figure S6). Leaf 15N recovery at the 3rd h and the 5th day collections was positively correlated with plant size (Supporting Information S1: Figure S7). However, plant size had little effects on twig N recovery (Supporting Information S1: Figure S8).
4 DISCUSSION
4.1 Foliar uptake of 15N-NO3− is more efficient than 15N-NH4+
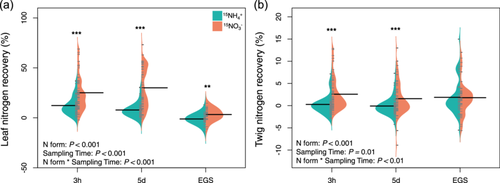
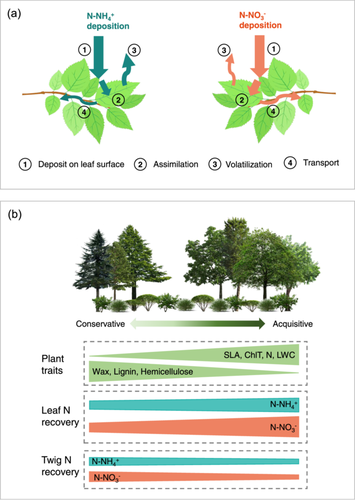
Our results showed that leaves of mature woody plants could uptake inorganic N directly from wet deposition (Figures 2 and 6). With time increased from 3 h to 5 days after 15N labelling, the average leaf 15N-NH4+ recovery decreased, whereas the leaf 15N-NO3− recovery increased (Figure 4). This indicated that for mature woody plants, foliar uptake of inorganic N from wet deposition occurs within a few hours, and the leaf NO3− absorption process potentially last several days. This might be because NO3− had a longer residence time on the leaf surface due to it being chemically more stable than NH4+. Furthermore, the leaves of most woody species assimilated 15N-NO3− more efficiently than 15N-NH4+ (Figures 2 and 6), which was consistent with several field studies conducted in mature natural forests (Bryan Dail et al., 2009; Fenn et al., 2013; Ferraretto et al., 2022).
Theoretically, because the cuticle is generally negatively charged due to the ionization of carboxyl groups and galacturonic acids, NH4+ should be more readily transported through cuticles than NO3− due to its positive charge (Fernández & Eichert, 2009; Ishfaq et al., 2022; Schönherr & Huber, 1977; Tyree et al., 1990). Its lower ionic molecular weight and smaller hydrated radius should also facilitate its foliar absorption (Schönherr & Schreiber, 2004; Tyree et al., 1990). However, previous studies have indicated that the efficiency of leaf N uptake also is largely influenced by meteorological conditions through their impact on the residence time of deposited N on the leaf surface and leaf physiological activities (Eichert & Fernández, 2012; Fernández & Eichert, 2009; Vallano & Sparks, 2008; Wuyts et al., 2015). High air temperature and PAR could cause more NH4+ to be released as gaseous (NH3) after being deposited on leaf surface (Wentworth et al., 2016). Accumulated NH4+ in the cytosol due to higher leaf photorespiration under high PAR conditions could also inhibit the diffusion rates of NH4+ through the cuticular penetration (Keys, 2006; Novitskaya et al., 2002). Previous studies showed that high PAR could increase nitrate reduction in the leaves (Chapin et al., 2011; Tischner, 2000), thereby accelerating NO3− diffusion rates into leaves.
Additionally, the hygroscopicity of N compounds could also affect the foliar uptake process. For the two chemical compounds used for our 15N labelling experiment, the lower deliquescent relative humidity (DRH) of NaNO3 compared with NH4Cl could result in higher permeability of NO3− than NH4+ under the ambient RH conditions (Burkhardt, 2010; Fernández & Eichert, 2009; Fernández et al., 2017). Those processes may together result in a higher initial 15N-NO3− recovery than 15N-NH4+.
4.2 Foliar N uptake regulated by plant traits
In addition to the chemical forms of N, leaf traits that affect leaf surface wettability and resource acquisition could significantly influence leaf 15N recoveries across species (Table 2 and Figures 2, 5, 6). We found that plants have higher leaf wax concentration had lower 15NO3− recovery at both 3 h and 5 days samplings, although this effect was not significant for 15NH4+. The main pathways for foliar N absorption can be related to the cuticle/wax layer of epidermal cells, trichomes, stomata, veins, and other epidermal structures (Fernández et al., 2021; Ishfaq et al., 2022). The chemical composition and structure of cuticle/wax layer affect hydrophobicity, porosity and the formation of hydrophilic pores, all of which can either facilitate or hinder the nutrient penetration (Fernández et al., 2021; Schönherr, 2006; Shepherd & Griffiths, 2006). Additionally, trichome density and the chemical heterogeneity of trichome surfaces have been shown to affect leaf water capture and solute permeability (Fernández et al., 2024; Schreel et al., 2020). However, we only measured the wax concentration of the leaves in our study. Future studies are needed to elucidate the mechanisms by which the physicochemical properties of leaf surfaces influence foliar N uptake.
Furthermore, our results showed that leaf N recovery was correlated with leaf traits and plant size (Figure 5, Supporting Information S1: Figure S7), although it was not influenced by phylogenetic history or mycorrhizal type (Supporting Information S1: Table S2 and Figure S3). Species exhibiting acquisitive traits (higher leaf N and ChlT concentrations and larger SLA) demonstrated higher foliar N uptake efficiency, likely due to their high N demand and physiological activity (Wright et al., 2004; Wuyts et al., 2015). Plant size of adult individuals is an important integrated index of plant traits, indicating the ability to compete for resources and whole plant fecundity (Díaz et al., 2016). The positive relationships between leaf N recovery with plant size (Supporting Information S1: Figure S7) suggested that species with large body might acquire more N through foliar uptake. When comparing functional groups, the mean leaf 15N recoveries of the more acquisitive broad-leaved species were 1.6–3.2 times higher than those of the more conservative conifers, which is consistent with previous studies on saplings (Adriaenssens et al., 2011; Garten et al., 1998; Wuyts et al., 2015). However, this study was conducted at the leaf scale on individual branches. Considering the differences in leaf phenology and canopy structure, the annual N uptake of the entire canopy of broad-leaved trees and conifers still needs to be fully estimated.
4.3 Soil and meteorological conditions had limited impacts on foliar N uptake
Besides plant traits, soil nutrient availability may also mediate the foliar N uptake process by influencing plant nutrient status. Previous pot experiments have found that soil N addition could enhance leaf N concentration and thus nitrate reductase activity, further promoting the foliar uptake of NO2 and inorganic N solution (Vallano & Sparks, 2008; Wuyts et al., 2015). However, in our study, the effects of soil fertility and soil texture on leaf N recovery were not significant (Table 1 and Supporting Information S1: Table S4, Figure S5). This could be due to the relatively narrow range of the soil physiochemical properties in our study.
Meteorological conditions are known to play an important role in regulating foliar N uptake (Fernández & Eichert, 2009; Fernández et al., 2021; Ishfaq et al., 2022). For example, high RH could stimulate foliar N uptake by slowing the drying of applied solute on leaf surface and improving the level of cuticle hydration (Fernández & Eichert, 2009; Fernández et al., 2017; Schönherr, 2001). Moderate air temperature and light levels could stimulate stomatal opening and enhance various physiological processes in plants, such as photosynthesis and transpiration, thereby increasing the rate of foliar N uptake (Fernández & Eichert, 2009). While these meteorological factors could impact the initial foliar uptake process of NH4+ and NO3− (see Section 4.1), they had limited effects on the variation in foliar N uptake across the 19 species in our study (Table 1). This may be attributed to the relatively stable meteorological conditions during the experiment. Further experiments are needed to gain deeper insights into the intricate interactions between metrological conditions, N forms and plant traits in regulating foliar N uptake.
The common garden approach enabled us to explore how leaf traits affect foliar N uptake under similar soil and meteorological conditions. However, it also introduces uncertainties when extrapolating our findings to natural forest ecosystems. Although most species in this study are widespread in North China and experience similar climatic conditions to those in the garden, the common garden still differs from their natural habitat. Factors like clustered growth, varied age classes, symbiotic relationships with microorganisms and variable soil fertility due to land-use history can contribute to these uncertainties. To gain a more comprehensive understanding of how plant traits, soil and metrological conditions interact to influence foliar N uptake, further field studies across different climate zones, alongside common garden experiments, are necessary.
4.4 The translocation of 15N taken up by leaves
In the root pathway, once absorbed, N will be transported from the roots to other organs, such as leaves, shoots and twigs (Nair et al., 2016; Wang et al., 2021). However, the extent to which the N absorbed by leaves is transported to other plant components remains unclear. In this study, we observed very low twig 15N recovery at 3 h and 5 days after 15N labelling (Figures 3 and 4). As 15N solution was applied on the leaf surface, the enriched 15N of twigs should originate from the transfer of 15N absorbed by the leaves. The low twig 15N recovery indicated that the translocation of leaf assimilated 15N to the rest of the tree was limited in a short period. Our findings were consistent with a previous study using a girdling method, which found that 15N uptake by canopy leaves was not translocated to other parts of the tree within 24 h (Ferraretto et al., 2022).
Divergent responses were observed between leaves and twigs regarding variations in the leaf economic spectrum. While leaf 15N-NO3− recovery showed an increasing trend, twig 15N-NO3− decreased as PC1 values of leaf economic spectrum increased (Figures 5 and 6). This divergence may be attributed to the rapid utilization of assimilated 15N by leaves for the physiological actives, particularly in species with high N demand. However, by the end of the growing season, the 15N retained by leaves in the growing season might be transported to woody tissues through resorption during leaf senescence (Vergutz et al., 2012; Wang et al., 2021). This is supported by the decrease in 15N recoveries and N concentrations in leaf litters, along with the higher N concentrations in twigs at the end of growing season compared with the peak growing season (Figures 2 and 4, Supporting Information S1: Table S3).
5 CONCLUSIONS
In summary, our study demonstrated that the leaves of mature woody plants can utilize inorganic N directly from wet deposition within a few hours. Most woody plant species recovered more 15N via leaves under 15NO3− treatment than that under 15NH4+ treatment (Figure 6). Despite potential biases, the consistent N application approach allows for reliable comparisons of foliar N uptake capacity across species, an aspect that has been lacking in previous research. Our findings highlight that species with higher leaf N concentration, larger SLA and lower wax concentration could absorb N deposited on leaf surface more efficiently, especially for NO3− (Figures 5 and 6). In addition, our findings also implied that N deposition might benefit the N supply of broad-leaved trees more than conifers during the growing season, as broad-leaved trees could acquire N more efficiently through foliar uptake.
It is worth noting that we only assessed foliar uptake of dissolved N (NH4+ and NO3−), potentially leading to an underestimation of total canopy N uptake. This limitation arises because leaves can also assimilate gaseous and particulate forms of N (Burkhardt, 2010; Harrison et al., 2000; Sparks et al., 2001). Furthermore, more thorough measurements of leaf surface characteristics are needed, along with studies on how canopy structure, phyllosphere microbiome, phenology, and background N deposition could influence whole canopy foliar N uptake (Adriaenssens et al., 2012; Fenn et al., 2013; Fernández et al., 2017; Guerrieri et al., 2021; Houle et al., 2015). Further research on canopy N processes is necessary to resolve these uncertainties and to gain a more complete understanding of how forests respond to atmospheric N deposition under climate change.
ACKNOWLEDGEMENTS
The authors thank Li Zhang and Shumin Zhang from Plant Science Facility of the Institute of Botany, Chinese Academy of Sciences for their excellent technical assistance in the laboratory work. This study was financially supported by the National Natural Science Foundation of China (32171500, 32125025, 31988102), Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020082).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




