CIPK11 phosphorylates GSTU23 to promote cold tolerance in Camellia sinensis
Abstract
Cold stress negatively impacts the growth, development, and quality of Camellia sinensis (Cs, tea) plants. CBL-interacting protein kinases (CIPK) comprise a pivotal protein family involved in plant development and response to multiple environmental stimuli. However, their roles and regulatory mechanisms in tea plants (Camellia sinensis (L.) O. Kuntze) remain unknown. Here we show that CsCBL-interacting protein kinase 11 (CsCIPK11), whose transcript abundance was significantly induced at low temperatures, interacts and phosphorylates tau class glutathione S-transferase 23 (CsGSTU23). CsGSTU23 was also a cold-inducible gene and has significantly higher transcript abundance in cold-resistant accessions than in cold-susceptible accessions. CsCIPK11 phosphorylated CsGSTU23 at Ser37, enhancing its stability and enzymatic activity. Overexpression of CsCIPK11 in Arabidopsis thaliana resulted in enhanced cold tolerance under freezing conditions, while transient knockdown of CsCIPK11 expression in tea plants had the opposite effect, resulting in decreased cold tolerance and suppression of the C-repeat-binding transcription factor (CBF) transcriptional pathway under freezing stress. Furthermore, the transient overexpression of CsGSTU23 in tea plants increased cold tolerance. These findings demonstrate that CsCIPK11 plays a central role in the signaling pathway to cold signals and modulates antioxidant capacity by phosphorylating CsGSTU23, leading to improved cold tolerance in tea plants.
1 INTRODUCTION
Low temperature (cold, freezing) is an abiotic stresses that adversely influence plant growth and development (Pearce, 2001). Plants have evolved a variety of regulatory mechanisms to tolerate unfavorable cold temperatures (Guy, 1990; Ruelland et al., 2009). Cold tolerance in plants depends on cold signal perception and cellular signal transduction pathways, with calcium (Ca2+) playing an important role. In response to cold stress, cold signaling is perceived by sensors, for example, COLD1 in Oryza sativa and alteration in membrane fluidity lead to an influx of Ca2+ to initiate a signaling cascade to mediate tolerance to lower temperature (Ma et al., 2015). Calcineurin B-like protein (CBL)-interacting protein kinases (CIPKs) play important roles in Ca2+ signal transduction. CIPKs contain a kinase domain at the N-terminal and a regulatory domain at the C-terminal. CIPKs are plant-specific serine-threonine (S/T) protein kinases, and SOS3-SOS2 was originally identified as a typical CBL-CIPK module in Arabidopsis thaliana (Liu & Zhu, 1998; Liu et al., 2000). Extensive studies have established that the CIPK network plays a crucial role in the evolution of plants in terms of physiology and adaptation to a variety of abiotic stresses (Kleist & Luan, 2016; Luan et al., 2009; Steinhorst et al., 2015; Zhou et al., 2015).
CIPKs play prominent roles in plant adaptation to cold conditions. In rice, a point mutation in the OsCIPK7 kinase domain leads to a conformational change, resulting in an open form of the protein kinase; this OsCIPK7 mutant showed increased 4°C chilling tolerance (Zhang, Guo, et al., 2019). OsCIPK03-overexpressing plants accumulate higher contents of soluble sugars and proline; specifically, OsCIPK03 overexpression significantly improves rice tolerance to 4°C low temperatures (Xiang et al., 2007). CaCIPK13-overexpressing tomato plants and CaCIPK13-silenced pepper plants exhibit increased and decreased tolerance to 4°C chilling stress, respectively (Ma et al., 2021). Overexpression of grape VaCIPK18 in Arabidopsis upregulates the C-repeat binding transcription factor (CBF) transcriptional pathway and reduces reactive oxygen species (ROS) levels, thus enhancing cold tolerance (Yu et al., 2022a).
Cold stress can affect multiple biological processes via ROS overaccumulation. Excessive ROS levels in cells pose hazards to a variety of cellular components, resulting in cell death (Apel & Hirt, 2004). Plants have developed both nonenzymatic and enzymatic antioxidant defense systems to modulate the over-accumulation of ROS caused by cold stress. Glutathione S-transferases (GSTs, EC 2.5.1.18), a superfamily of versatile enzymes, mostly catalyze the conjugation of reduced glutathione (GSH) to a variety of cytotoxic compounds, exhibiting a broad spectrum of functions in the resistance to cellular damage resulting from ROS and toxic substrates, as well as maintenance of the cellular redox system (Cummins et al., 2011; Sappl et al., 2009). In plants, substantial evidence has shown that tau class glutathione S-transferases (GSTUs) maintain ROS homeostasis under stresses (Zhang, Yang, et al., 2019). A null mutant of gstu7 exhibited decreased osmotic resistance during seed germination by accumulating higher levels of reduced GSH and decreased endogenous H2O2 in Arabidopsis (Wu et al., 2020). Ectopic GsGSTU13-overexpressing Medicago sativa lines also showed enhanced salt-alkaline tolerance (Jia et al., 2016). Alfalfa MsCML10 interacts with MsGSTU8, modulates ROS homeostasis, and contributes to cold tolerance (Yu, Wu, et al., 2022). Poncirus trifoliate PtrERF9 modulates PtrGSTU17 expression and GST activity to regulate ROS levels in response to cold stress (Zhang et al., 2022).
Tea plants (Camellia sinensis (L.) O. Kuntze) are a staple economic crop worldwide. As tea plants originate in tropical or subtropical areas, their growth and development are sensitive to low temperatures (Wang et al., 2020). Freezing stress in winter and cold spells in spring cause serious damage to tea yield and quality (Hao et al., 2018). Enhancing the ability of tea plants to withstand low-temperature stress is crucial for the tea industry. In a previous study, we found that the transcript abundance of several CsCIPK genes increased in abundance in the cold stress response of tea plants (Wang et al., 2020). In this study, to determine the functional role of CsCIPK11 in response to cold stress, CsCIPK11 was heterologously overexpressed in Arabidopsis and transiently suppressed in tea plants. We further screened and validated the interacting protein to explore the mechanism of CsCIPK11 in response to low-temperature stress. Our results will lay the theoretical foundation for breeding tea plants for cold resistance.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
The tea plant materials used in this study were all cultivated at the Tea Research Institute of the Chinese Academy of Agricultural Sciences, Hangzhou, China, including the cultivar ‘Longjing 43’ (LJ43), and three cold-resistant (#R1, #R2 and #R3) and three cold-susceptible (#S1, #S2 and #S3) tea plant accessions (Wang et al., 2022). The first leaves of six 3-year-old tea plant accessions (#R1, #R2, #R3, #S1, #S2 and #S3) at the top of the branch were collected on December 13 and January 17. For time-course expression analysis, 1-year-old potted tea plant seedlings of LJ43 were grown in the chamber at 12 h 22°C light/12 h 20°C dark, and were treated with 4°C for cold treatment. One bud with one leaf (1B1L) and mature leaf (ML) samples of tea plants were sampled at 0 h, 3 h, 6 h, 1 d, 3 d, 5 d and 7 d posttreatment, and after 2 days recovery. Three biological replicates were used for all experiments
The Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used to generate transgenic lines. To construct CsCIPK11-overexpressing (OE) lines, the CsCIPK11 (Cha11g008040) ORF was amplified and cloned into the vector pCAMBIA S1300 containing a super promoter (Lee et al., 2007). The primer sequences used for gene amplification are listed in Supporting Information: Table S1. For the freezing treatment, Arabidopsis seedlings were grown under 10 h 22°C light/14 h 20°C dark before cold treatment, 21-day-old seedlings were subjected to a cold acclimation (CA) treatment at 4°C for 4 day (with normal light dark cycle as above), then exposed to −10°C for 8 h; 21-day-old seedlings grown at 22°C served as control. The aerial parts of the Arabidopsis before and after −10°C treatment was sampled for relative electrolyte leakage (REL), Fv/Fm, and antioxidant capacity measurements.
Transgenic Nicotiana benthamiana plants with a nuclear marker H2B-RFP were used for subcellular localization and bimolecular fluorescence complementation (BiFC) assays (Li, Zhao, et al., 2017). Wild-type tobacco N. benthamiana was used for luciferase complementation imaging (LCI) analysis and protein degradation analysis. Tobacco seedlings were grown under a 16/8 h light/dark cycle (22/20°C) for 28 days before injection.
2.2 Total RNA isolation and quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted using an RNAprep Pure kit (Tiangen) following the manufacturer's instructions. Complementary DNA (cDNA) was produced using a PrimeScript™ RT reagent kit (TaKaRa). qRT-PCR was carried out using a Light Cycler 480 machine (Roch) with SYBR Green I Master Mix (Roche Basel). The relative expression levels of the genes were calculated to the CsPTB reference gene using the 2−∆∆CT method (Hao et al., 2014, Livak & Schmittgen, 2001). The qRT-PCR primer sequences are listed in Supporting Information: Table S2.
2.3 Subcellular localization
To determine the subcellular localization of CsCIPK11 and tau class glutathione S-transferase 23 (CsGSTU23, Cha09g013270) in plants, the ORF of CsCIPK11 and CsGSTU23 without a stop code was fused with a green fluorescent protein (GFP). CsCIPK11-GFP and CsGSTU23-GFP fusion proteins were expressed in the leaves of N. benthamiana expressing the nuclear marker H2B-RFP, as previously described (Peng et al., 2022). A Zeiss LSM710 confocal laser scanning microscope (Zeiss) was used for imaging.
2.4 Measurement of relative electrolyte leakage, Fv/Fm, antioxidant capacity, and GST enzymatic activity
The REL of tea plants and Arabidopsis was determined as previously described (Wang et al., 2019; Yao et al., 2020). Briefly, the 15 leaf discs of tea plant and aerial parts of Arabidopsis were collected, respectively, and placed into a 50 mL tube with 15 mL deionized water. The tubes were shaken at 200 rpm under 25°C for 2 h. The conductivity of the solutions before and after boiling was measured. Before Fv/Fm measurements, the plants were placed in the dark for 20 min, and the Fv/Fm values of the leaves were conducted on a FluorCam 7 instrument (Photon Systems Instruments). The clearance rate of free radicals and total antioxidant capacity were determined using DPPH and FRAP kits (Comin), according to the manufacturer's protocol. To measure the GST enzymatic activity in vivo, 35S-CsGSTU23 was transiently co-expressed with 35S-CsCIPK11 and the control vector in the leaves of N. benthamiana plants, respectively. After 3 days, the leaves were sampled, and GST enzymatic activity was measured using a GST activity test kit (Comin) following the manufacturer's instructions. To measure the effect of the mutation of site 37 on GST enzymatic activity, the Serine37 of CsGSTU23 was mutated to Alanine37. Proteins were expressed in Escherichia coli BL21(DE3) cells and purified using Ni-NTA Agarose Resin (Thermo Fisher Scientific). Then, the same amount of CsGSTU23 (WT; Serine37) and CsGSTU23 (37A, Alanine37) proteins were used for GST activity measurement.
2.5 CsCIPK11 gene suppression in tea plants
Antisense oligonucleotides (AsODN) and sense oligonucleotides (sODN) sequences were designed using Soligo software by inputting the CsCIPK11 cDNA sequence (Ding & Lawrence, 2003). The sequences are listed in Supporting Information: Table S3. The first leaves of young shoots were collected from ‘LJ43’ tea plants for sODN and AsODN treatments. The leaves were soaked in 1 mL of 20 μM AsODN or sODN solution and cultivated in a climatic chamber under a 12/12 h light/dark cycle (22/20°C) as previously described (Li et al., 2022; Zhao et al., 2023). After 24 h of incubation, the leaves were divided into two groups: one group was sampled immediately, and the other group was sampled after being subjected to −2°C for 2 h. In each group, 10 leaves were used as 10 replicates for Fv/Fm analysis, three leaves were used as three replicates for REL measurement, and 18 leaves were divided into three replicates and were used for total antioxidant capacity, free radical clearance rate, and expression analysis. The phenotype was recorded under recovery for 2 h at room temperature after −2°C treatment.
2.6 Yeast two-hybrid (Y2H) assay
Coding DNA sequences or truncated fragments of CsGSTU23 and CsCIPK11 were conducted into the prey vector pGADT7 and bait vector pGBKT7, respectively. The recombinant pGADT7 and vectors pGBKT7 were co-transformed into chemically competent AH109 yeast cells in specified combinations, following the manufacturer's protocol (Weidi). Positive transformants grown on SD-Trp/-Leu (DDO) medium were transferred to a screening medium (SD-Trp/-Leu/-His/-Ade, QDO) to confirm possible interactions.
2.7 BiFC and LCI assays
For the BiFC and LCI assays, Agrobacterium tumefaciens GV3101 strain was transformed with binary constructs, nYFP/cYFP and nLuc/cLuc were transiently expressed in the leaves of N. benthamiana plants expressing the nuclear marker H2B-RFP and wild-type N. benthamiana, respectively. After incubation for 3 days, BiFC assays were conducted using the Zeiss LSM710 confocal laser scanning microscope (Zeiss). The LCI assay was conducted on a chemiluminescence image analysis system (Tanon).
2.8 GST pull-down assay
GST or CsCIPK11-GST proteins were expressed in Escherichia coli BL21(DE3) cells and purified using Pierce Glutathione Agarose (Thermo Fisher Scientific). For the pull-down assays, 10 μg of GST or CsCIPK11-GST proteins and 10 μL glutathione agarose beads were incubated in 500 μL pull-down buffer at 4°C for 1.5 h with gentle shaking. Then, the agarose beads were collected and further incubated with CsGSTU23-His proteins at 4°C for 1.5 h with gentle shaking. Proteins bound to the agarose beads were released by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. The proteins were detected via immunoblotting using an anti-His antibody (Abmart).
2.9 Phosphorylation assays and LC-MS/MS analysis
For the phosphorylation assay, 5 μg CsCIPK11-GST and 5 μg CsGSTU23-His proteins purified from E. coli were incubated in a kinase reaction buffer (50 mM HEPES, 2 mM DTT, 10 mM MgCl2, 4.6 mM CaCl2, 5 mM EGTA and 50 μM ATP) at 25°C for 30 min. ADP production from reactions buffer was determined by an ADP-Glo assay kit (Promega). Briefly, the ADP-Glo reagent was used to stop the kinase reaction, and incubated at 25°C for 50 min. Then, the kinase detection reagent was added and incubated at 25°C for another 50 min. Luminescence was measured using a GloMax 20/20 system (Promega).
Protein phosphorylation levels were detected via immunoblotting, including a dephosphorylation reaction with the FastAP™ alkaline phosphatase (Thermo Fisher Scientific), phosphorylation reaction in vitro, incubation with the corresponding antibodies (anti-phospho-serine (anti-pSer) (Sigma) and anti-phospho-threonine (anti-pThr) (CST), and detection following protein separation using SDS-PAGE.
For the phosphorylation assay in vivo, 35S-CsCIPK11-GFP and empty vector were co-expressed with 35S-CsGSTU23-HA in N. benthamiana leaves for 3 days, respectively, and then the total proteins of N. benthamiana leaves were extracted and incubated with 10 µL of anti-HA agarose beads. After 4 h of gentle shaking at room temperature, the beads were washed three times with washing buffer. The immunoprecipitated proteins were then released from the agarose beads by boiling them in SDS-PAGE loading buffer. CsGSTU23-HA proteins were separated and collected via SDS-PAGE for further digestion with trypsin for 15 h. The phosphopeptides were analyzed using a timsTOF Pro2 system (Bruker Daltonics). Raw data were processed by PEAKS Studio version 10.6 (Bioinformatics Solutions Inc.). The species information in the database is Camellia sinensis (L.) O. Ktze. Database search parameters fragment ion mass tolerance error was 0.05 Da, parent ion mass tolerance error was 20ppm. Peptide card value was −10lgP ≥ 15.
2.10 Protein degradation measurement
To measure protein degradation in vivo, the 35S-CsGSTU23-Luc vector transformed into the A. tumefaciens GV3101-p19 strain was transiently co-expressed with 35S-CsCIPK11-GFP and 35S-GFP in the leaves of N. benthamiana plants. After incubation for 3 days, the protein level of CsGSTU23-Luc was measured using a dual-luciferase reporter assay system (Promega) on a Synergy H1 instrument (BioTek). Images were recorded using a chemiluminescence image analysis system (Tanon).
For cell-free degradation, CsGSTU23-His protein was expressed and purified from E. coli. The total proteins of the wild-type tobacco expressing 35S-GFP and 35S-CsCIPK11-GFP, respectively, were extracted. The protein concentration was determined by the BCA assay reagent (Kangwei). Each reaction mix contained 100 ng of CsGSTU23-His and 500 μg of total protein from tobacco. For the proteasome inhibitor experiments, 20 μM MG132 was added. The reaction mixes were incubated at 22°C and were sampled at 0, 0.5, 1, 3, 6, 12 and 24 h. The reactions were stopped by SDS-PAGE loading buffer and boiling at 98°C for 10 min.
2.11 Transient overexpression of CsGSTU23
CsGSTU23 was transiently overexpressed in the leaves of 1-year-old tea plant seedlings of #S3 accession according to a previously described method (Chen et al., 2023). One-year-old tea plant seedlings of #S3 were obtained by asexually cutting propagation. Thirty tea plants whose leaves expressed with 35S-CsGSTU23-GFP and 30 tea plants whose leaves expressed with 35S-empty vector (EV-GFP) were grown in a growth chamber under a 12/12 h light/dark cycle (22/20°C) for 2 days, then they were divided into two groups: one group was sampled immediately, and the other group was sampled after being subjected to −2°C for 4 h. In each group, three tea plants were used for phenotypic analysis, the second leaves of three tea plants were used for REL measurement, the second leaves of nine tea plants were divided into three replicates and were used for total antioxidant capacity, free radical clearance rate, and expression analysis, and the third leaves of 10 tea plants were used for Fv/Fm analysis. The phenotype was recorded under recovery for 6 h at room temperature after −2°C treatment.
2.12 Statistical analysis
All experimental data with more than three replicates were analyzed using IBM SPSS Statistics 21 software. Student's t-test and one-way analysis of variance (ANOVA) were used to calculate significant differences between pairs.
3 RESULTS
3.1 Expression pattern of CsCIPK11 and subcellular localization
CsCIPK11 is an ortholog of AtCIPK11 (63.1% identity). qRT-PCR was performed to determine the transcript abundance of CsCIPK11 in response to low temperatures. The expression levels of CsCIPK11 in one bud with one leaf (1B1L) and mature leaf (ML) of tea plants was significantly induced when subjected to 4°C within 3 h and 6 h, respectively, and reached peak expression at 5 days (9.23-fold) and 7 days (3.98-fold) posttreatment, respectively (Figure 1a). In addition, the induction of CsCIPK11 expression under 4°C treatment in 1B1L was higher at 1 day after treatment than that in ML.
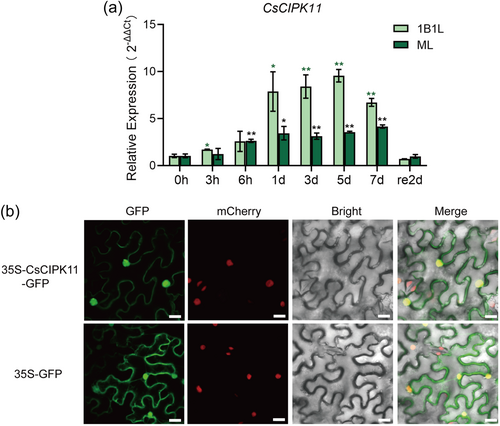
To investigate the subcellular localization of CsCIPK11, the 35 S CaMV-GFP construct of CsCIPK11 and the control vector (35 S CaMV-GFP) were transiently expressed in transgenic N. benthamiana leaves expressing H2B-RFP as a nuclear marker. The results showed that the CsCIPK11-GFP fusion protein was distributed throughout the cell, nucleus, and cell membrane, consistent with the expression of the 35S CaMV-GFP (Figure 1b).
3.2 Overexpression of CsCIPK11 improved cold tolerance in Arabidopsis
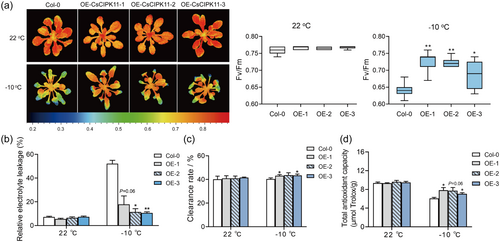
To test if CsCIPK11 plays a role in cold tolerance, transgenic Arabidopsis plants over-expressing CsCIPK11 were generated. Three independent OE lines (#1, #2 and #3) were selected for the cold tolerance assay (Supporting Information: Figure S1). No morphological differences were observed between the Col-0 and CsCIPK11-OE plants under normal conditions. After subjecting to −10°C for 8 h, CsCIPK11-OE plants maintained higher levels of chlorophyll fluorescence, with Fv/Fm values significantly higher than Col-0 plants (Figure 2a). The REL in the CsCIPK11-OE lines was significantly lower than those in Col-0 after −10°C treatment (Figure 2b). The free radical clearance rate and total antioxidant capacity in the CsCIPK11-OE lines were significantly higher than those in Col-0 after −10°C treatment (Figure 2c,d). Taken together, these results indicate that CsCIPK11-OE plants were more tolerant to low temperatures than Col-0 plants and that CsCIPK11 played a positive role in cold tolerance in transgenic Arabidopsis.
3.3 Transient knockdown of CsCIPK11 expression decreased cold tolerance in tea plants
To verify the role of CsCIPK11 in the cold response of tea plants, we suppressed CsCIPK11 expression in young tea plant leaves by soaking them in an antisense oligonucleotides (AsODN) solution and young leaves cultivating with sense oligonucleotides (sODN) served as control group. After incubation for 24 h, the relative expression level of CsCIPK11 in AsODN-CsCIPK11 leaves was significantly reduced by 26.82% and 60.22% compared to sODN before and after −2°C treatment, respectively (Figure 3a). The phenotypes were recorded after 2 h of recovery at 22°C. There were no differences observed in the young leaves of tea plants at 22°C; however, low-temperature injury was more severe in the CsCIPK11-silenced young leaves than in the controls after −2°C treatment (Figure 3b), as judged by a significantly lower chlorophyll fluorescence values (Figure 3c). Meanwhile, AsODN-CsCIPK11 leaves exhibited significantly lower Fv/Fm values (Figure 3d) and higher REL values (Figure 3e), as well as a reduced total antioxidant capacity (Figure 3f), and free radical clearance rate (Figure 3g) than the control under −2°C treatment. Taken together, these results indicated that CsCIPK11 positively modulates cold tolerance in tea plants.
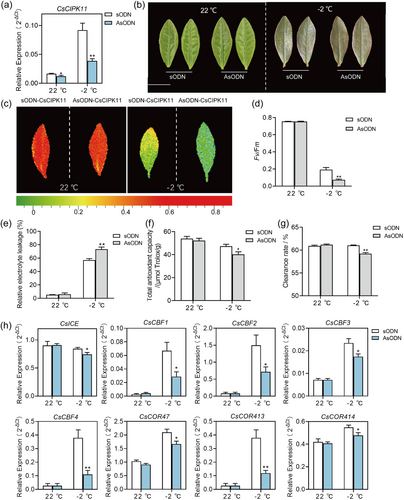
The ICE (inducer of CBF expression)—C repeat binding factor (CBF)—COR (cold-responsive genes) pathway plays a key role in cold stress response in plants (Shi et al., 2018). To explore the molecular mechanism of CsCIPK11 in regulating cold tolerance, the expression levels of genes involved in the ICE—CBF—COR pathway were determined. The expression levels of these genes showed no difference between AsODN-CsCIPK11 and sODN-CsCIPK11 under 22°C conditions. After −2°C treatment, the expression levels of CsICE, CsCBF1, CsCBF2, CsCBF3, CsCBF4, CsCOR47, CsCOR413 and CsCOR414 were significantly reduced in AsODN-CsCIPK11 compared with those in sODN-CsCIPK11 (Figure 3h). This suggests that CsCIPK11 participates in the regulation of the ICE—CBF—COR pathway.
3.4 CsCIPK11 interacted with the CsGSTU23 protein
To explore the molecular mechanism by which CsCIPK11 regulates the cold response in tea plants, yeast two-hybrid screening was performed with a tea plant cDNA library using CsCIPK11 as bait. Interestingly, CsGSTU23, which displays low expression in cold-sensitive tea plant cultivars (Wang et al., 2019), was identified as a candidate that interacted with CsCIPK11. To confirm the interaction of involving CsCIPK11-CsGSTU23, Y2H, LCI, BiFC and pull-down assays were performed. All yeast cells grew normally in the DDO medium. Yeast cells containing a combination of the CsCIPK11-BD and CsGSTU23-AD constructs grew effectively on QDO/X medium (Figure 4a). Pull-down assays showed that the CsGSTU23-His fusion protein bound to GST-CsCIPK11 but not to the negative control (GST) (Figure 4b). The BiFC assay showed that the yellow fluorescence (YFP) of CsCIPK11-CsGSTU23 complex was mainly present in the cell nucleus and cell membrane (Figure 4c). The LCI results showed that luminescence signals appeared only in the leaf regions co-expressing CsCIPK11-nLuc and CsGSTU23-cLuc (Figure 4d). These results demonstrate that CsCIPK11 interacts with CsGSTU23 in vitro and in vivo.

Bioinformatic analysis predicted that CsCIPK11 possesses an N-kinase domain and a C-regulatory domain and that CsGSTU23 contained a GSH-binding site at the N-terminus and a polypeptide binding site at the C-terminus (Figure 4e). Further LCI assays of the truncated sequence revealed that the kinase domain of CsCIPK11 and the N-GSH-binding domain of CsGSTU23 are necessary for the CsCIPK11-CsGSTU23 interaction (Figure 4f).
3.5 CsCIPK11 phosphorylated and stabilized the CsGSTU23 protein
As the N-kinase domain of CsCIPK11 interacts with the N-GSH-binding domain of CsGSTU23, we speculated that CsGSTU23 is a potential substrate for the phosphorylation of CsCIPK11 kinase. We analyzed the potential phosphorylation site of the CsGSTU23 N-terminus, with four Ser sites, one Thr site and one Tyr (Tyrosine) can potentially be phosphorylated (Figure 5a). Due to CIPK was S/T protein kinases, immunoblotting with anti-pSer and anti-pThr antibodies demonstrated that the GST-CsCIPK11 fusion recombinant protein could phosphorylate the CsGSTU23 protein at a Ser site, but not at the Thr site, of CsGSTU23 (Figure 5b). In vitro phosphorylation was measured using a luminescence-based kinase assay. The amount of ADP formed by ATP during the phosphorylation reaction was measured using a luminescence assay system (Zhang, Liu, et al., 2019). When CsCIPK11 was incubated with CsGSTU23, the production of ADP was significantly higher than that with CsCIPK11 and CsGSTU23 alone, indicating that CsGSTU23 was a substrate for CsCIPK11 kinase (Figure 5c).
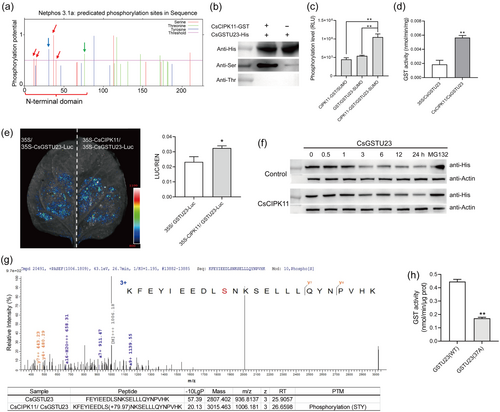
We further examined whether CsCIPK11 influenced the enzymatic activity and stability of CsGSTU23 protein. The GST enzymatic activity of N. benthamiana leaves co-expressed with 35S-CsCIPK11 and 35S-CsGSTU23 was significantly higher than that of leaves co-expressed with the empty vector and 35S-CsGSTU23, indicating that CsCIPK11 contributed to increased GST activity in vivo (Figure 5d). Protein degradation assays were also performed. Briefly, the 35S-CsGSTU23-Luc protein was co-expressed with equal amounts of 35S-CsCIPK11-GFP and 35S-GFP control proteins in N. benthamiana leaves. The luciferase assay showed that CsGSTU23-Luc protein levels were higher when co-expressed with CsCIPK11-GFP than when co-expressed with the control GFP protein (Figure 5e). Cell-free degradation also showed that CsGSTU23-His protein was more stable in protein extracts of 35S-CsCIPK11 tobacco than in those of the empty control (Figure 5f). These results indicated that the CsCIPK11-mediated interaction of CsGSTU23 increased the stability of CsGSTU23.
To identify the CsCIPK11-mediated phosphorylation sites of CsGSTU23, phosphorylated CsGSTU23 was subjected to LC-MS/MS analysis. One CsGSTU23 phosphopeptide incorporating the Ser37 site was found to be phosphorylated by CsCIPK11 (Figure 5g). Mutations at Ser37 site from Serine (S) to Alanine (A) resulted in a significant reduction in GST activity of CsGSTU23 (Figure 5h), indicating that the phosphorylation of Ser37 is critical for its enzymatic activity.
3.6 The transient overexpression of CsGSTU23 increased cold resistance in tea plants
To investigate the cold-responsive pattern of CsGSTU23 expression in tea plants, qRT-PCR analysis revealed that transcript abundance of CsGSTU23 was significantly induced by 4°C low temperatures in 1B1L and ML, and its induction in ML was earlier and higher than in 1B1L (Figure 6a). Meanwhile, the expression level of CsGSTU23 in cold-resistant tea plant accessions was notably higher than in the cold-susceptible accessions (Wang et al., 2022) (Figure 6b). These results suggest that CsGSTU23 plays an important role in cold tolerance in tea plants. The subcellular localization of CsGSTU23 was observed in the nucleus and cell membrane, similar to that of CsCIPK11 (Figure 6c).
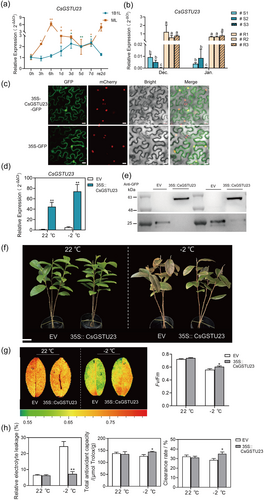
To further characterize the biological function of CsGSTU23 in tea plants, we designed experiments to suppress and increase its expression. Despite several attempts we did not obtain an effective AsODN to knock down CsGSTU23 in tea plants. Transient overexpression of CsGSTU23 in tea leaves was performed as described previously (Chen et al., 2023). qRT-PCR analysis revealed that the expression of CsGSTU23 in OE leaves was significantly overexpressed (44.8 times higher) than in EV control leaves (Figure 6d). Western blot analysis revealed the overexpression of CsGSTU23-GFP protein with a molecular weight of 60 kDa in 35 S CaMV-CsGSTU23 leaves, and the overexpression of EV-GFP protein with a molecular weight of 26 kDa in EV leaves (Figure 6e). After 4 h of treatment at −2°C, EV plants were more wilted and showed severe frost damage compared to OE plants (Figure 6f). Consistent with this phenotype, chlorophyll fluorescence imaging, Fv/Fm values (Figure 6g), total antioxidant capacity, and the free radical clearance rate (Figure 6h) were all significantly improved in OE leaves. In contrast, the REL value was significantly lower in OE leaves than in EV leaves (Figure 6h). These results indicate that the transient overexpression of CsGSTU23 leads to substantially decreased cold susceptibility in tea plants.
4 DISCUSSION
In this study, we experimentally confirmed the role of CsCIPK11 in cold temperature tolerance of tea plant. This conclusion is based on a variety of experimental investigations, (i) CsCIPK11 contributes to cold tolerance in transgenic Arabidopsis, as exhibited by increased resistance, decreased damage to cellular membrane functions and photosystems, and improved antioxidant ability in CsCIPK11-overexpressing transgenic Arabidopsis than in Col-0 plants (Figure 2), (ii) CsCIPK11 downregulation in tea plants led to greater susceptibility to cold treatment (Figure 3), (iii) CsCIPK11 regulated the expression of ICE—CBF—COR pathway genes (Figure 3h), leading to improved cold tolerance in tea plants, consistent with the role of the ICE—CBF—COR pathway in cold response in plants (Shi et al., 2018). It has been reported that AtMPK3/AtMPK6 negatively regulated AtCBF expression and freezing tolerance in Arabidopsis through phosphorylating and destabilizing the AtICE1 protein (Li, Zhao, et al., 2017). We hypothesized that CsCIPK11 may target other transcription factors that regulate the CBF signaling pathway, which needs further study in the future.
Cold stress is usually accompanied by the overproduction and accumulation of ROS, which are necessary to induce the system acquired adaptation to cold stress (Mittler et al., 2004). Our data showed that CsCIPK11 significantly modulated the antioxidant capacity of Arabidopsis and tea plants under low-temperature conditions (Figures 2c,d and 3f,g), indicating that CsCIPK11 can efficiently protect tea plants from cold-induced oxidative stress. ROS homeostasis depends primarily on the AsA-GSH pool and ROS-scavenging enzymes (Noctor et al., 2016). GSTU, a detoxification enzyme, alleviates cellular damage caused by ROS or toxic substrates. In tea plants, GST family members have been identified genome-wide, and RNA sequencing data have shown that CsGSTUs are associated with various stresses (Cao et al., 2022). However, the upstream regulatory role of GSTs in the cold tolerance response in tea plants is largely unknown. In currents study, we showed that CsCIPK11 directly targeted the cytosolic glutathione S-transferase CsGSTU23. A series of experiments showed that CsCIPK11 interacted with CsGSTU23 both in vivo and in vitro (Figure 4). These data indicate that CsGSTU23 may function as a downstream substrate of CsCIPK11 to mediate cold tolerance in tea plants.
Antioxidant enzymes play vital roles in signal transduction and ROS homeostasis. The crosstalk between Ca2+ and ROS signaling pathways in response to abiotic stress has also been widely reported (Ravi et al., 2023). Arabidopsis calcium-dependent protein kinase (CPK) 8 phosphorylates the antioxidant enzyme AtCAT3 at Ser261 to increase catalase activity and mediate drought tolerance (Zou et al., 2015). Rice OsCPK24 responds to cold stress by phosphorylating OsGrx10, a glutathione-dependent thiol transferase, while the specific phosphorylation sites of OsGrx10 are not elaborated (Liu et al., 2018). Tomato CPK28 phosphorylates the Thr59 and Thr164 sites of APX2, an ascorbate peroxidase, and improves thermotolerance in tomato plants, and the Thr59 and Thr164 sites of APX2 are important for APX activity (Hu et al., 2021). The involvement of GSTU phosphorylation in stress response is currently poorly reported in plants. In this study, CsCIPK11 phosphorylates CsGSTU23 at Ser37 in vivo, resulting in enhancing CsGSTU23 protein stability and enzymatic activity (Figure 5), which help us understand the function and mechanism of GSTU in response to cold stress in plant.
The identification and characterization of the role of CsGSTU23 in the cold tolerance of tea plants is critical to further understanding the molecular mechanism of CsCIPK11. The subcellular localization of CsCIPK11 and CsGSTU23 is consistent with the reports that CIPK and GST were localized in the cell membrane and nucleus (Meng et al., 2021; Zhang et al., 2021). The consistent subcellular localization of CsCIPK11 and CsGSTU23 further indicate that the CsCIPK11-CsGSTU23 module mediates cold tolerance in tea plants (Figure 6). Our previous study showed that CsGSTU8/19/23/25/27 displayed higher expression levels in cold-resistant cultivars than in cold-susceptible cultivars, which was related to higher ROS-scavenging capacity and less severe phenotypic damage in the field (Wang et al., 2019). The data presented here show that CsGSTU23 is a cold-induced gene, and the difference in CsGSTU23 expression levels between cold-resistant and cold-susceptible tea plant accessions was consistent with the phenotypic performance at low temperatures in winter (Figure 6a,b) (Wang et al., 2022). OsGST (Os01g27260), which contains more than one non-synonymous SNP in the functional domain, is important for the adaptation of ROS metabolism to low temperatures in rice (Zhang et al., 2016). Therefore, identification of CsGSTU23 may be an important genetic target for breeding chilling-tolerant tea plant varieties. The overexpression of CsGSTU23 in tea plants enhanced cold tolerance (Figures 6f–h), further confirming its pivotal role in cold resistance. In the future study, we will delve into the role of CsGSTU23 in the selection of cold-resistant varieties of tea plants.
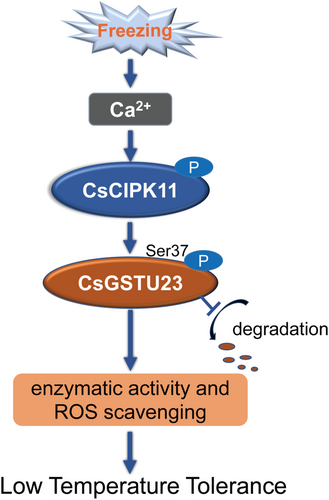
In conclusion, the data in the present study elucidated the positive role of CsCIPK11 in the cold tolerance of tea plants, combined with the CsCIPK11-mediated targeting of CsGSTU23, in the modulation of ROS homeostasis (Figure 7), which may be a new target for cold stress management and to facilitate the breeding of cold-tolerant tea cultivars.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32372774 and 31870685), the Special Project of Zhejiang Province (2020R52036), China Agriculture Research System of MOF and MARA (CARS-19-01A) and the Chinese Academy of Agricultural Sciences through an Innovation Project for Agricultural Sciences and Technology (CAAS-ASTIP-2021-TRICAAS).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.




