Photosynthetic capacity in middle-aged larch and spruce acclimates independently to experimental warming and elevated CO2
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Abstract
Photosynthetic acclimation to both warming and elevated CO2 of boreal trees remains a key uncertainty in modelling the response of photosynthesis to future climates. We investigated the impact of increased growth temperature and elevated CO2 on photosynthetic capacity (Vcmax and Jmax) in mature trees of two North American boreal conifers, tamarack and black spruce. We show that Vcmax and Jmax at a standard temperature of 25°C did not change with warming, while Vcmax and Jmax at their thermal optima (Topt) and growth temperature (Tg) increased. Moreover, Vcmax and Jmax at either 25°C, Topt or Tg decreased with elevated CO2. The Jmax/Vcmax ratio decreased with warming when assessed at both Topt and Tg but did not significantly vary at 25°C. The Jmax/Vcmax increased with elevated CO2 at either reference temperature. We found no significant interaction between warming and elevated CO2 on all traits. If this lack of interaction between warming and elevated CO2 on the Vcmax, Jmax and Jmax/Vcmax ratio is a general trend, it would have significant implications for improving photosynthesis representation in vegetation models. However, future research is required to investigate the widespread nature of this response in a larger number of species and biomes.
1 INTRODUCTION
Boreal forests represent a key component within the global carbon cycle as, through photosynthesis, they absorb a significant amount of carbon from the atmosphere annually (Beer et al., 2010; Houghton, 2007). Accurate representation of photosynthesis of boreal forests within terrestrial biosphere models (TBMs) is, therefore, important to reliably predict both current and future global carbon cycling and associated climatic conditions (Rogers et al., 2022). The boreal region has already warmed more than twice than the global average (IPCC, 2013), and predictions suggest that some regions could potentially warm by 6°C by 2100 compared to a global mean of ~4°C (IPCC, 2021). The Farquhar–von Caemmerer–Berry (FvCB; Farquhar et al., 1980) model of C3 photosynthesis is widely used to derive the two key parameters representing underlying biochemical processes of photosynthesis, the maximum rate of Rubisco carboxylation (Vcmax) and the maximum rate of electron transport (Jmax) (Farquhar et al., 1980; von Caemmerer, 2000; Wullschleger, 1993). The Vcmax and Jmax are key parameters in many TBMs to simulate current and future terrestrial carbon uptake and sequestration (Knauer et al., 2023; Mercado et al., 2018; Oliver et al., 2022; Rogers, Serbin, et al., 2017; Rogers, 2014). However, many of the TBMs do not currently incorporate long-term acclimation responses of both Vcmax and Jmax to climate change variables such as warming and elevated CO2 (and their combination) (Lombardozzi et al., 2015; Rogers, Serbin, et al., 2017, 2022; Smith & Dukes, 2013), largely due to the lack of these data and particularly for the boreal region (Rogers et al., 2022; Stinziano et al., 2019). Such a knowledge gap limits the ability of these models to reliably forecast the feedback between boreal forest carbon cycling and future climate.
The least-cost framework (Prentice et al., 2014; Smith et al., 2019) was recently used to predict acclimation of photosynthetic capacity (Vcmax and Jmax) to warming and elevated CO2 from first-principles (Jiang et al., 2020; Smith & Keenan, 2020; Wang et al., 2020). This framework suggests that Vcmax and Jmax when measured at a given standard temperature (e.g., 25°C) should decrease in warm-grown plants compared to cool-grown counterparts as plants operating in warm growth conditions can achieve optimal carbon assimilation rates with relatively lower photosynthetic protein content (e.g., Rubisco and chlorophylls) (Lu et al., 2020; Maire et al., 2012; Wang et al., 2020; Yamori et al., 2014). But when assessed at growth temperature (Tg), both Vcmax and Jmax should increase with growth temperature but with a lower slope compared to the short-term temperature responses (Smith et al., 2019; Smith & Keenan, 2020; Wang et al., 2020). However, Vcmax should increase more strongly than Jmax with growth temperature due to a greater allocation of resources to Rubisco carboxylation compared to electron transport, as a mechanism to counteract increase in photorespiration associated with rising temperatures (Smith & Keenan, 2020). Therefore, this higher investment in Rubisco carboxylation should lead to a decrease in the Jmax/Vcmax ratio with rising in growth temperature (Smith & Keenan, 2020). Similarly, plants grown under elevated CO2 conditions should reduce Vcmax and Rubisco content since under elevated CO2 conditions Rubisco is less limited by substrate availability (i.e., CO2), and thus high carbon assimilation rates can be achieved with relatively lower Rubisco protein content compared to ambient CO2-grown plants (Smith & Keenan, 2020). In contrast to Vcmax, the least-cost framework predicts slightly higher rates of Jmax with elevated CO2 which should lead to higher Jmax/Vcmax ratio in elevated CO2-grown plants compared to ambient CO2-grown counterparts (Smith & Keenan, 2020).
In the literature, the acclimation responses of photosynthetic capacity to warming are mixed and thus partly disagree with the least-cost optimality theory of photosynthetic capacity. Most previous meta-analyses using both warming experiments and studies with seasonal/spatial natural temperature variability largely indicate a lack of change of Vcmax (Kattge & Knorr, 2007; Kumarathunge et al., 2019; Way & Oren, 2010; Crous et al., 2022) and Jmax (Kattge & Knorr, 2007; Way & Oren, 2010) at a reference temperature of 25°C to increasing growth temperature, with a few exceptions in some datasets reporting a decrease in either Vcmax25 (Wang et al., 2020) or Jmax25 (Crous et al., 2022; Kumarathunge et al., 2019). However, when measured at prevailing growth temperatures both Vcmax and Jmax consistently increase (Scafaro et al., 2017; Smith & Dukes, 2017; Smith & Keenan, 2020; Way & Oren, 2010), and the Jmax/Vcmax ratio commonly decreases with warming (Crous et al., 2022; Kattge & Knorr, 2007; Kumarathunge et al., 2019; Smith & Dukes, 2017). Similarly, warming responses of photosynthetic capacity rates at a common temperature in boreal tree species are mixed. In controlled greenhouse/glasshouse experiments on seedlings, Vcmax and Jmax at a standard temperature decreased with warming (Way & Sage, 2008a, 2008b; Dusenge, Madhavji, et al., 2020) but not in (Murphy & Way, 2021), while experimental studies with freely-rooted boreal juvenile (Bermudez et al., 2021; Stefanski et al., 2020) and mature (Lamba et al., 2018) mainly reported no change in Vcmax and Jmax. When measured at growth temperature, Vcmax increased in response to warming but not for Jmax (Murphy & Way, 2021), and the Jmax/Vcmax ratio generally decreases with warming in some studies (Dusenge, Madhavji, et al., 2020; Stefanski et al., 2020; ; Bermudez et al., 2021; Murphy & Way, 2021). However, there are still limited studies that have investigated the response of photosynthetic capacity to warming in mature trees naturally growing in the field.
The acclimation responses of Vcmax to long-term exposure to elevated CO2 is similar to that predicted by the least-cost optimality model, with Vcmax decreasing in elevated-CO2 grown plants compared to ambient CO2-grown counterparts (Ainsworth & Long, 2005; Leakey et al., 2009; Smith & Keenan, 2020). However, the acclimation responses of Jmax to elevated CO2 vary between empirical observations and predictions based on least-cost optimality. Empirical studies commonly reported a decrease in Jmax with elevated CO2 (Ainsworth & Long, 2005; Leakey et al., 2009), while the least-cost theory predicts no change or even slightly positive responses (Smith & Keenan, 2020). These differential responses between Vcmax and Jmax result in the Jmax/Vcmax ratio being less positive than predicted by optimality (Smith & Keenan, 2020). Studies conducted on boreal tree species reported mixed responses. The majority of studies, which focused on seedlings in controlled growth conditions, reported a lack of acclimation of Vcmax and Jmax to elevated CO2 (Dusenge, Madhavji, et al., 2020; Kellomaki & Wang, 1996; Murphy & Way, 2021; Ofori-Amanfo et al., 2020; Wang, 1996). However, two other studies—one examining both Vcmax and Jmax in seedlings (Bigras & Bertrand, 2006) and another focusing solely on Vcmax in mature Norway spruce (Lamba et al., 2018)—found strong acclimation of these parameters to elevated CO2. However, more studies investigating the impact of elevated CO2 on Vcmax and Jmax in boreal mature trees in the field are still needed.
Given that ongoing climate warming is primarily driven by rising atmospheric CO2 concentration, it is imperative to examine their combined impacts on Vcmax and Jmax to improve further the predictive capability of TBMs (Dusenge et al., 2019; Norby & Luo, 2004; Smith & Dukes, 2013). However, no study has evaluated predictions of the least-cost optimality theory under warming and elevated CO2. The main reason is that there are far fewer studies examining the potential interactive effects of warming and elevated CO2 on Vcmax and Jmax, limiting the theory's validation (Smith & Keenan, 2020). Nevertheless, utilizing the least-cost optimality approach, Smith and Keenan (2020) predicted the response of photosynthetic nitrogen as the sum of simulated nitrogen invested into Rubisco and bioenergetics. Photosynthetic nitrogen concentration can be used as a proxy for rates of Vcmax and Jmax at a standard temperature, as they indirectly reflect the active photosynthetic protein content (Ellsworth et al., 2022; Rogers, 2014). Smith and Keenan's (2020) predictions showed that photosynthetic nitrogen decreased with both warming and elevated CO2, but growth temperature did not affect the slope of response to elevated CO2. These predictions suggest that the responses of Vcmax and Jmax rates to atmospheric CO2 levels will not be significantly influenced by growth temperature.
Results from the few studies that explore the combined effects of warming and elevated CO2 are largely derived from highly controlled experiments on seedlings, and their findings are mixed, making it challenging to draw consistent conclusions. Some of these studies showed that Vcmax and Jmax at a standard temperature only acclimate to warming but not elevated CO2 (Crous et al., 2013; Dusenge, Madhavji, et al., 2020; Ghannoum et al., 2010; Kellomaki & Wang, 1996), acclimated to elevated CO2 but not to warming (Fauset et al., 2019; Ghannoum et al., 2010; Lamba et al., 2018), or no acclimation to any of these two environmental factors (Murphy & Way, 2021; Wang, 1996). The remaining set of studies exposed seedlings to only combined warming and elevated CO2 without including treatments of their separate effects (e.g., either warming or elevated CO2), limiting the assessment of potential interactive effects. This latter category of studies demonstrated that seedlings grown under both warming and elevated CO2 showed decreased Vcmax and Jmax compared to control (both ambient CO2 and temperature) tree species (Slot et al., 2021; Wang et al., 2022, 2023, 2024). However, there is a need for more data on the responses of Vcmax and Jmax to warming and elevated CO2, especially in mature trees growing naturally in the field, while also separately manipulating these two factors to explore their potential interactive effects.
With the goal to improve our understanding on how photosynthesis in North America's boreal forests respond to combined warming and elevated CO2, we assessed the responses of rates of both Vcmax and Jmax to 2 years of warming combined with 1 year of elevated CO2 in two canopy tree species, black spruce (Picea mariana) and tamarack (Larix laricina), growing in the field at the southern range of their natural distribution. This study is part of the Spruce and Peatland Responses Under Changing Environments (SPRUCE; https://mnspruce.ornl.gov) long-term experiment, which uses whole-ecosystem heating, spanning from 3 m belowground up to 7 m aboveground using large octagonal open-top enclosures (Hanson et al., 2017). We recently published a companion study (Dusenge et al., 2023) detailing the response of the thermal optima of net photosynthesis (ToptA), Vcmax (ToptV) and Jmax (ToptJ) to warming and elevated CO2, and rates of net photosynthesis at their respective prevailing growth conditions (Ag). In summary, we found that the ToptA of both species increased with warming. However, these increases were not proportional to the warming, as ToptA only rose by 0.26–0.35°C per 1°C of warming. Interestingly, these small shifts in ToptA were largely influenced by concurrent changes in ToptV and ToptJ. Furthermore, Ag increased with warming in elevated CO2 black spruce, while remaining relatively constant in ambient CO2-grown black spruce and in both ambient and elevated CO2-grown tamarack with warming. Our current study delves into the detailed responses of the rates of the photosynthetic biochemical processes, Vcmax and Jmax, aiming to provide further insight into the observed rates of net photosynthesis in the SPRUCE experiment after 2 years of treatments. Based on both the least-cost optimality theory and previous research, the following four hypothesized predictions were tested:
H1..At a standard temperature, photosynthetic capacity (Vcmax and Jmax) will decrease with warming but will increase with growth temperature.
H2..At a standard temperature, photosynthetic capacity will be lower in plants grown under elevated CO2 conditions compared to those grown under ambient CO2 conditions.
H3..Photosynthetic capacity will be lower in trees exposed to both warm and elevated CO2 conditions with comparable responses to their independent effects.
2 MATERIALS AND METHODS
2.1 Site description and experimental design
The current study was conducted at the Oak Ridge National Laboratory's SPRUCE project site located at the U.S. Forest Service's Marcell Experimental Forest, in Minnesota, USA (47°30.476′ N; 93°27.162′ W). At the SPRUCE site, the dominant tree species is Picea mariana (black spruce) mixed with less abundant Larix laricina (tamarack). The SPRUCE experiment uses five temperature treatments (ambient or +0, which serves also as the control, +2.25°C, +4.5°C, +6.75°C, and +9°C above the ambient) established in a regression-based design (Hanson et al., 2017). These temperature treatment levels are controlled within 10 large octagonal open-top enclosures with an interior surface area of 114.8 m2, and a sampling area of 66.4 m2. In addition to the temperature treatments, there are also elevated CO2 treatments, with five enclosures exposed to an ambient-CO2 atmosphere, while the other five have an elevated CO2 atmosphere that range between +430 and 500 ppm above the ambient. The whole-ecosystem warming treatments were initiated 15 August 2015, while the CO2 treatments were introduced a year later, on 15 June 2016. Overall, the targets of the temperature treatment levels and CO2 concentrations were successfully achieved (Supporting Information S1: Figure S1).
2.2 Plant material sampling and gas exchange measurements
The data used in this study were conducted between 18–30 June and 15–30 August 2017. The daytime temperatures (4:00–20:30) recorded by a climate station established at the SPRUCE site were 18.97°C and 18.02°C, for June and August, respectively. We studied individuals of 1.9–7.6 m height of the two mixed-age (up to ~45 years old) canopy tree species, black spruce and tamarack. For black spruce, one branchlet of each tree and in each plot was collected and 1-year old needle cohorts (i.e., developed in 2016) from each branchlet was measured. For tamarack, fully expanded current year foliage (i.e., developed in 2017) was used. In the June field campaign, three trees in each plot were sampled, but we decided to reduce the number of harvested branches down to two trees in the August campaign, to reduce the damage that may be caused by overharvesting in this long-term experiment. For tamarack, the same number of branchlets from different trees in each plot was used, except in one plot (in ambient CO2 and +0) where there was only one tamarack tree to be sampled. All the data were collected on sun-exposed branchlets cut using a pruning pole. After cutting, branchlets were placed in water, and recut under water to avoid xylem transport disruption and stomatal closure.
The collection of branches took place during the early morning hours, specifically between 4 and 5 am on the day of measurement. Subsequently, these branches were carefully placed in water-filled containers within a plastic cooler. They were then transported from the field site located in Marcell, Minnesota to specialized walk-in growth chambers situated at the University of Minnesota in St. Paul, where the subsequent measurements were carried out. Before starting the measurements, branchlets were re-cut again under water. It has been demonstrated that the impact of cutting and the duration between cutting and gas exchange measurements do not exert a significant influence on stomatal conductance in conifers (Akalusi et al., 2021; Dang et al., 1997). Between 10:00 and 20:00 during the June and August field campaigns, seven portable photosynthesis systems (Li-COR 6400 XT, 6400-18 RGB light source, and 6400-22 opaque conifer chamber; LI-COR Biosciences) were employed to carry out gas exchange measurements. Measurements of net CO2 assimilation rates (A) were conducted under saturating light conditions at 1800 µmol m−2 s−1, with variations in air CO2 concentrations performed sequentially (400, 300, 200, 50, 400, 500, 600, 800, 1200, 1600, and 2000 µmol mol−1) to generate A–Ci curves. These A–Ci curves were conducted at five different leaf temperatures (Tleaf) in the following order: 15°C, 25°C, 32.5°C, 40°C and 45°C, which produced 96A – Ci temperature response curves after data quality checks (Dusenge, Ward, et al., 2020). To attain the desired Tleaf for each target, we conducted all measurements within a walk-in chamber. This setup ensured that both the entire branch and the Li-COR IRGA (Infrared Gas Analyser) sensor were exposed to the specified temperature for a minimum of 30 min before start-up measurements at that particular temperature. In addition, this approach effectively reduced measurement errors caused by the internal thermal gradient which occurs when the Li-COR and the leaves are exposed to different temperatures, a phenomenon that had been previously documented with LI-6400 instruments (Garen et al., 2022).
Following the gas exchange measurements, we utilized ImageJ software (NH) to calculate the projected leaf area of the sampled needles. Subsequently, we adjusted for the needle area before conducting the analyses of the gas exchange data. Furthermore, we examined the collected needle tissues for elemental nitrogen concentrations (N) using equipment from Costech Analytical Technologies, Inc.
2.3 Parameterization of photosynthetic capacity
2.4 Statistical analyses
To evaluate the effects of temperature and elevated CO2 on photosynthetic capacity (Vcmax and Jmax) and their ratios at various conditions—including the reference temperature of 25°C (Vcmax25, Jmax25 and Jmax25/Vcmax25), the thermal optimum of each process (Vcmaxopt and Jmaxopt), and the mean growth temperature (Vcmaxg and Jmaxg)—as well as leaf nitrogen and leaf mass per unit area (LMA), we started with a linear mixed-effect model. In this model, warming and elevated CO2 treatments, along with species, were designated as fixed effects, while the month during which the campaign was conducted was designated as the random effect. All analyses were run on the plot means with n = 1–4 trees/plot. The process of choosing the ultimate statistical model occurred in two stages, following the methodology outlined by Zuur et al. (2009). We first evaluated whether a random factor was required by comparing the model with the random intercept (i.e., month) and the one without any random structure. For this first step, we used the gls function in the model without the random structure and the lme function with the “Restricted maximum likelihood—REML” method in the model with the random structure, and both gls and lme functions are from the nlme R package (Pinheiro et al., 2023). We excluded the model featuring both random slope and intercept structures from the comparison, as our preliminary analyses suggested that it was over-parameterized. Thereafter, the model with the adequate random structure was selected based on the lowest akaike information criterion (AICc) (Supporting Information S1: Tables S1 and S2). When the model with random structure was not significantly different from the model without the random structure, we proceeded the analyses with a simple linear regression model. Following the determination of an appropriate random structure, we proceeded to choose the appropriate fixed effect structure. This involved considering both the model with only the main effects (i.e., warming, elevated CO2, and species) without interactions and models incorporating all possible combinations of main effects and their interactions. The latter selection was done by comparing models with different structures using the “maximum likelihood—ML” method within the gls function from the nlme R package (Pinheiro et al., 2023). Similarly, the best fixed effect structure was selected based on the lowest AICc value using AICcmodavg R package (Mazerolle, 2023) (Supporting Information S1: Tables S1 and S2). Last, three-way repeated measures ANOVA tests were used to analyse the main effects of growth temperature, growth CO2, leaf temperature and their interactions on the responses of Vcmax, and Jmax for each species using the lmerTest package (Kuznetsova et al., 2017). The random structure of this three-way repeated measures ANOVA involved leaf temperature nested within each measured tree, which was in turn nested within each experimental plot, and further nested within each month. To derive p value and respective statistical significance for each factor and interactions, we used the Type II Wald F tests with Kenward–Roger degrees of freedom (DF). All analyses were conducted in R (R Core Team, 2024).
3 RESULTS
Except for the ratio of Jmax to Vcmax at the thermal optimum and growth temperature, there were no significant differences across the two field campaigns (June and August 2017) on other measured leaf traits. Therefore, results of all other traits were lumped across June and August months. For all the studied photosynthetic traits, we did not find any interactive effects of warming and elevated CO2 on their responses to these environmental factors, therefore, results below are reported for warming and elevated CO2 separately.
3.1 Temperature responses
The temperature response of Vcmax was significantly affected by warming in both species (Supporting Information S1: Figures S2, S3; Table S4). Furthermore, Vcmax was affected by warming differently when estimated at different reference temperatures. At a standard temperature of 25°C (Vcmax25), Vcmax25 was not affected by warming in both species (Figure 1a,b; Supporting Information S1: Table S3). By contrast, Vcmax estimated at both the thermal optimum (Vcmaxopt; Figure 2a,b) and at growth temperature (Vcmaxg; Figure 3a,b), significantly increased with warming in both species (Supporting Information S1: Table S3). Across species, Vcmaxopt increased by 3.3 µmol m−2 s−1 per 1°C warming (Supporting Information S1: Table S3), while Vcmaxg increased by 3.65 μmol m−2 s−1 per 1°C warming (Supporting Information S1: Table S3).
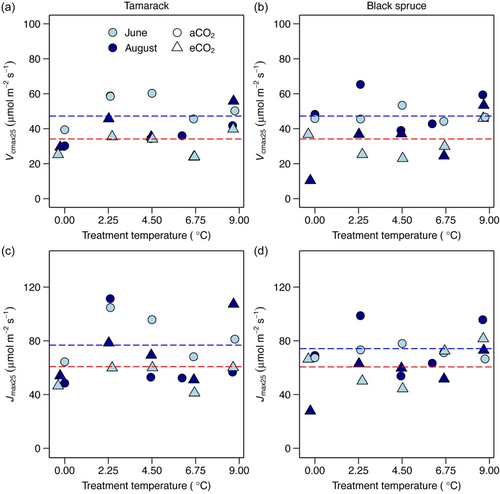
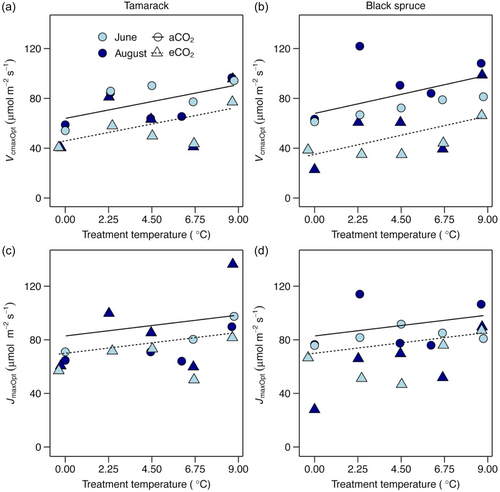

By contrast, the temperature response of Jmax was largely not affected by growth temperature in both species (Supporting Information S1: Figures S4, S5; Table S4). However, similar to Vcmax, Jmax also responded differently when estimated at different reference temperatures. Jmax at 25°C, Jmax25, was not affected by warming in both species (Figure 1c,d; Supporting Information S1: Table S3). Across the two species, Jmax at the thermal optimum, Jmaxopt, increased by 2.2 μmol m−2 s−1 per 1°C warming (Figure 2c,d; Supporting Information S1: Table S3), while Jmax at the prevailing growth temperature, Jmaxg, marginally (p = 0.064) increased by 2 μmol m−2 s−1 per 1°C warming (Figure 3c,d; Supporting Information S1: Table S3).
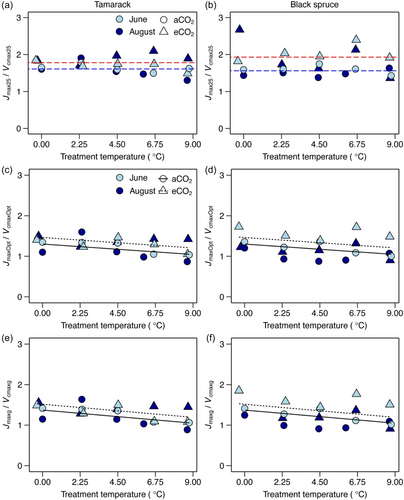
The ratio of Jmax to Vcmax mirrored that of both Vcmax and Jmax. At 25°C, warming did not affect this ratio (Figure 4; Supporting Information S1: Table S3). By contrast, the Jmax to Vcmax ratio at both their thermal optima (Jmaxopt/Vcmaxopt) and prevailing growth temperature (Jmaxg/Vcmaxg) decreased with warming (Figure 4). Specifically, Jmaxopt/Vcmaxopt decreased by 0.025 per 1°C warming, while for the Jmaxg/Vcmaxg, the slope was slightly steeper, decreasing on average by 0.032 per 1°C warming across the two species (Supporting Information S1: Table S3).
3.2 Elevated CO2 responses
The temperature response of both Vcmax and Jmax was not significantly affected by elevated CO2 in either species (Supporting Information S1: Figures S2–5; Table S4). Moreover, responses of both Vcmax and Jmax estimated at any of the three reference temperatures (25°C, thermal optima and growth temperature) were consistent across the two species, as they were all significantly lower in plants grown under elevated CO2 growth conditions compared to those grown under ambient CO2 (Figures 1-3; Supporting Information S1: Table S3). The Vcmax25, Vcmaopt, and Vcmaxg decreased by 28%, 36%, and 35%, respectively, in trees grown under elevated CO2 compared to their ambient-CO2 counterparts. For Jmax, both Jmax25 and Jmaxopt significantly decreased by 15% and 19%, respectively, while Jmaxg decreased by 14%, but this decrease was not statistically significant, potentially driven by observed substantial variations in this trait (p = 0.12; Figures 1-3; Supporting Information S1: Table S3). The ratio of Jmax25/Vcmax25, Jmaxopt/Vcmaxopt, and Jmaxg/Vcmaxg increased by 16%, 18%, and 17%, respectively, in elevated CO2-grown trees compared to those growing under ambient CO2 conditions (Figure 5; Supporting Information S1: Table S3).
3.3 Leaf nitrogen and leaf mass per unit area
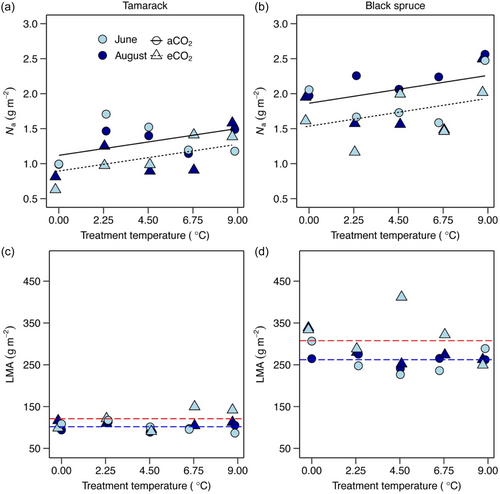
Warming and elevated CO2 treatments had similar effects on leaf nitrogen concentration on area basis (Na) and leaf mass per leaf area (LMA) in both tamarack and black spruce (Figure 5; Supporting Information S1: Table S3). Leaf Na was significantly impacted by warming and elevated CO2 (without interaction) (Figure 5a,b; Supporting Information S1: Table S3). In response to warming, leaf Na increased on average 0.043 g m−2 per 1°C of warming across the two species. In response to elevated CO2, leaf Na was, on average, 20% lower in elevated CO2-grown trees compared to those in ambient CO2 conditions across the two species. By contrast, LMA was significantly affected by elevated CO2 but not by warming across species (Figure 5c,d; Supporting Information S1: Table S3). Across the two species, LMA was, on average, 18% higher in elevated CO2 trees than in ambient CO2 counterparts.
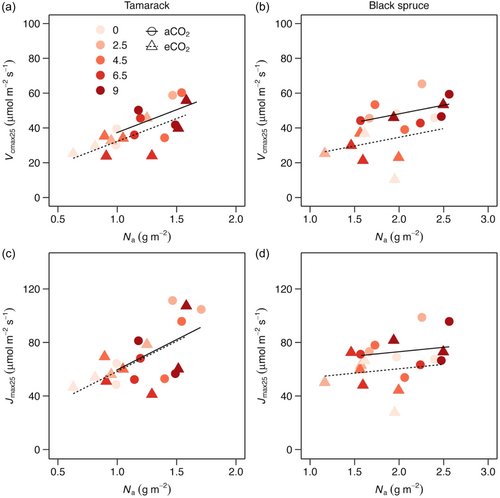
3.4 Relationship between photosynthetic capacity and leaf nitrogen
Both Vcmax25 and Jmax25 were positively related to leaf Na in both species (Figure 6; Supporting Information S1: Table S4). To further explore whether leaf nitrogen have played a role in the response of photosynthetic capacity at a common leaf temperature of 25°C to the treatments, we normalised Vcmax25 and Jmax25 to leaf Na. In both species, both Vcmax25/N and Jmax25/N did not significantly vary across warming treatments (Figure S6; Supporting Information S1: Table S3), suggesting that the lack of thermal acclimation of photosynthetic capacity could not largely be attributed to warming-induced effects on leaf Na. By contrast, Vcmax25/N and Jmax25/N were not significantly different between ambient and elevated CO2-grown trees (Supporting Information S1: Table S3), suggesting that acclimation of both Vcmax25 and Jmax25 to elevated strongly influenced changes in leaf Na in elevated CO2-grown trees.
4 DISCUSSION
In this study, we report findings from a field study that investigated the acclimation of photosynthetic capacity (Vcmax and Jmax) to warming and elevated CO2 after 2 years of a whole-ecosystem experimental warming (up to +9°C above ambient temperature) combined with 1 year of elevated CO2 (+430–500 ppm above ambient atmospheric CO2) in mature trees of North America's boreal conifers (black spruce and tamarack) at their southern range of natural distribution. We found that rates of Vcmax and Jmax when measured at a common temperature of 25°C did not show any acclimation (i.e., did not change) to warming in either tamarack or black spruce (Figure 1). However, when measured at their thermal optima and prevailing growth temperature, both Vcmax and Jmax increased with warming (i.e., acclimated) (Figures 2 and 3), suggesting that the ability to detect the thermal acclimation of photosynthetic capacity may partly depend on the chosen reference temperature. We also found that leaf Na content, Vcmax and Jmax all decreased in concert to elevated CO2 (i.e., acclimated) at any of the three reference temperature (Figures 1-3, 5; Supporting Information S1: Table S3). The Jmax/Vcmax ratio decreased with warming (i.e., acclimated) at the thermal optima of the two processes and at prevailing growth temperature, but remained constant at 25°C. In response to elevated CO2, Jmax/Vcmax ratio increased with elevated CO2 (i.e., acclimated) in both species and at the three reference temperatures (Figure 4). We did not find any interactive effect of warming and elevated CO2 on any studied trait of photosynthetic capacity.
4.1 Temperature responses of photosynthetic capacity
In both species, Vcmax25 and Jmax25 did not significantly change with warming (Figure 1), partly rejecting our first hypothesis (H1), which proposed that both Vcmax25 and Jmax25 should decrease with warming according to the least-optimality framework (Smith & Keenan, 2020; Wang et al., 2020). However, our results are in agreement with findings from meta-analyses (Crous et al., 2022; Kattge & Knorr, 2007; Kumarathunge et al., 2019; Way & Oren, 2010) and also those from recent experimental warming field studies on boreal and temperate seedlings (Bermudez et al., 2021; Stefanski et al., 2020), and European mature boreal conifers (Lamba et al., 2018). Nitrogen (N) is a key nutrient influencing variation of Vcmax and Jmax rates (Ellsworth et al., 2022), and changes in leaf N were shown to be related to the thermal acclimation of Vcmax and Jmax in some studies (Crous et al., 2018; Dusenge, Madhavji, et al., 2020, 2021; Scafaro et al., 2017; Way & Sage, 2008a). In our study, leaf N increased with warming, a response likely influenced by increases in soil nitrogen availability due to warming, as recently reported at our experimental site (Iversen et al., 2023). However, after normalizing Vcmax25 and Jmax25 to leaf N (Supporting Information S1: Figure S6), photosynthetic capacity exhibited similar response to non-normalized values (i.e., no change), indicating that nitrogen have relatively little influence on thermal acclimation of these two processes. Recently, Stefanski et al. (2020) and Bermudez et al. (2021) suggested that the lack of thermal acclimation of Vcmax25 and Jmax25 commonly observed in boreal experimental warming field studies may largely be due to modest warming treatments (~3 – 4°C above ambient) typically applied (Bermudez et al., 2021; Lamba et al., 2018; Smith et al., 2020; Stefanski et al., 2020). However, our study, which utilized incremental warming of 2.25°C up to 9°C—with the 9°C warming (and atmospheric CO2 of >800 ppm) considered a likely scenario under the business as usual in some higher latitude regions by 2100 (IPCC, 2021), indicates that the lack of discernable acclimation of photosynthetic capacity to warming in realistic field conditions may be less dependent on the level of warming applied. Future studies that investigate warming effects on underlying photosynthetic biochemistry (e.g., Rubisco content and activation state, and photosynthetic pigments content) in field settings are still needed to fully understand the causes of this commonly observed lack of thermal acclimation of photosynthetic capacity at a given standard temperature, which also contradicts predictions of the least-cost optimality framework.
In contrast to photosynthetic capacity measured at 25°C, photosynthetic capacity measured at both the thermal optima of the respective process components (VcmaxOpt and JmaxOpt) and prevailing growth temperature increased with warming (Figures 2 and 3), suggesting a positive acclimation response to warming in the studied species (Way & Yamori, 2014). These findings partly support H1, which proposed that both Vcmaxg and Jmaxg should increase with warming according to the least-optimality framework (Smith et al., 2019; Smith & Keenan, 2020; Wang et al., 2020), and are consistent with results from previous studies (Scafaro et al., 2017; Smith & Dukes, 2017; Smith & Keenan, 2020; Way & Oren, 2010). This observed positive acclimation of photosynthetic capacity at the thermal optima and growth temperature suggests that Vcmax and Jmax have acclimated to warming despite not being seen at the commonly used standard temperature of 25°C. In our companion SPRUCE study (Dusenge et al., 2023), we also found that the thermal optima of Vcmax and Jmax in these species increased with warming, indicating a strong thermal acclimation of photosynthetic biochemistry in these boreal conifers. Overall, our findings indicate that the ability to detect the thermal acclimation of Vcmax and Jmax depends on the chosen reference temperature and parameter of interest, since the shape of instantaneous temperature response of photosynthetic capacity and its acclimation response to increased growth temperature can be seen through several important temperature sensitivity parameters such as the activation energy (initial slope of the temperature response; EaVcmax and EaJmax), thermal optima (ToptVcmax and ToptJmax), and deactivation energy (Kumarathunge et al., 2019; Way et al., 2014; Yamori et al., 2014) of these key processes.
The ratio of Jmax to Vcmax (Jmax/Vcmax) is another key parameter used for modelling photosynthesis within TBMs (Rogers, Medlyn, et al., 2017). At 25°C, Jmax/Vcmax was constant across warming treatments, however, at both the thermal optima of the two processes and at prevailing growth temperature, Jmax/Vcmax decreased with warming (i.e., acclimated) (Figure 4). Our results at 25°C, therefore, contradict findings from several studies (Bermudez et al., 2021; Dusenge et al., 2015, Dusenge, Madhavji, et al., 2020; Smith & McNellis, & Dukes, 2020; Stefanski et al., 2020) and meta-analysis (Crous et al., 2022; Kattge & Knorr, 2007; Kumarathunge et al., 2019) that commonly report a decrease in Jmax25/Vcmax25 to warming. However, a few other studies also did not observe a decrease of Jmax25/Vcmax25 with warming (Crous et al., 2018; Scafaro et al., 2017). Constant Jmax25/Vcmax25 in our study could largely be attributed to the observed lack of effect of warming on either Vcmax25 or Jmax25. However, responses at the thermal optimum and growth temperature are consistent with findings of previous studies (Murphy & Way, 2021; Smith & Dukes, 2017) and the least-cost optimality framework (Smith & Keenan, 2020). Decrease of the Jmax/Vcmax with warming is proposed to be a mechanism that reduces photorespiration that simultaneously increases with rising growth temperature (Dusenge et al., 2019; Sage & Kubien, 2007) by allocating relatively more resources to Rubisco carboxylation (i.e., Vcmax) compared to the electron transport (i.e., Jmax) process (Dusenge et al., 2021; Smith & Keenan, 2020).
4.2 Elevated CO2 responses of photosynthetic capacity
In our study, photosynthetic capacity generally acclimated to elevated CO2, by decreasing in trees exposed to elevated CO2 regardless of the reference temperature (Figures 1-3; Supporting Information S1: Table S3), and this response agrees with our second hypothesis (H2). Both Vcmax and Jmax strongly acclimated to elevated CO2, with Vcmax showing relatively stronger acclimation response to elevated CO2 (−28% to –36%) compared to Jmax (−14% to −19%). Observed reductions in Vcmax and Jmax in our study are largely consistent with findings from meta-analyses, which are dominated by studies on temperate tree species and crops (Ainsworth & Long, 2005; Ainsworth & Rogers, 2007; Leakey et al., 2009; Smith & Keenan, 2020), but partly contrast predictions of the least-cost optimality theory which predicts rather slight positive responses of Jmax to elevated CO2 (Smith & Keenan, 2020). Consequently, the ratio of Jmax to Vcmax at all three reference temperatures was higher (16%–17%; Figure 4, Supporting Information S1: Table S3) in trees exposed to elevated CO2 compared to those growing in ambient atmospheric CO2 conditions. The latter findings are consistent with results from a few previous studies conducted mainly on seedlings in highly controlled growth conditions (Crous et al., 2013; Dusenge, Madhavji, et al., 2020; Smith & Keenan, 2020). There has been relatively fewer studies on long-term CO2 responses of photosynthetic capacity of mature boreal conifer trees in realistic field conditions. Before our study, we are aware of only one field study on mature trees of Norway spruce which showed that Vcmax strongly acclimated to elevated CO2 (i.e., −23%), but the study did not investigate the responses of Jmax (Lamba et al., 2018). Therefore, our current study, further generally adds results on long-term acclimation of Jmax and Jmax/Vcmax ratio to elevated CO2 for the mature boreal conifers.
4.3 Responses of photosynthetic capacity to combined warming and elevated CO2
Our study did not find any interactive effect of temperature and elevated CO2 on Vcmax and Jmax at any reference temperature (Supporting Information S1: Table S3), supporting our third hypothesis (H3). Our findings also support the predictions of the least-cost optimality framework, where a strong interaction of warming and elevated CO2 on rates of photosynthetic capacity is not expected, and that responses of photosynthetic capacity to combined warming and elevated CO2 should be comparable to their independent effects (see Figure 4 in Smith & Keenan, 2020). Several previous studies also did not find any interactions of warming and elevated CO2 on either Vcmax, Jmax, instead showing that Vcmax and Jmax at a standard temperature acclimate to: (1) warming but not elevated CO2 (Crous et al., 2013; Dusenge et al. 2020; Ghannoum et al., 2010; Kellomaki and Wang, 1996); (2) elevated CO2 but not warming (Fauset et al., 2019; Ghannoum et al., 2010; Lamba et al., 2018); or (3) neither of these two factors (Murphy & Way, 2021; Wang, 1996). Clearly, there is an urgent need for more studies that focus on unraveling the biochemical mechanisms underlying the responses of Vcmax and Jmax to warming and elevated CO2. Such studies are essential for improving our understanding of photosynthetic responses and modelling under both warming and elevated CO2 growth conditions.
In summary, our study showed that the photosynthetic capacity (Vcmax and Jmax) of mature trees of North American boreal conifers responded independently to warming and elevated CO2 when exposed to both environmental factors. Our results are consistent with several previous studies that predominantly focused on younger trees, suggesting that ontogeny minimally influences these parameters' responses to global change factors. Furthermore, our results add to growing empirical evidence that the representation of photosynthesis within TBMs under both warming and elevated CO2 could incorporate their effects independently. However, our study was confined to two boreal conifer species. Therefore, we advocate for future research encompassing a broader spectrum of boreal species, diverse plant functional types, and other biomes to understand the widespread nature of the observed responses.
CODE AVAILABILITY
The R codes used for analyses for each figure included in this paper can be accessed at https://doi.org/10.6084/m9.figshare.23685984.v2.
ACKNOWLEDGEMENTS
Research was sponsored by the Biological and Environmental Research Program in the Office of Science, U.S. Department of Energy managed by UT- Battelle, LLC, for the U.S. Department of Energy under contract DEAC05-00OR22725. M.E.D., J.M.W., E.J.W., D.A.M., A.W.K. and P.J.H. were supported under this contract. E.J.W. also acknowledges support from USGS Climate Research and Development Program. P.B.R., A.S., R.B., and R.A.M acknowledge funding support by the U.S. NSF Biological Integration Institutes grant DBI-2021898. D.A.W. acknowledges funding from the NSERC Discovery and Strategic programs (RGPIN/04677-2019 and STPGP/521445-2018), the Research School of Biology at the Australian National University, and the U.S. Department of Energy contract No. DE-SC0012704 to Brookhaven National Laboratory. Notice: This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan). The DOI link for the data set used in this paper can be accessed at https://doi.org/10.25581/spruce.056/1455138 (Dusenge, Ward, et al., 2020) and https://doi.org/10.6084/m9.figshare.23685984.v2. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Figshare at https://doi.org/10.6084/m9.figshare.23685984.v2. The raw and processed (i.e., mean values used to generate each figure in the paper) photosynthetic capacity data generated in this study have been deposited in the figshare database and can be accessed at https://doi.org/10.6084/m9.figshare.23685984.v2. The complete leaf gas exchange data, including the data used in this paper, are also available through the SPRUCE project website at https://doi.org/10.25581/spruce.056/1455138.




