Hydraulic plasticity and water use regulation act to maintain the hydraulic safety margins of Mediterranean trees in rainfall exclusion experiments
Abstract
Hydraulic failure due to xylem embolism has been identified as one of the main mechanisms involved in drought-induced forest decline. Trees vulnerability to hydraulic failure depends on their hydraulic safety margin (HSM). While it has been shown that HSM globally converges between tree species and biomes, there is still limited knowledge regarding how HSM can adjust locally to varying drought conditions within species. In this study, we relied on three long-term partial rainfall exclusion experiments to investigate the plasticity of hydraulic traits and HSM for three Mediterranean tree species (Quercus ilex L., Quercus pubescens Willd., and Pinus halepensis Mill.). For all species, a homeostasis of HSM in response to rainfall reduction was found, achieved through different mechanisms. For Q. ilex, the convergence in HSM is attributed to the adjustment of both the turgor loss point (Ψtlp) and the water potential at which 50% of xylem conductivity is lost due to embolism (P50). In contrast, the maintenance of HSM for P. halepensis and Q. pubescens is related to its isohydric behavior for the first and leaf area adjustment for the latter. It remains to be seen whether this HSM homeostasis can be generalized and if it will be sufficient to withstand extreme droughts expected in the Mediterranean region.
1 INTRODUCTION
Over the last decades, many studies have been conducted to better understand the mechanisms responsible for tree dieback following severe water stress (Barigah et al., 2013; Choat et al., 2018; Sperry et al., 2002; Tyree & Sperry, 1988). Among the different processes involved in plant mortality under drought (Allen et al., 2015; Anderegg et al., 2015), hydraulic failure has been identified as one of the key triggers of tree death (Adams et al., 2017; Anderegg et al., 2016; Arend et al., 2021; McDowell et al., 2008). During drought, soil and atmospheric drying result in an increase in xylem water tension (i.e. a decrease in plant water potential). Excessive water tension promotes the occurrence of xylem cavitation, which causes the rupture of water columns between roots and leaves by embolism. Hydraulic failure occurs when the water transport in the plant is severely impaired by embolism (Tyree & Sperry, 1989), causing different damages to living tissues that may prevent the recovery of the tree when drought ends and eventually kill the tree (Mantova et al., 2022).
The timing and likelihood of hydraulic failure during drought is influenced by various physiological characteristics that control plant desiccation resistance (Choat et al., 2018; Duursma et al., 2019; Ruffault et al., 2022). The xylem vulnerability to cavitation is a crucial trait that defines the rate of spread of embolism with water potential decrease. It is derived from vulnerability curves relating increased xylem embolism (e.g. the loss of hydraulic conductance) with decreasing xylem water potentials (Cochard et al., 2008, 2013; Cochard, 2002). The main parameter of this vulnerability curve is the water potential causing 50% of hydraulic conductance loss (P50), which is negatively correlated with survival time during drought and the dryness of species habitat (Lens et al., 2016; Martin-StPaul et al., 2017). The rate of water potential decline during drought (i.e. plant desiccation dynamics resistance) depends, among other factors, on water loss regulation through stomatal control (Martin-StPaul et al., 2017). Thus, the earlier stomata close after the drought onset, the more plants are able to limit the decrease in their water potential and preserve xylem conduits from cavitation. The water potential at turgor loss point (Ψtlp) can be used as a surrogate of the water potential at which stomata close (Brodribb & Holbrook, 2003; Martin-StPaul et al., 2017), given that stomata close once guard cells lose turgidity. In addition, plant residual transpiration, occurring through incompletely closed stomata or the cuticle (Machado et al., 2021), also determines the rate of the plant-drying process and the hydraulic risk (Duursma et al., 2019).
The hydraulic safety margin (HSM) is an integrative trait used to evaluate the risk of hydraulic failure under drought, with a higher HSM indicating a lower risk. Two versions of HSM have been proposed. The first one corresponds to the difference between the minimum xylem water potential (e.g. Ψmidd min) reached by a plant and the threshold water potential causing severe xylem embolism (e.g. P50) (Choat et al., 2012). According to this definition, HSM represents the hydraulic risk associated to the water stress actually experienced by the plant. In the second definition, Ψmidd min is replaced by the water potential causing stomatal closure (Martin-StPaul et al., 2017). This second version of HSM integrates the degree to which stomatal control can prevent hydraulic risk. Since the minimum potential achieved by a plant depends on stomatal control, the two definitions of HSM are closely linked. The HSM has been shown to be conserved across forest tree species worldwide (Choat et al., 2012), as a result of the coordination of multiple traits optimizing the xylem safety-efficiency trade-off with species habitat (Franklin et al., 2023; Guillemot et al., 2022; Martin-StPaul et al., 2017; Pivovaroff et al., 2018). However, there has been little investigation into the intraspecific variations of the HSM in response to drier conditions. To our knowledge, the only publication focusing on this topic reports narrower HSM for Mediterranean species occurring at the dry edge of their geographical distribution (Alon et al., 2023).
Understanding the impact of plant trait plasticity on plant desiccation dynamics and HSM is crucial for predicting hydraulic risk in the current context of rapid climate change (Jinagool et al., 2018). Yet, limited research has thus far investigated the adjustment of multiple traits after long periods of increased drought. Some studies suggested that leaf traits, such as Ψtlp change more in response to sustained drought than the xylem vulnerability to cavitation (Bartlett et al., 2012; Torres-Ruiz et al., 2019), which is sometimes invariant (Lobo et al., 2018; Rosas et al., 2019) and sometimes plastic (Bert et al., 2021; Herbette et al., 2021; Lemaire et al., 2021). For the Mediterranean species Quercus ilex, past studies have reported limited plasticity of xylem vulnerability to cavitation (Limousin et al., 2010, 2022; Martin-StPaul et al., 2013). Additionally, it is important to note that, until recently, the methods used to measure xylem vulnerability to cavitation in long-vessel branches, such as those of Q. ilex, were affected by a bias due to open vessel methodological artefact (Martin-StPaul et al., 2014; Torres-Ruiz et al., 2014). Similarly, the methods used to estimate leaf hydraulic vulnerability curves based on leaf hydraulic conductance were also biased by the integration of an extra-xylary water pathway (Li et al., 2020; Limousin et al., 2022; Trifiló et al., 2016). Hence, it is important to revisit the impact of plant trait plasticity on plant desiccation dynamics with up-to-date and unbiased methods, such as Cavitron (Cochard, 2002) or optical vulnerability (Brodribb et al., 2016) techniques.
In this study, we aimed to assess whether, and by which mechanisms, the HSM of a given species can change with increasing drought conditions. We focused on three of the most widespread tree species in the Mediterranean basin, namely Pinus halepensis Mill., Quercus ilex L. and Quercus pubescens Willd. Those species are being monitored in three long-term partial rainfall exclusion experiments set up in natural forests in southern France: the Puéchabon (P hereafter) experimental site (a Q. ilex forest), the Oak Observatory at the Haute-Provence Observatory (O3HP hereafter) (a Q. pubescens forest) and the Font-blanche (FB hereafter) site (a mixed forest of P. halepensis and Q. ilex). In those three sites, we measured and compared hydraulic traits in adults of the three species under control and aggravated drought conditions.
2 MATERIALS AND METHODS
2.1 Sites description
The study was conducted on three experimental forest sites located in the French Mediterranean region (Figure 1) that stand out by their vegetation types: the FB forest is composed of P. halepensis in the overstory and Q. ilex in the understory, the oak observatory at the O3HP is dominated by Q. pubescens and the P site by Q. ilex. The climate of the sites is Mediterranean, with wet, mild winters and dry, hot summers (Supporting Information S1: Figure S1). The subsoil of all sites is composed of a hard limestone bedrock. The soils of the FB and P sites are similar and classified as silty clay loam, while the O3HP site is a clay-loam soil. They have a high proportion of rocks and soil depth varies between 35 and 50 in FB and P and 30–40 cm in O3HP. On all sites, a rainfall exclusion experiment has been implemented for several years (15, 9 and 6 years respectively for P, FB and O3HP sites at the time of this study excluding approximately 30% of the precipitation reaching the ground. At FB and P, the system excluding precipitation is passive, with polyvinyl chloride gutters hung under the tree canopies. At O3HP, rainfall exclusion is achieved with a mobile rainout shelter deployed manually only during some rainfall events from spring to autumn (Figure 1). Table 1 provides more details on site characteristics and rainfall exclusion treatments. On each site, meteorological variables are monitored, including half-hourly precipitation, radiation, air temperature and humidity.

| Font-blanche | Puéchabon | O3HP | |
|---|---|---|---|
| Location | 43°14′27″N, 5°40′45″E | 43°44′29″N, 3°35′45″E | 43°56′115″ N, 05°42′642″ E |
| Precipitation (mm)/temperature (°C) (annual mean value between 2016–2019) | 615/14.2 | 1033/14.0 | 871/12.9 |
Soil characteristics: type, depth (cm), available soil water (mm) |
Silty clay loam, 20–50, 160 | Silty clay loam, 30–70, 140 | Clay-loam, 30–40, NA |
| Vegetation type | Evergreen Pinus halepensis and Quercus ilex forest | Evergreen Q. ilex forest |
Deciduous Quercus pubescens forest |
| Basal area (m2/ha) (mean 2018) | 29.7 | 27.2 | 21 |
| Tree height (mean 2018) | 13.5 for P. halepensis 6.5 for Q. ilex |
5.5 | 5 |
| Rainfall exclusion device, treatment area (m2), year of the start of the exclusion | Gutters, 625, 2009 | Gutters, 140 × 4 replicates, 2003 | Mobile rainout shelter, 300, 2012 |
| Precipitation exclusion ratio | ≈30% | ≈30% | ≈30% |
| References | Moreno et al. (2021) | Limousin et al. (2009) | Genard-Zielinski et al. (2018) |
- Abbreviation: O3HP, Haute-Provence Observatory.
2.2 Water potentials in the field
Water potentials were taken from Moreno et al. (2021) for P. halepensis and Q. ilex at FB, from Limousin et al. (2022) for Q. ilex at P and from Genard-Zielinski et al. (2018) for Q. pubescens at O3HP. Predawn (Ψpd) and midday (Ψmidd) water potentials were measured from 2015 to 2019 in each site (Table 2) during summer using Scholander pressure chambers (different brands depending on year and site). The samplings were performed on several dates from the onset to the end of summer (between two and six measurements per year depending on year and site). At each site and date, measurements were made on twigs (FB and P sites) or leaves (O3HP site) of at least three trees per species and per treatment (control/rainfall exclusion). One to three samples per tree were measured depending on site and date during the 2 h preceding sunrise for Ψpd and between 2:00 and 4:00 PM for Ψmidd. Ψmidd were measured on transpiring twigs/leaves. For Ψpd, the data from the date with the lowest values recorded per species were selected for each year to compute the extreme annual predawn water potentials (Ψpd min). The same applied to Ψmidd, to compute the lowest midday water potentials (Ψmidd min).
| Measurements | Pinus halepensis | Quercus pubescens | Quercus ilex FB | Quercus ilex P |
|---|---|---|---|---|
| Trees water potentials Ψpd min (MPa) | From 2013 to 2018 | 2014, 2015, 2018 | From 2013 to 2018 | From 2015 to 2018 2017 |
| Ψmidd min (MPa) | 2018 | 2015 | 2017 | 2017 |
| Pressure volume curves: Ψtlp (MPa), π100 (MPa) | March 2018 | September 2019 | March 2018 | March 2018 |
| gmin (mmol s−1 m−2) | March 2018 | September 2019 | March 2018 | March 2018 |
| Xylem vulnerability to cavitation: P50 (MPa) | March 2018, October 2018 | September 2019 | Onset of summer 2019 | Onset of summer 2019 |
| Native stem xylem embolism (%) | March 2018 | — | March 2018 | March 2018 |
- Abbreviations: FB, Font-blanche; P, Puéchabon sites.
2.3 Vulnerability to cavitation
Because xylem vessel length in the two oaks species can reach up to 1 m (Martínez-Vilalta et al., 2002), we were unable to employ the cavitron technique with oaks, due to the bias for long-vessel species when branch samples are shorter than the vessel length (Beikircher et al., 2010; Cochard et al., 2013; Martin-StPaul et al., 2014; Sergent et al., 2020). Instead, we used the optical technique (Brodribb et al., 2016) to measure the xylem vulnerability to cavitation in the leaves. We opted for the leaf optical technique rather than the stem optical technique, as it does not require to remove tissue limiting potential bias caused by damaged xylem if not well executed. No branches used to measure vulnerability curve were flushed to remove native embolism, as such procedure can bias vulnerability curves (Petruzzellis et al., 2023) due to open-vessel artefact. Drawing from the findings of Li et al. (2020), and our own observation of similar P50 using the optical method and X-ray microtomography for Q. ilex stems (Sergent et al., 2020) and cavitron for Q. pubescens branches (personal data given by Delzon) (Supporting Information S1: Figure S3), we assumed that hydraulic segmentation did not occur in the two oak species. Anyway, despite the use of different hydraulic techniques across species, it is important to note that the aim of this study is to evaluate intraspecific plasticity in hydraulic traits, rather than interspecific drought tolerance.
During summer 2019, 4–5 branches more than 1 m long of Q. ilex and Q. pubescens sampled from different trees, were harvested in each control and rainfall exclusion treatments of the three studied sites. Branches were placed in humidified bags and stored in a dark cold room with the cut end in water for at least one night to rehydrate. Once fully rehydrated, branches were allowed to dehydrate progressively on the lab bench (bench dehydration technique). One or two mature leaves per branch, still attached to the branch, were placed on a flatbed light transmission scanner (Epson V850). The leaves were kept flat using microscope slides attached to the scanner glass with transparent tape. Leaf scans at a resolution of 2400 dpi were recorded in light transmission mode every 5 min while the branch was progressively dehydrating. Concomitantly, xylem water potential was measured on bagged leaves of the same branch using a Scholander pressure chamber (PMS instrument), approximately every 3 h throughout branch desiccation (a minimum period of 3 days being enough to reach 100% emboli in the xylem, Supporting Information S1: Figure S4).
Once the branch was fully desiccated and the xylem water potential was no longer measurable with Scholender pressure chamber, all images were analysed to detect cavitation events as changes in light transmittance between successive scans (http://www.opensourceov.org/). Image J (FIJI, Schindelin et al., 2012) was used to reveal embolism events by overlapping successive leaf scans and calculating the pixel area of embolized vessels. Vulnerability curves were constructed for each leaf by plotting the relative cumulative embolized area (corresponding to the cumulative embolized area divided by the total embolized area at the end of leaf dehydration) as a function of leaf water potential and fitting the same sigmoid function as for P. halepensis.
2.4 Pressure volume curves and minimum leaf conductance to water vapour
Pressure–volume curves (hereafter PV curves) were used to characterize different foliar traits related to the maintenance of leaf turgor and hydration (Bartlett et al., 2012; Tyree & Hammel, 1972). For each species, two well-lit twigs per tree were collected from the outer crown of 4–6 trees in the control and exclusion treatments. The sampling took place in March 2018 for P. halepensis and Q. ilex at FB and P sites and in September 2019 for Q. pubescens at O3HP site. In all cases, once collected, twigs were bagged and placed in a cooler at 4°C until reaching the laboratory. Once in the lab, each twig end was recut under water with a razor blade, then put overnight in a cool chamber in distilled water to rehydrate. Half of the samples were used to estimate the leaf mass area and the other half to perform the PV curves.
PV curves were established using the bench drying method proposed by Hinckley et al. (1980). Briefly, the weight and the water potential of the twigs were measured all along their dehydration using a precision balance (FS-220, resolution 0.1 mg) and a Scholander pressure chamber (PMS instrument), respectively. To overcome oversaturation of rehydrated twigs, the first measurement of water potential and weight was removed, and full turgor weight was extrapolated from the regression between twig weight and water potential before the turgor loss point. The osmotic potential at full turgor (π100) and at the turgor loss point (Ψtlp), the relative water content at turgor loss point (RWCtlp), the symplastic water fraction at full turgor (Fs) and the bulk modulus of tissue elasticity (ε) were obtained by plotting the inverse of water potential (−1/Ψ, in MPa) against twig water saturation deficit (1 − relative water content, in %). PV curves data used for Q. ilex are the same as those reported in Limousin et al. (2022).
2.5 Hydraulic safety margins
Two types of hydraulic safety margins (HSM) were computed at each site and for both treatments by subtracting either (i) the minimal midday water potentials (Ψmidd min) to P50, following the approach outlined by Choat et al. (2012), or (ii) the turgor loss point, as a surrogate as water potential causing stomatal closure to P50 as in Martin-StPaul et al. (2017).
To avoid damaging trees, the samples collected to construct hydraulic vulnerability curves were taken from different trees than those sampled for leaf water potential and turgor loss point measurements. Therefore, it was not possible to calculate the HSM at the tree level. Instead, we determined both HSM versions at the species and treatment levels using a nonparametric bootstrap approach. For each species and treatment (control and rainfall exclusion), we randomly sampled, with replacements, five values of P50 and Ψmidd min or Ψtlp and determined five HSM as the difference between these values. This process was repeated 1500 times to obtain a distribution of HSM values for each site and treatment that was subsequently used for statistical comparisons.
2.6 Native stem xylem embolism measured by X-ray microtomography
Native stem xylem embolism was estimated for Q. ilex and P. halepensis trees in both rainfall exclusion and control treatments at FB and P. The sampling took place in March 2018. Four to eight well-lit branches of more than 1 m were collected depending on the sites, the treatments and the species considered. Once collected, branches were immediately recut under water to avoid cutting artefacts.
The cut surface of the branches was kept underwater until reaching the laboratory. Then, short segments of branches (diameter <0.7 cm and length <4 cm) were cut under water, plunged in liquid paraffin and stored in a cold chamber at 4°C until analysis. Measurements were performed following the protocol described in Cochard et al. (2015). Samples were inserted in an X-ray microtomograph (Nanotom 180 XS; GE) and analysed using a field of view of 5 × 5 × 5 mm3, X-ray voltage of 60 kV, current of 240 µA and a scan time of 21 min. The final spatial resolution of the three-dimensional (3D) images was 2.5 µm after 3D reconstruction. For each sample, one transversal 2D slice was extracted from the middle of the branch using VGStudio Max© software (Volume Graphics). The surface area of embolized conduits was estimated from slices using the software ImageJ (Schneider et al., 2012).
As Q. ilex is a semiring-porous species with log-normal distribution of xylem conduit sizes, we measured the surface area of large embolized vessels (contributing the most to hydraulic conductivity) to estimate their mean diameter and their corresponding hydraulic conductivity. The maximal hydraulic conductivity was estimated thanks to a second scan we performed on the same branch sample once it was fully embolized. The level of embolism was calculated as the ratio between estimated native hydraulic conductivity and maximum hydraulic conductivity.
2.7 Statistics
To assess whether plant and leaf traits were different between treatments we used t test for all traits measured. For the HSM, we made a Student's t test for each of the 1500 subsamples obtained via bootstrapping. We considered that rainfall exclusion and control treatments were significantly different if the probability of having a p value > 0.05 among the 1500 t tests realized was lower than 0.05. Differences in parameters derived from xylem vulnerability curve (slope and P50; Supporting Information S1: Tables S2 and S3, Figure S5), π100, Ψtlp and gmin between rainfall exclusion and control treatments were found to be similar for Q. ilex in both FB and P sites (Limousin et al., 2022). Hence, to increase the statistical power in testing for a rainfall exclusion effect on trait values, as in Limousin et al. (2022) we decided to combine the data from both sites for this particular species. All statistical analyses were performed with R software (3.5.2, R Development Core Team 2018).
3 RESULTS
3.1 Rainfall exclusion impacts on tree water status
Variations in the response of Ψpd min to rainfall exclusion treatments were observed among different tree species and sites (Figure 2). For Q. pubescens at O3HP, Ψpd min tended to be more negative for trees growing in the rainfall exclusion treatments (p value = 0.005) until 2015 (2 years after the onset of the rainfall exclusion). After that, Ψpd min was similar to that of control trees (p value > 0.1). For P. halepensis at FB, Ψpd min were similar across years, except in 2015 (p value = 0.05), when they were slightly more negative in the rainfall exclusion treatments (Figure 2). It should be noted, however, that if we consider the whole seasonal water potential dynamic rather than the extreme values, significantly more negative water potentials appear in rainfall exclusions at lower levels of water stress (Genard-Zielinski et al., 2018; Moreno et al., 2021). Moreover, soil water content taken at shallow ground level (<50 cm depth), tended to be more negative in the rainfall exclusion treatment than in the control (Genard-Zielinski et al., 2018; Moreno et al., 2021). All of these findings attest to the effectiveness of the exclusion treatments for these two species.
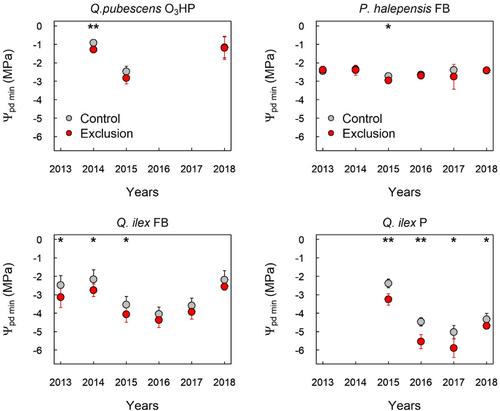
Concerning Q. ilex at both FB and P sites, Ψpd min tended to be more negative in the rainfall exclusion treatments (Figure 2, p value < 0.05 in FB site until 2015; p value < 0.006 in P site until 2016, then p value < 0.05).
3.2 Effects of rainfall exclusion on hydraulic and leaf traits
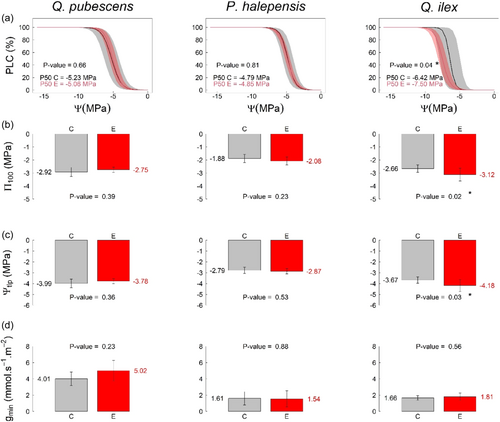
No significant differences in P50, π100, Ψtlp nor gmin, were found between the rainfall exclusion and control treatments for Q. pubescens and P. halepensis (Figure 3, p value > 0.2 for each of these traits). For Q. ilex, P50, π100 and Ψtlp were significantly more negative for trees growing in the rainfall exclusion (p value = 0.04 for P50; p value = 0.02 for π100; p value = 0.03 for Ψtlp), whereas gmin remained unchanged (p value > 0.05).
3.3 Effect of rainfall exclusion on species HSM and xylem embolism
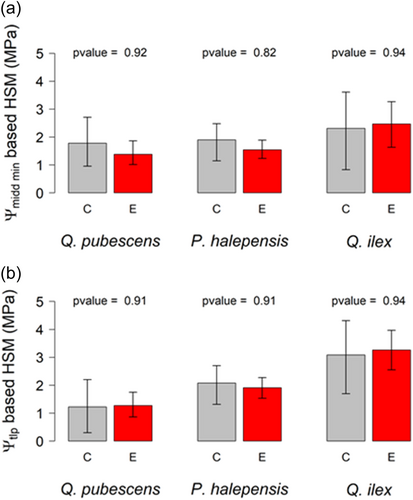
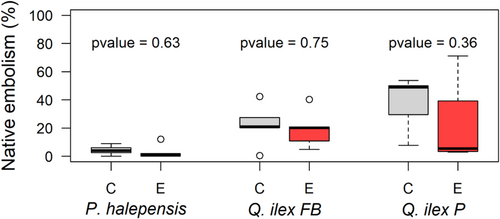
For all species, we did not observe any statistical difference in HSM between rainfall exclusion and control trees, based on either Ψmidd min (Figure 4a, p value > 0.8 for each species) or Ψtlp (Figure 4b, p value > 0.9 for each species). Levels of native stem xylem embolism were also similar in both treatments for P. halepensis or Q. ilex, the only species analysed (Figure 5).
4 DISCUSSION
In the current context of rapid global change, tree capacity to acclimate to increasing drought appears crucial for the maintenance of forest structure, composition and function. Under drier conditions, hydraulic failure risk should increase for trees if they cannot adjust key hydraulic traits involved in the hydraulic safety. In the present study, we aimed to prospect the ability of three functionally different species (i.e. with different drought response strategies, clades, phenology, etc.) to modify these traits under increasing drought conditions and to assess the hydraulic risk ensued. Among the three studied species, only Q. ilex exhibited significant plasticity in the hydraulic traits prospected, but all three species maintained the HSM. In the following, we first discuss the effects of the hydraulic adjustments of Q. ilex for its drought tolerance capacity. Then, we argue about the likely causes leading to a lack of hydraulic adjustment under rainfall exclusion treatment for both P. halepensis and Q. pubescens. Finally, we discuss the consequences of HSM homoeostasis in response to drier conditions for the hydraulic risk of the species.
4.1 Homoeostasis of HSM due to plasticity in Q. ilex
Q. ilex reached lower values of both Ψpd min and Ψmidd min in the rainfall exclusion treatments of both FB and P sites (Figure 2 and Supporting Information S1: Figure S6). Thus, the higher water stress experienced by this species in response to increased drought could be the trigger for P50 and Ψtlp adjustments towards more negative values (Figure 3 and Supporting Information S1: Figure S7).
Until now, studies focusing on long-term drought acclimation of Q. ilex had concluded that no plasticity of hydraulic vulnerability occurred for adult trees undergoing rainfall exclusion, in either branches (Limousin et al., 2010; Martin-StPaul et al., 2013) or in leaves (Limousin et al., 2022). Our results contradict those previous works. This could be explained by the facts that (1) previous estimates of P50 in the xylem of branches were possibly biased by the open-vessel artefact (Martin-StPaul et al., 2014; Torres-Ruiz et al., 2014); (2) estimations of xylem vulnerability to cavitation in branches include several wood rings, which may hide xylem plasticity occurring after a specific aggravated drought and (3) the rehydration kinetics method used to calculate leaf hydraulic conductance may not reflect only the xylem vulnerability to cavitation but also an extra-xylary component in the leaf mesophyll (Li et al., 2020; Limousin et al., 2022; Trifiló et al., 2016). The optical technique (Brodribb et al., 2016) used in this study for Q. ilex and Q. pubescens appears as a good solution to overcome these limitations. It allows the estimation of hydraulic vulnerability in the xylem only and in leaves, that are shorter lived, and therefore less affected than branches, by the potential biases due to regrowth and cavitation fatigue, or by methodological issues related to the flushing of native stem xylem embolism and vessels open at both ends (Gauthey et al., 2020).
In addition to the adjustment of xylem vulnerability to cavitation, our findings also highlighted that turgor-related traits also changed for this species under aggravated water stress conditions. Indeed, for Q. ilex trees growing in the rainfall exclusion treatments, lower π100 values were reported (Limousin et al., 2022), which translate into a significant reduction of Ψtlp (Bartlett et al., 2012). As stomatal closure is realized through the loss of stomata guard cells' turgidity, Ψtlp could be used as a surrogate of the point of stomatal closure (Martin-StPaul et al., 2017). This suggests that Q. ilex trees from the rainfall exclusion treatments could close their stomata at lower water potentials than trees from the control treatments thus allowing them to maintain more photosynthetic activity or root growth under similar drought conditions. This adjustment may lead to an increased risk of xylem embolism if the vulnerability to cavitation, P50, is not also reduced. The apparent coordinated plasticities of Ψtlp, that is lowered by 0.5 MPa and of P50 that is lowered by 1 MPa, could be beneficial to leaf gas exchange, without affecting the hydraulic safety margin (Figure 4b). This maintenance of the HSM in spite of drier conditions seems confirmed by the similar levels of native stem xylem embolism observed in the Q. ilex trees growing inside the rainfall exclusion treatments and those growing under control conditions (Figure 5). Limousin et al. (2022) showed that Fs increased significantly in the rainfall exclusion treatments, suggesting that lower Ψtlp do not result from internal cell water loss but rather from the accumulation of osmolytes. Hence, we can assume that, besides its action on stomatal control, osmotic adjustment in the exclusion treatments could also play a role in limiting cell dehydration and hence drought-induced damages on leaves, which is crucial for this evergreen species with a leaf lifespan of two to 3 years (La Mantia et al., 2003).
Regarding the minimal leaf conductance (gmin), which is implied in water loss after stomatal closure (Duursma et al., 2019), we observed no plasticity in response to long-term increased drought for Q. ilex or any of the other species (Figure 3). This suggests either that gmin is not or little plastic, or that more precise methods, such as the drought box (Billon et al., 2020), should be used to detect plasticity in this trait.
4.2 Homoeostasis of HSM despite lack of plasticity of hydraulic traits prospected in P. halepensis and Q. pubescens
The most likely explanation for the lack of adjustments in hydraulic traits for P. halepensis and Q. pubescens is that these species experienced similar levels of extreme water stress under both control and rainfall exclusion treatments, and that that was no advantage in increasing HSM. This hypothesis appears plausible for P. halepensis, for which Ψpd min were similar between rainfall exclusion and control trees (Figure 2). Such result is consistent with its behaviour in response to drought. Indeed, P. halepensis is an isohydric species that strongly regulates its transpiration at the onset of drought, allowing it to limit soil water consumption and maintain both Ψpd and Ψmidd (Figures 2, 3 and Supporting Information S1: S6) over a safe threshold of −3 MPa (Moreno et al., 2021). Recently, Moreno et al. (2024) proposed that such isohydric behaviour is the result of two mechanisms: (i) its stomatal closure that happened at relatively high level of water stress and (ii) its ability to isolate itself from the ground and the atmosphere. Hence, its water use strategy seems sufficient in itself to limit hydraulic damages caused by rainfall reduction, without requiring xylem adjustments, at least under our experimental conditions. The fact that native stem xylem embolism was similar between rainfall exclusion and control treatments (Figure 5 and Supporting Information S1: Figure S6) further supports this idea.
For Q. pubescens, given its anisohydric behaviour (Damesin & Rambal, 1995; Poyatos et al., 2008), we expect trees from the rainfall exclusion to be exposed to more negative water potentials. In 2014, 2 years after the onset of the rainfall exclusion experiment, a significant difference between Ψpd of control and rainfall exclusion trees was observed. This result attests the effectiveness of the rainfall exclusion, which is in line with the significant reduction in soil moisture measured in the rainfall exclusion treatments (Genard-Zielinski et al., 2015, 2018). Nevertheless, for the following studied years, Ψpd min was similar between treatments. This result, which seems at first sight contradictory, probably underpins other adjustments in unprospected traits. Supporting Information S1: Figure S8 shows that in 2010, the amount of leaf produced by trees in the rainfall exclusion treatment was 1.5 higher than that of the control treatment, suggesting a higher leaf area index in the rainfall exclusion plot. Since 2012 (onset of the rainfall exclusion experiment), throughout the years, the difference between the two treatments narrowed, until leaf productions in both treatments were similar in 2018 onward. This result shows that trees in the exclusion plot have adjusted their leaf area, and therefore their water demand, and hence water potential decrease. Other adjustments could also have occurred as, for example, an increase of rooting depth induced after aggravated water stress, as proposed by Martin-StPaul et al. (2013), enabling trees to access deeper soil water and thus limiting their drought exposure. Likewise, earlier leaf senescence could also occur in trees facing drier conditions (Wu et al., 2022). These additional adjustments occurring at the whole tree scale could be sufficient to dampen the rainfall exclusion effect on extreme tree water stress and prevent hydraulic damages. Further investigations are however needed to identify the other traits implied in the homoeostasis of hydraulic risk for this species.
4.3 The risk of maintaining the HSM for species vulnerability under future climatic conditions
Although achieved through different means, our data report a maintenance of HSM under experimental 30% rainfall exclusion for the three studied species (Figure 4). This suggests that no matter the drought response strategy adopted by these species, trees exposed to drier conditions adjusted functionally to preserve this trait. One might have expected that, to reduce hydraulic risk, trees could have adjusted to increase HSM values, through the development of a more cavitation-resistant xylem. A reasonable explanation that could be advanced to explain this lack is the need for the plant to optimize fluxes relative to the hydraulic risk and that can be achieved through a myriad of strategies. The maintenance of HSM in response to plasticity that we reported in this study is in line with metanalysis from Choat et al. (2012), which reports a worldwide convergence of forest vulnerability to drought (estimated through tree HSM) at the interspecific level independently of the level of drought. The fact that trees seem to maintain HSM despite several years of increasing drought calls into question the natural capacity of forests to withstand accelerating climate change. To what extent HSM can be maintained in the future and whether this maintenance holds at global scale are still open questions. It remains to be known how much future extreme drought or heat waves may affect acclimated and nonacclimated trees. Mechanistic process-based models (Blackman et al., 2019; Martin-StPaul et al., 2017; Ruffault et al. 2023), able to characterize hydraulic risk in various conditions, they would enable to assess how the combination of several traits could translate into different levels of risk in a warmer, drier future.
ACKNOWLEDGEMENTS
Myriam Moreno was supported both by (i) the French Environment and Energy Management Agency (ADEME) and INRAE in the form of a PhD scholarship and (ii) by the INRAE in the form of a Postdoctoral fellowship. J. R.-C. acknowledges the “Ministerio de Educación Cultura y Deporte” for a “José Castillejo” mobility grant. The work has also received support from H2020 under Grant Agreement No. 862221 (FORGENIUS) and by Agence Nationale pour la Recherche (MixForChange, ANR-20-EBI5-0003; TAW-tree, ANR-23-CE01-0008-01) and the Metaprogramme ACCAF Drought&Fire. The Puéchabon, Font-Blanche and O3HP experimental sites all belong to the French national research infrastructure ANAEE-France (ANR-11-INBS-0001), with Puéchabon and Font-Blanche also belonging to the French network of ICOS Ecosystem stations (Integrated Carbon Observation System ERIC) and O3HP and Puéchabon belonging to the SEE-life label from CNRS (Long term monitoring of life in Ecology and Evolution). Puéchabon is further supported by the OSU OREME (UMS 3282). Measurements at Font-Blanche were supported by the ECODIV department of INRAE. O3HP is also supported by the OSU Pytheas, especially the Observatoire de Haute Provence, the Experimentation and Observation Node from IMBE (especially Jean-Philippe Orts for water potential measurements) and the Fédération de Recherche 3098 ECCOREV (ECosystèmes COntinentaux et Risques EnVironnementaux) (especially Ilja Reiter for climate-related captors and climatic data management). The authors thank the Phenobois Plateform (INRAE, Clermont-Ferrand-France) for X-ray microtomography; Amélie Tournant and Pierre-Jean Dumas for their help in the extraction of data of xylem vulnerability curves; Arnaud Jouineau and Jean-Phillipe Orts for their help in collecting samples.
Open Research
DATA AVAILABILITY STATEMENT
Data are incorporated into the article and the supplementary material.




