Discrimination of relatedness drives rice flowering and reproduction in cultivar mixtures
Abstract
The improvement of performance and yield in both cultivar and species mixtures has been well established. Despite the clear benefits of crop mixtures to agriculture, identifying the critical mechanisms behind performance increases are largely lacking. We experimentally demonstrated that the benefits of rice cultivar mixtures were linked to relatedness-mediated intraspecific neighbour recognition and discrimination under both field and controlled conditions. We then tested biochemical mechanisms of responses in incubation experiments involving the addition of root exudates and a root-secreted signal, (–)-loliolide, followed by transcriptome analysis. We found that closely related cultivar mixtures increased grain yields by modifying root behaviour and accelerating flowering over distantly related mixtures. Importantly, these responses were accompanied by altered concentration of signalling (–)-loliolide that affected rice transcriptome profiling, directly regulating root growth and flowering gene expression. These findings suggest that beneficial crop combinations may be generated a-priori by manipulating neighbour genetic relatedness in rice cultivar mixtures and that root-secreted (–)-loliolide functions as a key mediator of genetic relatedness interactions. The ability of relatedness discrimination to regulate rice flowering and yield raises an intriguing possibility to increase crop production.
1 INTRODUCTION
When two or more plant species occur together and interact, intraspecific competition generally must be stronger than interspecific competition to allow coexistence. This pattern is mainly generated by resource partitioning and niche divergence minimising interspecific interactions (Chesson, 2000; Tilman, 1982), whereas conspecifics interact more intensely because of their similarity. However, beneficial plant–plant interactions, such as conspecific cooperation and heterospecific facilitation, also frequently occur in natural and managed ecosystems. Neighbours such as N-fixing species or closely related cultivars within a crop species may improve soil nutrient availability and reduce plant competition, improving performance and yield in species and cultivar mixtures (Anten & Chen, 2021; Li et al., 2016; Xia et al., 2016; Yang et al., 2018). The net outcomes of an intercropping (multiple species) or cultivar mixtures (single species) involve multiple biotic and abiotic factors, but the central driver must be neighbour interactions.
Neighbour-dependent benefits of mixed cropping systems have been attributed to niche complementarity, facilitation and within-species diversification in interspecific interactions (Li et al., 2021; Ryan, 2021; Tilman, 2020; Yang et al., 2019; Yu et al., 2021), but relatedness-mediated intraspecific interactions have rarely been invoked (Fréville et al., 2022; Yang et al., 2018). The ability to detect relatedness would allow plants to discriminate closely from distantly related neighbours and allow plants to optimise response strategies to the composition of their local neighbourhood (Anten & Chen, 2021; Dudley et al., 2013; Mazal et al., 2023). Such a relatedness-mediated identity recognition within a species has been observed not only at the individual, population and biotype levels (Ding et al., 2023; Dudley & File, 2007; Lepik et al., 2012; Torices et al., 2018) but also cultivar level (Pezzola et al., 2020; Xu et al., 2021; Yang et al., 2018).
Kin recognition in crop plants can result in less intraspecific competition, maximising stand performance and increasing yield (Kiers & Denison, 2014; Murphy et al., 2017; López Pereira et al., 2017; Yang et al., 2018). Therefore, kin recognition in crop plants may be a potential mechanism responsible for performance and yield in cultivar mixtures (Anten & Chen, 2021; Fréville et al., 2019; Yang et al., 2018). Rice (Oryza sativa L.) is a principal grain crop comprised of numerous cultivars with varying degrees of genetic relatedness. Natural populations have considered progeny of the same mother as siblings, progeny of different mothers as strangers (Dudley & File, 2007; Lepik et al., 2012; Torices et al., 2018). Artificial selection generates rice cultivars that are genetically and morphologically uniform whether they are progeny of the same or different mothers. In the case of highly self-pollinating same cultivar, the coefficient of relatedness is very close to 1 (i.e., r ≈ 1; Mazal et al., 2023; Pezzola et al., 2020; Simonsen et al., 2014). Therefore, genetic relatedness in self-pollinated rice is better represented by closely related cultivars as within cultivar variation is minimal (Xu et al., 2021; Yang et al., 2018).
Kin recognition allows behaviours toward kin groups that promote the survival and reproduction of relatives (Hamilton, 1964; Lehmann & Perrin, 2002). Reducing the energy devoted to competitive root systems allows for greater allocation to reproduction in kin groups (Bhatt et al., 2011; Belter & Cahill Jr, 2015; Biedrzycki & Bais, 2022). In some species, flowering time and reproductive success may depend on the relatedness of neighbours (Bawa, 2016; López Pereira et al., 2017; Shivaprakash & Bawa, 2022; Sun et al., 2021; Torices et al., 2018, 2021). Improvement of grain yields in rice cultivar mixtures can be achieved by selection for root behaviour and biomass allocation in response to closely related cultivars (Fang et al., 2013; Xu et al., 2021; Yang et al., 2018). However, flowering time, the other critical component of rice yields, has not been explored in cultivar mixtures.
Kin recognition in plants involves both physical and chemical signals (Crepy & Casal, 2015; Wang et al., 2021). Most evidence suggests that root exudates act as the primary signal of relatedness (Biedrzycki et al., 2010; Semchenko et al., 2014; Yang et al., 2018), as recognition can take place under root segregation or before physical contact occurs (Goddard et al., 2020; Yang et al., 2018). Root exudates contain signalling molecules such as (–)-loliolide, one of the apocarotenoids produced by the oxidative breakdown of carotenoids (Murata et al., 2019). The oxidative breakdown of carotenoids can form a diverse family of small apocarotenoid molecules, including two primary phytohormones, abscisic acid and strigolactones, as well as anchorene, β-cyclocitral, α-ionone and (–)-loliolide. These signalling molecules are vital to regulating plant growth, development and stress response, and both plant–plant and plant–herbivore interactions (Moreno et al., 2021). Recent studies have shown that root-secreted (–)-loliolide acts as a signalling molecule in plants (Kong et al., 2018; Li et al., 2020; Wang et al., 2023). (–)-Loliolide also acts as an inducer of herbivore and pathogen resistance in plants (Li et al., 2023b; Murata et al., 2019). Furthermore, root-secreted (–)-loliolide modulates both belowground defence and aboveground flowering in tobacco (Nicotiana benthamiana) and Arabidopsis (Li et al., 2023a). (–)-Loliolide has been found to be a general signal of plant stress (Li et al., 2023b), with actions similar to known plant hormones (Frost, 2023). However, much work remains to determine whether (–)-loliolide operates with an endogenous capacity to initiate signal transduction pathways and modulate phytohormone crosstalk networks. In particular, (–)-loliolide's role in relatedness-mediated neighbour discrimination in rice cultivar mixtures has not been considered.
This study used a relatedness gradient of rice cultivars to assess the underlying mechanisms that generate flowering and grain yield in cultivar mixtures. To achieve this, a series of field and controlled experiments were carried out with two sets of rice cultivars, each comprising a focal cultivar, a cultivar closely related to the focal cultivar, and a cultivar distantly related to the focal cultivar. We used these experiments to (1) assess relatedness-mediated impacts on rice flowering time and grain yield in the field and greenhouse, (2) examine the role of (–)-loliolide in driving these changes, and (3) determine changes in gene expression that drive root and flowering responses to neighbour relatedness.
2 MATERIALS AND METHODS
2.1 Plant materials, soil and chemicals
Seven rice (Oryza sativa) cultivars, Huagan-3, Huagan-8, Huafeng, Lingyou-6173, Lingyou-6365, Lingyou-3826 and Liaojing, were used for this study. The pairs Huagan-3/Huagan-8 and Lingyou-6173/Lingyou-6365 were bred from reciprocal parents or identical male sterile, represent the closest relatedness and have the ability for kin recognition at the cultivar level (Xu et al., 2021; Yang et al., 2018). Huafeng and Lingyou-3826 were obtained from other parents or male sterile lines that do not have consanguinity ties with the two closely related pairs. These cultivars were divided into two sets of genotypes, indica-inbred and indica-hybrid. In the indica-inbred rice set, Huagan-3 was the focal cultivar. Huagan-8 and Huafeng were the closely related and distantly related cultivars, respectively. In the indica-hybrid rice set, Lingyou-6173 was the focal cultivar with Lingyou-6365 and Lingyou-3826 as the closely related and distantly related cultivars, respectively. In addition, the japonica-inbred cultivar Liaojing was used as a more distantly related cultivar for both sets. The relatedness of cultivars in both sets has been confirmed by genetic distances to the focal cultivar with simple sequence repeat markers (Yang et al., 2018). The seeds of two common paddy weeds Echinochloa crus-galli and Leptochloa chinensis were collected from local rice fields for use in weed competition experiments.
Soil was collected at Nanling Experimental Station of Rice Research, Anhui Province, China (30°56′52″ N, 118°23′15″ E), which located in the low mountains on the southern side of the Yangtze River. Soil was collected randomly from the surface (0–10 cm) of a paddy field. The soil is a typical fluvaquent, Etisol (US taxonomy) with pH 5.6, organic matter of 24.5 g·kg-1, total nitrogen of 1.4 g·kg-1, available phosphourus of 29.3 mg·kg-1 and available potassium of 56.8 mg·kg-1. Soil samples were air-dried, sieved (2 mm mesh) to remove plant tissues, and used in the series of greenhouse experiments described below.
(–)-Loliolide was isolated from root exudates using previously developed methods (Kong et al., 2018) and verified with its authentic standard obtained from Yuanye Biology Corporation (Shanghai, China). Organic solvents and other chemicals were purchased from China National Chemical Corporation (Beijing, China) and were of the highest purity available.
2.2 Field trials
Two paddy fields were selected at the experimental station described above during the 2021 growing season. The paddies had previously been planted with rice for several seasons. Both fields were divided into a series of 3 × 5 m plots arranged in a completely randomised design of two rice sets and four combinations with four replicates accounting for 32 plots. Plots were separated by trenches with at least 30 cm discard strips on each side. Each focal cultivar was paired with itself (the same cultivar) or one of the closely or distantly related cultivars. The pairs were planted in randomly selected plots at a density of 50 plants/m2 by direct seeding in a field. Cultivar mixtures were conducted in a fully crossed design at a 1:1 ratio. All plots received fertiliser (N, P2O5 and K2O at the rates of 7.5, 9.0 and 8.5 g/m2, respectively) 1 day before the fields were saturated with water. All other field management followed the rules of the rural administration for the local rice industry. Rice growth was checked weekly before flowering. The number of days from sowing when the first flower appeared in the main panicle was recorded daily in 256 haphazardly selected plants for each focal cultivar and 128 plants for each neighbour cultivar during the flowering stage. Finally, grain yields were recorded at mature stage.
2.3 Pot-culture experiments
Four experiments were conducted in a greenhouse with 20–30°C night and day temperatures and 65%–90% relative humidity. Each experiment was conducted in a completely randomised design with five replicates for all treatments and control for a total of 140 pots. Seeds were surface-sterilised with 5% H2O2, placed in Petri dishes with moistened filter paper, and germinated in an environmental chamber at 28°C in the dark.
A cultivar mixture experiment was conducted to evaluate the performance of focal rice cultivars to neighbour relatedness in a series of 15 (diameter) × 20 cm (height) plastic pots containing 4 Kg of soil with base fertiliser (60 mg N·kg-1, 30 mg P·kg-1, 90 mg K·kg-1) in each pot. Three germinated seeds of the focal cultivar were spaced uniformly in the centre of each pot while three seeds of the neighbour cultivar were sown in the surrounding area. Pots with monocultures in the same planting pattern served as controls. All pots were placed in the greenhouse, watered daily and their positions randomised weekly. Focal cultivars in 1/3 of pots were sampled at both the seedling and flowering stages, and their shoots and roots used for the quantification of (–)-loliolide, or for quantitative real-time PCR analysis of flowering-related genes as described below. In the remaining 1/3 of pots, flowering time and grain biomass were recorded.
An exudate incubation experiment investigated the impact of root exudates from the cultivars of varying relatedness on focal cultivar flowering time and grain yield. Root exudates of rice cultivars were collected hydroponically. Sterilised and germinated rice seeds were sown in nursery seedling plates and placed in a growth chamber at 22°C with a 14 h light and 10 h dark photoperiod. Seedlings of each cultivar at the 3-leaf stage were inserted into a styrofoam float, transplanted to a hydroponic container (12 cm diameter × 5 cm height) containing 500 ml distilled water and placed in a growth chamber. After 7 days, the solution was filtered with sterile filter paper (GE Healthcare Whatman) and yielded the root exudates for use in subsequent experiments: Six pre-germinated seeds of each focal cultivar were sown in a series of pots (15 cm diameter × 20 cm height) containing 4 kg soil as described above and placed in the greenhouse. The pots were divided into four relatedness groups. Each group was watered with 300 ml of the root exudates from the same cultivar, closely or distantly related cultivars once every 2 days. Pots treated with sterile distilled water served as controls. Finally, flowering time and grain yield were determined.
A weed competition experiment addressed endogenous (–)-loliolide induction of rice in response to common paddy weeds. Germinated seeds of each focal rice cultivar and two weeds (Echinochloa crus-galli and Leptochloa chinensis) were sown in a plastic pot (12 cm × 10 cm × 10 cm) containing 800 g of soil. Three germinated seeds of rice were placed uniformly in the centre of each pot, and three seeds of one weed were sown surrounding them. Pots with rice monocultures in the same planting pattern served as controls. The pots were placed in the greenhouse and water daily. Rice seedlings were sampled at the 3-leaf stage for the quantification of (–)-loliolide as described below.
A whole-growth-period-sampling experiment was to detect temporal changes in the level of (–)-loliolide in roots, leaves, and rhizosphere soils with varying growth stages. One germinated rice seed was sown into a plastic pot (15 cm diameter × 15 cm height) containing 2 kg of the soil described above. Pots were placed in the greenhouse, watered daily and their positions randomised weekly. Roots, shoots, and rhizosphere soil were sampled in three pots each at the 1-leaf, 3-leaf, 5-leaf, tillering, elongation, booting, heading, flowering, and mature stages. Rhizosphere soil was collected from the soil portion tightly adhering to roots by taking the plants from the soil and shaking off the loosely adhering soil (Guo et al., 2011). Plant and soil samples were taken for the quantification of (–)-loliolide as described below.
2.4 Rhizobox experiments
To examine root growth and placement patterns of focal cultivars in response to different neighbour cultivars, a series of window rhizoboxes were made of a 10 (width) × 200 (length) × 300 mm (height) polyvinyl chloride box containing 800 g of soil. Each focal cultivar was paired with the same cultivar, or closely and distantly related cultivars with five replicates. The rhizoboxes were divided into two groups. The first group was in the presence of neighbour cultivars. To remove edge effect on root growth, each rhizobox was vertically divided into four equal parts, and two germinated seeds of the focal cultivar were sown in the left while two neighbour cultivar seeds were sown in the right (Figure 2a). The second group were sown only with a focal cultivar in the centre of each rhizobox (Figure S2a) and were incubated with the root exudates of one of cultivars with varying genetic relatedness every 3 days. Incubation was carried out with two syringes 5 cm away on each side of the seedling at a depth of 2 cm. The left syringe injected root exudates while the right injected 1/10 Hoagland solution to control for the effect of nutrition solution in the root exudates.
All rhizoboxes were placed in the greenhouse. When the rice reached the 3-leaf stage, rhizoboxes were opened and the central root systems of focal cultivar were processed with WINRHIZO (Regent Instruments, Quebec, Canada) to determine horizontal asymmetry in root length. The roots were subsequently freeze-dried for biomass determination.
2.5 (–)-Loliolide incubation
Surface-sterilised rice seeds were germinated in a growth chamber at 28°C until their roots reached 1 cm in length. Three seedlings of focal cultivars were transferred into a 50 ml bottle with 1/2 Hoagland solution, each with three replicates. The bottles were placed in a growth chamber at 28°C with a 14 h-light/10 h-dark photoperiod. After 7 days, rice roots were incubated with (–)-loliolide at a concentration of 50 μM (Li et al., 2023b). Roots incubated without (–)-loliolide served as controls. Roots and shoots were sampled at different times after the start of incubation, and samples were used for the RNA-seq and quantitative real-time PCR analysis described below.
2.6 Quantitative analysis of (–)-loliolide
The quantification of (–)-loliolide was performed by liquid extraction/solid-phase extraction, followed by high-performance liquid chromatography (HPLC). Plant tissues and rhizosphere soils were each freeze-dried and ground with liquid nitrogen. 250 mg of the resulting powder was extracted with 10 ml of a MeCN (acetonitrile)-H2O-HOAC mixture (90:9:1, v/v/v), and vortexed for 5 min at 25°C. After the solution was centrifuged at 2800 × g for 10 min, the supernatant was filtered with a 0.22 μm nylon syringe filter (Sterlitech, Kent, WA, USA). The filtrates of tissue and extracts of soil were evaporated to dryness individually under vacuum. Dry residues were dissolved in 50% aqueous methanol and loaded onto reversed phase C18 Sep-Pak cartridges (Waters, Co., Milford, MA, USA), equilibrated with water, and eluted with MeOH. The MeOH fraction was concentrated with nitrogen gas to a final volume of 100 μl. The concentrated samples were subsequently subjected to HPLC analysis (Waters 1525) equipped with a C18 reverse-phase column (Hypersil 100 mm×4.0 mm, 5 μm) and a diode array UV detector at 220 nm. Elution was performed with a mixture of 1% acetic acid and MeOH (70:30, v/v) at a constant flow rate of 1.0 ml min-1 at 35°C. The peak of (–)-loliolide was identified by its retention time (9.8 min) and coelution with an authentic standard (Yuanye Biology Co., Shanghai, China). Working standard solutions ranging from 0.1 mg/L to 50 mg/L were prepared to establish a calibration curve. (–)-Loliolide was quantified by regression analysis of the peak areas against standard concentrations.
2.7 RNA-seq and transcriptome
Rice samples at 1 h from the (–)-loliolide incubation experiment described above were used for RNA-seq with three biological replicates. The extraction of total RNA used Trizol reagent (Invitrogen, CA, USA) following the manufacture's protocol. Library construction and sequencing were carried out by Lianchuan Biotechnology Corporation (Hangzhou, China) on an Illumina Hiseq. 4000 platform. The clean reads were mapped to the reference genome (Du et al., 2017).
2.8 Quantitative real-time PCR
The expression levels of genes involved in flowering [Heading date 3a (Hd3a), Rice flowering locus T 1 (RFT1), Early heading date 1 (Ehd1), Days To Heading on chromosome 8 (DTH8), GIGANTEA (GI), Grain number, plant height and heading date 7 (Ghd7)] in rice were analysed with quantitative real-time PCR. Rice shoots were sampled at the 3-leaf stage in the mixed-cultivar experiment, and at 0, 3, 6, 12 and 24 h in the (–)-loliolide incubation experiment described above. Total RNA was extracted from samples using a RNA extraction kit (Tiangen Biotech Co., Beijing, China), following the manufacturer's protocol. Agarose gel electrophoresis and spectrophotometry were used to determine the quality and concentration of RNA. First-strand cDNA synthesis was made with a cDNA synthesis kit (Tiangen Biotech Co., Beijing, China). qPCR was conducted with a Talent qPCR premix kit (Tiangen Biotech Co., Beijing, China) and StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The total reaction mixture consisted of 10 μl 2×Talent qPCR PreMix, 0.6 μl forward and reverse primers, 2 μl 50×ROX Reference Dye, 2 μl diluted cDNA, and 4.8 μl RNase-free ddH2O. Reaction conditions consisted of 95°C for 3 min followed by 40 cycles of 30 s at 95°C, 30 s at 52°C, and 30 s at 72°C. The relative level of expression was calculated using the comparative CT method (Schmittgen & Livak, 2008). The UBQ was used as an internal control. Each sample was represented by three biological replicates, and each biological replicate by three technical replicates. Primer sequences are provided in Table S1.
2.9 Data analyses
Data were presented as means ± SE from at all replicates for each experiment and determination. The normality and homogeneity of variances were verified before statistical analysis. The effects of weed neighbours and developmental stages on the (–)-loliolide concentration in rice were analysed using one-way ANOVA. The effects of relatedness level and rice set on the flowering time, grain yield, (–)-loliolide concentration, root biomass and root length of focal cultivars were examined using two-way ANOVA. Tukey's honestly significant difference (HSD) test was conducted for multiple comparisons if ANOVA terms were significant. In addition, a simple Pearson's correlation was used to test whether the (–)-loliolide concentration across focal rice cultivars correlated with root biomass and flowering time. All data analyses were performed with SPSS v.25.0 programme (SPSS, Chicago, IL).
For gene expression analysis, the transcript abundance of each gene was normalised by the fragments per kilobase per million fragments mapped reads (FPKM) method. Differentially expressed genes (DEGs) were selected that had log2 (fold change) >1 or <-1 with statistical significance (p < 0.05) using the edge package in R (R Foundation for Statistical Computing, Vienna, Austria). The identified DEGs were subjected to enrichment analysis of the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway.
3 RESULTS
3.1 Genetic relatedness determines rice flowering and grain yield in cultivar mixtures
When focal cultivars (Huagan-3 and Lingyou-6173) were each paired with themselves (the same cultivar) or their closely and distantly related cultivars in paddy fields, flowering time and grain yield significantly varied with relatedness of paired cultivars. The presence of the same and closely related cultivars advanced flowering time 1–3 days (Figure 1a), and increased grain yields by at least 5% (Figure 1b) over target plants with more distantly related neighbours. The two focal cultivars responded similarly to relatedness in flowering time (interaction was nonsignificant) but the response was greater in Huagan-3 for yield (a significant cultivar × relatedness interaction) in a field setting (Figure 1, Table S2).
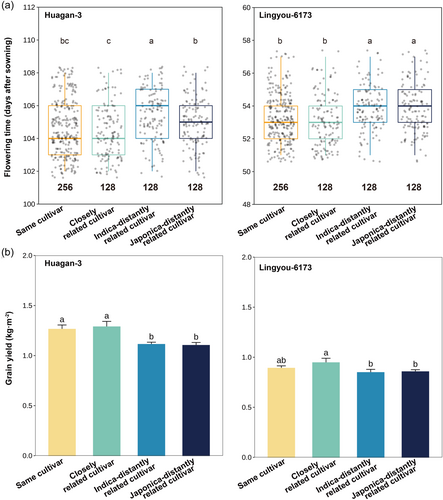
The greenhouse trial yielded consistent changes in flowering time and grain yield directly associated with relatedness as the field trial (Figure S1). Focal cultivars growing with closely related cultivars significantly earlier flowering and increased grain yield compared to those growing with distantly related cultivars (Table S2), indicating overall identical responses in the field and lab with the exception that yield responded similarly to relatedness across focal cultivars (a nonsignificant cultivar × relatedness interaction).
3.2 Root growth and distribution in response to genetic relatedness
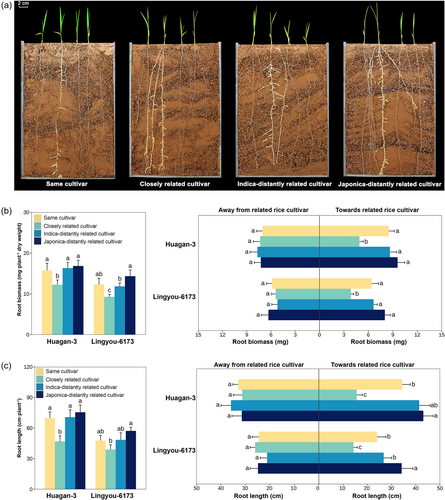
When rice cultivars interacted, root growth and distribution varied significantly with relatedness (Figure 2a, Table S3). Focal cultivars growing with closely related cultivars had reduced root biomass (Figure 2b) and root length (Figure 2c), increasing both when grown with distantly related cultivars. In particular, focal cultivars’ root placement changed with neighbour relatedness, growing towards the roots of distantly related neighbours. However, focal cultivars tended to avoid the roots of themselves or closely related cultivars (Figure 2b, c). These root placement patterns were consistent across focal cultivars and should minimise root competition in closely related cultivar mixtures.
3.3 Root exudates mediate neighbour relatedness discrimination
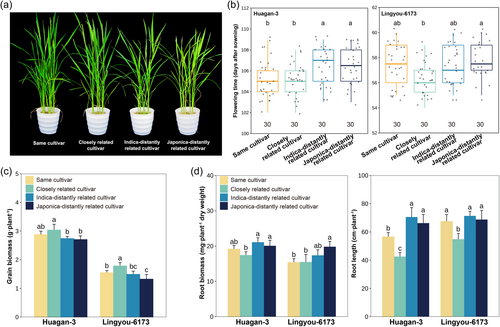
The observed yield, root behaviour, and flowering time responses appear to be mediated by neighbour's root exudates and dependent on their relatedness. Similar to the physical presence of neighbours, flowering time and grain yield significantly varied with application of root exudates of different relatedness cultivars (Table S4). Application of root exudates from the same cultivars and closely related cultivars resulted in faster flowering (Figure 3a, b), greater grain yield (Figure 3c), and optimised root growth and placement patterns in focal cultivars (Figure 3d & Figure S2) than when they were exposed to root exudates of distantly related cultivars.
3.4 The role of (–)-loliolide in relatedness-mediated neighbour discrimination
Rice plants consistently produced more (–)-loliolide in response to the presence of unrelated neighbours regardless of whether they were intraspecific or interspecific competitors. As expected, the concentration of (–)-loliolide in focal cultivars significantly increased with genetic distance of neighbours (Figure 4a & Table S5). The most distant japonica cultivar led to the highest (–)-loliolide concentration, followed by the indica-distantly related cultivars in both rice sets. Focal cultivars grown by themselves or with closely related cultivars contained the lowest (–)-loliolide concentrations (Figure 4a). Interestingly, the signal transduction of relatedness by (–)-loliolide also extended to interspecific competition. The presence of the competing paddy weeds Echinochloa crus-galli and Leptochloa chinensis also induced (–)-loliolide production in rice (Figure 4b).
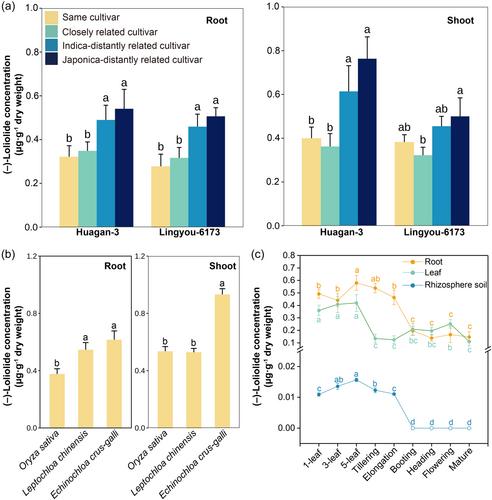
The activity of (–)-loliolide as a relatedness signal was greatest at early developmental stages when competition may be most important. The production and release of (–)-loliolide in rice significantly varied with developmental stage ((Figure 4c & Table S6). (–)-Loliolide was produced and released in early growth stages, dramatically declining as plants matured (Figure 4c). There were significantly positive relationships between (–)-loliolide concentrations and both root biomass and flowering time in rice cultivar mixtures (Figure S3), implying that (–)-loliolide stimulated root growth and delayed flowering.
3.5 Transcriptomic profiles of rice flowering and roots in response to (–)-loliolide
To address the regulatory networks and molecular mechanisms associated with flowering time and root behaviour, we performed a transcriptome analysis in rice exposed to (–)-loliolide. RNA-seq identified a total of 1780 (598 upregulated and 1182 downregulated) and 2323 (1649 upregulated and 674 downregulated) differential expressed genes (DEGs) in rice roots and shoots after incubation with (–)-loliolide (Figure S4a). Among these DEGs, 46 DEGs genes were related to root growth, of which 20 genes were upregulated and 26 genes were downregulated. KEGG analysis showed that most of the enriched genes were in the zeatin biosynthesis, RNA transport, and brassinosteroid biosynthesis pathways (Figure S4b). The enrichment of DEGs related to the zeatin biosynthesis pathway suggest that (–)-loliolide could directly promote root growth by upregulating zeatin biosynthesis.
Similarly, 33 genes were related to flowering (24 upregulated and nine downregulated). These were mainly enriched in circadian rhythm, MAPK signalling and proteasome pathways (Figure S4c). The 21 genes (17 upregulated and four downregulated) with significant changes in the circadian rhythm pathway included: CONSTANS-LIKE (OsR498G0306913700; OsR498G0815539300), GHD7 (OsR498G0713935400; OsR498G1019192800), PRR73 (OsR498G0305710000), PRR95 (OsR498G0917850800), TCP7 (OsR498G0204385100), RUP2 (OsR498G0202844500; OsR498G0261422600), and FT (OsR498G0917792400) (Figure S4c).
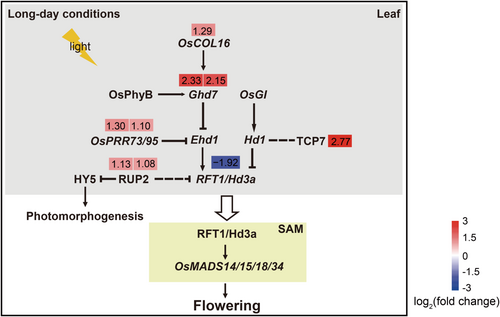
To clarify the effect of (-)-loliolide on rice flowering, key genes in the circadian rhythm pathway were screened by transcriptome analysis and mapped onto a signalling pathway. Under long-day conditions, the negative flowering regulators OsCOL16, Ghd7, TCP7, OsPPR73 and OsPPR95 were significantly upregulated in leaf, inhibiting the expression of RFT1/Hd3a which codes florigen. Moreover, the upregulation of RUP2 also repressed photomorphogenesis (Figure 5). This analysis indicates that (–)-loliolide exposure may directly inhibit rice flowering by regulating the expression of flowering-related genes.
3.6 (–)-Loliolide regulates rice flowering gene expression
Similar to the results of the cultivar mixture and transcriptome analysis, the presence of distantly related rice cultivars delayed flowering. Compared to plants grown with distantly related cultivars, the expression levels of positive flowering regulators Hd3a, RFT1 and Ehd1 were increased in focal cultivars grown with closely related cultivars. However, the expression of the negative flowering regulators DTH8, GI and Ghd7 were reduced in focal cultivars grown with closely related cultivars (Figure 6a).

In incubation experiments, (–)-loliolide upregulated or downregulated the expression of key flowering genes based on their functional roles and focal cultivar identity over a time-course (Figure 6b & Table S7). In general, (–)-loliolide inhibited the expression of positive regulators and promoted the expression of negative regulators related to flowering in Huagan-3 after 6 h incubation (Figure 6b). (–)-Loliolide also inhibited the expression of positive regulators and promoted the expression of negative regulators related to flowering in Lingyou-6173 at 12 h (Figure 6b). Therefore, (–)-loliolide may delay the flowering time of rice by modulating the expression of flowering-related genes in response to neighbour relatedness.
4 DISCUSSION
Ecological and evolutionary approaches to improving crop cultivar mixtures have been commonly used throughout the world (Creissen et al., 2016; Reiss & Drinkwater, 2018; Wuest et al., 2021; Yang et al., 2019). Preferentially reducing competitive effects on relatives may be a potential mechanism responsible for yield of crop cultivar mixtures (Fréville et al., 2019; Yang et al., 2018). Kin recognition and conspecific cooperation among crop plants are predicted to increase yield by reducing intraspecific competition and shifting resource allocation to reproduction (Anten & Chen, 2021; Bawa, 2016; Murphy et al., 2017; Yang et al., 2018). Improving our understanding of the processes at play in kin recognition is key to develop cultivar mixtures that increase grain yields in the limited area suitable for agriculture. From rice cultivar mixtures in paddy fields and the greenhouse, we found that yields of closely related cultivar mixtures were consistently greater than distantly related cultivar mixtures. The benefits were generated by reduced root competition, accelerated flowering, and lowered (–)-loliolide levels in closely related cultivars.
An increasing number of studies have shown that genetic relatedness elicits changes in root behaviour and aboveground flowering (Bhatt et al., 2011; Goddard et al., 2020; Torices et al., 2018, 2021). Kin recognition can result in differences in growth and biomass allocation, particularly in root growth and placement (Belter & Cahill, 2015; Fang et al., 2013; Xu et al., 2021; Yang et al., 2018). Root behaviour and plasticity allow plants to adapt to their local environment. The ability to form proliferative and intrusive roots when competing with neighbouring individuals is likely essential in natural systems, increasing access to soil resources (Wang et al., 2023; Weiner, 2004). However, such root behaviour may be disadvantageous in cropping systems with plants allocating more biomass to root systems, reducing yields (Gersani et al., 2001; Homulle et al., 2022). Therefore, modifying root behaviour to minimise crop–crop competition is key to creating more productive and resilient agroecosystems. In the current study, closely related cultivar mixtures reduced root growth and intrusive behaviour, lowered root competition and increased investment towards reproduction.
Flowering time is key to a plant's reproductive strategy, and known cues are mostly large-scale environmental phenomenon (Song et al., 2013). Several studies have shown that flowering behaviour depends on the relatedness of neighbours, and that behaviour is expected to affect reproductive success (Falik et al., 2014; Lankinen et al., 2013; Torices et al., 2018). Our study documented that rice growing with closely related cultivars had accelerated flowering relative to those growing with distantly related cultivars. Early flowering plants are favoured by phenotypic selection on flowering phenology (Munguía-Rosas et al., 2011), providing a linkage to the evolution of plant reproductive strategies. Acceleration of rice flowering with closely related neighbours likely reflects the competitive costs incurred by plants growing with distantly related neighbours. This may represent an effective strategy for increasing grain yield, particularly for self-pollinated plants such as rice, that do not rely on outcrossing for successful pollination.
Plants perceive and respond to chemical cues emitted from neighbours, and accordingly adjust growth, reproduction and defensive strategies (Kong et al., 2018; Kong et al., 2024; Li et al., 2020). A few studies have shown that root exudates mediate kin recognition in plants (Biedrzycki et al., 2010; Semchenko et al., 2014; Yang et al., 2018). Root exudates also determine root behaviour, flowering and reproduction in interacting plants in both intraspecific and interspecific interactions (Falik et al., 2014; Li et al., 2023a; Semchenko et al., 2014; Wang et al., 2021). The current study documents this phenomenon in self-pollinating rice mediated by intraspecific neighbour relatedness. Furthermore, kin recognition and discrimination involve signalling interactions among intraspecific neighbours (Anten & Chen, 2021; Biedrzycki & Bais, 2022; Goddard et al., 2020). A specific root-secreted allantoin has a role in kin recognition in rice lines (Yang et al., 2018). However, the nitrogen-rich allantoin may not be the signal of relatedness but rather the effect of the underlying signal (Wang et al., 2021). The current study indicates that the signalling interactions shown in rice cultivar mixtures function through a ubiquitous signalling chemical (–)-loliolide. The production of this signal was significantly reduced in the presence of closely related cultivars but increased when growing with distantly related cultivars. (–)-Loliolide is a ubiquitous phytochemical cue that serves both exogenous and endogenous roles (Frost, 2023; Li et al., 2023b). This study extends the functional role of (–)-loliolide as a signal of relatedness that indirectly indicates neighbour genetic relatedness in rice cultivar mixtures.
Rice responds to identity of non-kin competitors by increasing (–)-loliolide and root production. These responses require rice to invest resources into metabolic networks, resulting in reduced grain yields and delayed flowering when in competition with distantly related cultivars or paddy weeds. In response to the presence of closely related and distantly related cultivars or exposure to (–)-loliolide, expression of key rice flowering genes was significantly altered. Ghd7, DTH8 and GI suppress rice flowering (Cai et al., 2019; Hayama et al., 2002). In contrast, Hd3a, RFT1 and Ehd1 are positive regulators of rice flowering (Komiya et al., 2008; Komiya et al., 2009; Zhao et al., 2015). Distantly related cultivars reduced the expression levels of positive flowering regulators Hd3a, RFT1 and Ehd1, but increased the expression of negative flowering regulators DTH8, GI and Ghd7 in focal cultivars. Similarly, the results of RNA-seq transcriptome and qRT-PCR of rice incubated with (–)-loliolide both demonstrated that (–)-loliolide promoted negative flowering regulators and suppressed positive flowering regulators. Therefore, flowering time of rice may be delayed by (–)-loliolide directly via the regulation of flowering genes and perhaps indirectly via allocation of resources toward competitive responses.
It is worthy of remark while the yields of the same cultivar monocultures and closely related cultivar mixtures were equivalent in the current study, changes in some traits suggest mixtures could be advantageous in some conditions. Root growth was lowest with closely related neighbours with fewer intrusive roots produced (Figure 2), a pattern mimicked in plants solely exposed to root exudates (Figure 3). Furthermore, gene expression in plants grown with closely related cultivars was distinctly different from control plants (Figure 6). These results clearly indicate the ability to identify and differentially respond to close relatives in ways that should minimise competition, and therefore, increase grain yield.
5 CONCLUSIONS
Yield improvements in combinations of two cultivars are easily transferable to low-input management systems. However, most successful mixtures have been established from traditional practices or were assessed from experimental combinations built haphazardly. We currently lack effective strategies to a priori select mixture components to achieve agricultural benefits. This study provides compelling evidence that genetic relatedness plays a crucial role in determining the outcome of intraspecific plant–plant interactions in rice cultivar mixtures. In particular, a ubiquitous signalling (–)-loliolide may act as a mediator to identify suitable closely related pairings from cultivars with varying degrees of genetic relatedness at a biochemical level. Although the linkage of (–)-loliolide to neighbour identity needs refined, the predictive potential of this signalling chemical is very intriguing, with large-scale societal implications. If (–)-loliolide production is a reliable signal, it may contribute to designing beneficial cultivar mixtures and perhaps be extended to intercropping or mixed-species systems. A further understanding of (–)-loliolide's action mechanism may allow the application of kin recognition and discrimination principle to increase crop yields.
ACKNOWLEDGEMENTS
Authors sincerely thank two anonymous referees for their constructive comments that substantially improved this manuscript. This work was supported by the National Natural Science Foundation of China (31672040).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Sequence Read Archive (SRA) database at http://www.ncbi.nlm.nih.gov, reference number PRJNA767712. The RNA-seq data have been deposited in Sequence Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov) and can be found with Accession No. PRJNA767712. Other data supporting the findings of this paper are available from the corresponding author on request.




