FveDREB1B improves cold tolerance of woodland strawberry by positively regulating FveSCL23 and FveCHS
Abstract
Cold stress has seriously inhibited the growth and development of strawberry during production. CBF/DREB1 is a key central transcription factor regulating plant cold tolerance, but its regulatory mechanisms are varied in different plants. Especially in strawberry, the molecular mechanism of CBF/DREB1 regulating cold tolerance is still unclear. In this study, we found that FveDREB1B was most significantly induced by cold stress in CBF/DREB1 family of diploid woodland strawberry. FveDREB1B was localized to the nucleus, and DREB1B sequences were highly conserved in diploid and octoploid strawberry, and even similar in Rosaceae. And FveDREB1B overexpressed strawberry plants showed delayed flowering and increased cold tolerance, while FveDREB1B silenced plants showed early flowering and decreased cold tolerance. Under cold stress, FveDREB1B activated FveSCL23 expression by directly binding to its promoter. Meanwhile, FveDREB1B and FveSCL23 interacted with FveDELLA, respectively. In addition, we also found that FveDREB1B promoted anthocyanin accumulation in strawberry leaves by directly activating FveCHS expression after cold treatment and recovery to 25°C. DREB1B genes were also detected to be highly expressed in cold-tolerant strawberry resources ‘Fragaria mandschurica’ and ‘Fragaria nipponica’. In conclusion, our study reveals the molecular mechanism of FveDREB1B-FveSCL23-FveDELLA module and FveDREB1B-FveCHS module to enhance the cold tolerance of woodland strawberry. It provides a new idea for improving the cold tolerance of cultivated strawberry and evaluating the cold tolerance of strawberry germplasm resources.
1 INTRODUCTION
Cold stress seriously affects the normal life activities of plants and determines their distribution (Wang, Li, et al., 2023). Especially for economic crops growing in temperate areas, low temperature has an impact on their growth and development, and even causes plant death, production reduction, and brings huge losses to agricultural production (Wu et al., 2021). Cold stress mainly includes freezing stress below 0°C and chilling stress above 0°C (Shen et al., 2023). After plants suffer from freezing stress, the intercellular spaces freeze and lose water, and the organizational structures are broken, showing symptoms such as wilting, cracking, drying, and so forth, and dying in severe cases (Waqas et al., 2021). After plants are subjected to chilling stress, photosynthesis is reduced, the fluidity of cell membranes is poor, and the absorption and transport of nutrients by roots are affected, which hinders the transport of photosynthetic products and mineral nutrients to the growing organs, making the growing organs of plants thin, degenerate or die due to insufficient nutrients (Guo et al., 2018; Hund et al., 2008). Hence, the investigation of the molecular mechanism underlying plant response to cold stress holds significant practical implications.
In the process of long-term struggle with nature, plants have evolved a unique response mechanism to low temperature. A period of nonlethal cold treatment can make plants more resistant to cold, a process also known as cold acclimation (Ding et al., 2019). Plants sense and transmit cold signals via cold sensors, such as cell membranes, Ca2+ channels, and COLD1 proteins, and then activate multiple regulatory networks in response to cold stress (Zhang et al., 2019). At present, research on low-temperature signal transduction pathways can be roughly divided into CBF/DREB1 (C-REPEAT BINDING FACTOR/DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN 1) dependent pathway and CBF/DREB1 independent pathway, which are carried out independently and have cross-effects in the regulation of plant cold tolerance (Li et al., 2022; Xie et al., 2018). Among them, CBF/DREB1-dependent pathway is the main pathway to regulate cold acclimation and cold tolerance of plants, which refers to the low-temperature signal transduction pathway centred on the CBF/DREB1 transcription factors (Song et al., 2021).
CBFs/DREB1s belong to the DREB subfamily of the AP2/ERF transcription factor family and respond to cold stress, by specifically binding to the CRT/DRE (CCGAC) motif on the promoters of downstream target genes to activate their expression (Feng et al., 2020). When plants are subjected to cold stress, cold signals activate a variety of transcriptional regulatory processes, as well as posttranslational modification processes such as phosphorylation, ubiquitination, myristoylation and sumoylation, thereby inducing CBFs/DREB1s transcription (Ding et al., 2020; Tang et al., 2020). Subsequently, CBFs/DREB1s activate the expression of COR (COLD REGULATED) genes, accumulating protective substances such as osmotic regulators and cryoprotective proteins to promote cold acclimation and improve cold tolerance (Shi et al., 2018). At present, the function of CBF/DREB1 transcription factors regulating plant cold tolerance has been reported in a variety of plants (Artlip et al., 2014; Li et al., 2022; Xie et al., 2018). However, studies on the regulatory mechanism mainly focus on the transmission of cold signals to CBFs/DREB1s (Ding et al., 2020), and the mechanism by which CBFs/DREB1s regulate downstream genes to affect plant cold tolerance has only been reported in a few plants. For example, in Arabidopsis thaliana, AtCBF1 can promote glycosyltransferase genes AtUGT79B2/B3 expression by directly binding to their promoters, and then increase anthocyanin content, thereby enhancing the cold tolerance of Arabidopsis (Li et al., 2016); AtCBF1 and AtCBF3 activated the expression of AtGA2ox3/6 and AtGA2ox7, respectively, which decreased the level of active GA and inhibited the degradation of DELLA protein, and at the same time, AtCBF1 can directly promote the expression of AtRGL3 to accumulate DELLA protein, thereby improving the cold tolerance of Arabidopsis by delaying its growth and development (Achard et al., 2008; Zhou et al., 2017). In addition, CmCBF4 promotes the expression of arginine decarboxylase gene CmADC by directly binding to its promoter, and then promotes the synthesis of putrescine (Put) to improve the cold tolerance of melon seedlings (Li et al., 2022). These studies revealed that the mechanisms of CBF/DREB1 regulating plant cold tolerance are diverse, and relatively few reports have been found in other plants.
Strawberry as an important berry fruit tree, its producing area has been extended to all over the world, and the cold climate conditions have seriously inhibited the growth of strawberry (Davik et al., 2021). Therefore, it is necessary to study the molecular mechanism regulating cold tolerance of strawberry, but so far, there are only a few studies on cold-induced CBFs/DREB1s in strawberry. For example, it was found that FaCBF1 expression was upregulated under cold stress in octoploid cultivated strawberry ‘Fengxiang’ (Fragaria × ananassa) (Zhang et al., 2014). Gu et al. (2013, 2015) speculated that RdreB1BI of rice enhanced cold tolerance of strawberry (F. ananassa Duch. cv. Benihoppe) by promoting photosynthesis and accumulating defensive-related proteins, and regulated the expression of cold-tolerance related genes and anthocyanin biosynthesis genes through proteomic and RNA-sequencing analysis, respectively. Moreover, in revealing that low temperature activates FveMYB10 phosphorylation and FveCHS1 degradation induced by FveMAPK3, thereby inhibiting anthocyanin accumulation in woodland strawberry (Fragaria vesca) fruits, and it was also found that FveCBF1 and FveCBF3 were upregulated in FveMAPK3 overexpressed woodland strawberry fruits (Mao et al., 2022). However, the molecular mechanism of CBFs/DREB1s regulating cold tolerance of strawberry remains unclear.
In this study, we found FveDREB1B was most significantly induced by cold stress in CBF/DREB1 transcription factors of woodland strawberry. FveDREB1B directly binds to the FveSCL23 promoter to activate its expression. Meanwhile, FveDREB1B and FveSCL23 interact with FveDELLA, respectively, to stabilize DELLA proteins, thereby delaying flowering and enhancing cold tolerance of woodland strawberry. In addition, FveDREB1B also activated the expression of FveCHS by directly binding to its promoter, which promoted the accumulation of anthocyanins in woodland strawberry leaves after cold treatment and recovery at 25°C, thereby enhancing the cold tolerance of woodland strawberry. Therefore, we revealed the molecular mechanism by which FveDREB1B enhances cold tolerance of strawberry, which provides theoretical support for cold tolerance breeding and cold tolerance resource evaluation.
2 MATERIALS AND METHODS
2.1 Plant materials and treatments
The diploid woodland strawberry (F. vesca) ‘Yellow Wonder (YW, without runners)’ and ‘Hawaii 4 (H4, with runners)’ and different ploidy strawberry resources (Supporting Information: Table S1) were planted on the solar greenhouse of Shenyang Agricultural University, China. Shoot apical meristems (SAMs) and different organs of potted plants, and leaves of tissue culture seedlings were stored at −80°C.
GA affects the differentiation of SAMs, especially plays a decisive role in the formation of inflorescence branches and runners, so SAMs of potted strawberry plants are selected for the determination of GA content. The determination of GA content was carried out by Ruiyuan Biotechnology Co. LTD.
Tobacco (Nicotiana benthamiana) was planted on the substrate of peat: vermiculite = 1:1. One-month-old tobacco leaves were used for experiments. Strawberry and tobacco plants grew under long day at 25°C.
Cold treatment was carried out in a 4°C thermostat refrigerator, and sufficient light was provided by adding light strips to maintain 10 h of light per day. The temperature outside the solar greenhouse was queried and counted according to the ‘Tianqi’ network (https://lishi.tianqi.com/). At 7:00 in the morning and 12:00 in the noon, the temperature in the solar greenhouse was recorded and counted according to the thermometer values.
2.2 Phylogenetic analysis and sequence alignment
Based on the NCBI (https://www.ncbi.nlm.nih.gov/nucleotide/) and GDR (https://www.rosaceae.org/) databases, we obtained the coding sequences of FveDREB1B (FvH4_6g18090/FvYW_6g18090), FaDREB1B (F×aYL_631g0031040), FveDREB1D (FvH4_7g28950), FveDREB1E (FvH4_5g01440), FveSCL23 (FvH4_6g39610), FveCXE15 (FvH4_6g21100), FveCHS (FvH4_7g01160), FveCHI (FvH4_7g25890), FveF3H (FvH4_1g11810), FveDFR (FvH4_2g39520), FveANS (FvH4_5g01170), FveUFGT (FvH4_3g19220), FnilDREB1B (FnYN6G016750.1_fn_v1.0), FmaDREB1B (chr6:17967618.17966995), FviDREB1B (Fvir6:11570582.11569998), FnipDREB1B (FNI_icon04358861.1.g00001.1), FpeDREB1B (chr6:13994739.13994146), FnuDREB1B (Fnub6:6831743.6831120), ForDREB1B (FOR_iscf00153742.1:875.258), FvirDREB1B (hap1-6A:9421276.9421869), FchDREB1B (hap2-6A:9903276.9903809), FveGA2ox (FvH4_3g05530) and 9 FveDELLAs (Supporting Information: Table S2). Promoters (1500 bp) of FveSCL23, FveCXE15, FveCHS, FveCHI, FveF3H, FveDFR, FveANS, FveUFGT and FveGA2ox were obtained according to the GDR website.
According to the NCBI website, we found the homologous protein sequences of FveDREB1B in various species, including Pistacia vera (XP_031281742), Vitis vinifera (XP_010654049), Rosa chinensis (XP_024189499), Prunus avium (XP_021810133), Prunus mume (XP_008241428), Prunus persica (XP_007204774), Prunus dulcis (XP_034224862), Prunus yedoensis (PQM39018), Malus domestica (XP_028948628), Pyrus × bretschneideri (XP_009352989), Ziziphus jujuba (XP_015901948), Juglans regia (XP_018812676), Carya illinoinensis (XP_042943391), Quercus robur (XP_050242026), Quercus lobata (XP_030924994), Quercus suber (XP_023913339), Manihot esculenta (XP_021619158), and Cajanus cajan (XP_020223690).
Using DNAMAN 6.0 software, these sequences were compared. Using TBtools 1.0.7 software and the NCBI website, the conserved motifs and domains were analysed and drawn. Using MEGA 6.0 and TBtools 1.0.7 software, the phylogenetic tree was constructed.
2.3 Gene cloning and vector construction
Using modified CTAB method, the total DNA and RNA of different strawberry organs were extracted based on Chang et al. (2007). The cDNA was obtained by RNA reverse transcription using RNA reverse transcription kit (TaKaRa). Promoter sequences were cloned from DNA of ‘YW’ and ‘H4’ SAMs, and coding sequences were cloned from their cDNA. The related primers are shown in Supporting Information: Table S3. Various fusion vectors involved in this study were constructed according to Luo et al. (2024).
2.4 Acquisition and phenotypic identification of transgenic strawberry
FveDREB1B coding region was inserted into the pRI101-AN vector to construct the 35S::FveDREB1B vector. Based on Zhao et al. (2016), the 35S::FveDREB1B-RNAi vector was constructed with a 333 bp fragment from the CDS of FveDREB1B using RNA interference (RNAi) technology.
When tissue culture seedlings of ‘YW’ and ‘H4’ grew 3-4 leaves, Agrobacterium-mediated transformation was performed based on Li, Zhang, et al. (2017). Using semiquantitative verification and RT-qPCR, transgenic strawberry plants were identified according to Dong et al. (2021).
The tissue culture seedlings of transgenic strawberry were cultured in 1/2 MS rooting medium for 1 month. The roots were observed and their length and number were investigated. The roots were scanned with a Plant root scanning system (WinRHIZO). Transplanting transgenic strawberry plants were used for phenotypic observation and morphological index investigation. The related primers are shown in Supporting Information: Table S3.
2.5 RT-qPCR analysis
For potted strawberry plants, SAMs are young organs, and the quality of nucleic acid extracted from them is higher than that of mature leaves, so SAMs are used for RT-qPCR detection. The expressions of genes were detected by UltraSYBR Green Mixture Kit (ComWin) with the QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The Fve26S of woodland strawberry serves as the internal control gene. The related primers are shown in Supporting Information: Table S3. The method was used to calculate the expression of genes. Three biological and technical replicates were performed.
2.6 Subcellular localization
According to Dong et al. (2021), the pRI101-FveDREB1B-GFP fusion vector was constructed. The related primers are shown in Supporting Information: Table S3. The bacterial suspension of Agrobacterium tumefaciens strain EHA105 containing different vectors were transferred to N. benthamiana leaves, respectively. Confocal fluorescence microscopy (Leica DMi8 A) was used to observe GFP fluorescence signals.
2.7 Determination of physiological indexes and DAB/NBT staining
Based on Shin et al. (2007), total anthocyanin contents in leaves of strawberry plants were extracted and measured at A530 and A600 by ultraviolet spectrophotometer. The leaves of strawberry plants were ground with liquid nitrogen, and chlorophyll was extracted from them with a 95% ethanol solution. After mixing the extract and sample, let it stand in the dark for 2 h. The mixtures were then centrifuged to extract supernatants. The absorption values of supernatants at 646 and 663 nm were measured by ultraviolet spectrophotometer. The calculation formula of chlorophyll content is ‘Total chlorophyll content (mg/g) = (12.21 × A663-2.81 × A646 + 20.13 × A646-5.03 × A663) × V/1000 × W’. Here, W (g) represents the weight of strawberry leaves ground with liquid nitrogen, and V (mL) represents the total volume of the added extract.
According to Dahro et al. (2016), the electrolyte leakage (EL) rates were determined. The contents of PRO, MDA and H2O2 were determined by visible spectrophotometer according to the instructions of the proline (PRO), malondialdehyde (MDA) and hydrogen peroxide (H2O2) content determination kit (Grace, Suzhou, Jiangsu Province, China). The activity of APX was determined by ultraviolet spectrophotometer according to the instruction of the ascorbate peroxidase (APX) activity determination kit (Grace, Suzhou, Jiangsu Province, China). Based on Huang et al. (2013), DAB (3,3’-diaminobenzidine) and NBT (nitro blue tetrazolium) histochemical staining were carried out.
2.8 RNA sequencing analysis
The SAMs of cold-treated (4°C) FveDREB1B overexpressed plants (OE) and WT-Y plants were used for RNA sequencing (RNA-seq). The extraction of RNA from SAMs and the detection of RNA purity, concentration and integrity were performed by Biomarker Biotechnology Co. LTD (Beijing, China). The construction and quality control of the cDNA library were also completed by Biomarker (BMK), and the Illumina platform (Illumina, San Diego, CA, USA) was used to sequence the library. Fragaria_vesca_v4.0.a1 genome was used as the reference genome for sequence alignment and subsequent analysis. As an index, FPKM was used to detect gene expressions and the levels of transcripts. Data analysis was performed through the BMKCloud platform.
2.9 Yeast one-hybrid (Y1H) assay
The CDS of FveDREB1B was inserted into the pGADT7 vector to create the pGADT7-FveDREB1B fusion vector. Sequence fragments containing DRE elements (P, P1, and P2) on the promoters of FveCHS, FveSCL23 and FveCXE15 were linked to the pAbAi vector to create the pAbAi-pFveCHS(P), pAbAi-pFveSCL23(P1) and pAbAi-pFveCXE15(P2) vectors. The related primers are shown in Supporting Information: Table S3. According to Li, Xu, et al. (2017), the pGADT7-FveDREB1B and pAbAi-pFveCHS(P)/pAbAi-pFveSCL23(P1)/pAbAi-pFveCXE15(P2) fusion vectors were transferred to Y1H yeast to cultivate.
2.10 EMSA
The coding region of FveDREB1B was linked to the pEGX4T vector with the GST label to construct the FveDREB1B-GST vector. The probe sequence (40 bp) was isolated from FveSCL23 promoter and consisted of the DRE element. The related primers are shown in Supporting Information: Table S3. FveSCL23-Probe represents the probe sequence labelled with biotin, and competitor represents the probe sequence that was not labelled by biotin. FveSCL23-Probe and competitor were synthesized by Sangon Biotechnology Co. LTD (Shanghai, China). The method of EMSA was performed using the Chemiluminescent EMSA Kit (Beyotime, Shanghai, China).
2.11 Luciferase (LUC) reporter assay
According to Luo et al. (2024), the pRI101-FveDREB1B and pFveCHS::LUC/pFveSCL23::LUC/pFveCXE15::LUC containing FveCHS/FveSCL23/FveCXE15 promoter (1500 bp) vectors were constructed. The bacterial suspensions of A. tumefaciens strains EHA105 containing the vectors mentioned above were mixed according to the requirements and then injected into N. benthamiana leaves. After 2 days in the dark, the live fluorescence imager (Lb985, Berthold, Germany) was used to detect luciferase signals. The dual luciferase reporter gene assay kit (Beyotime, Shanghai, China) was used to detect the transcriptional activity. The related primers are listed in Supporting Information: Table S3.
2.12 β-glucuronidase (GUS) reporter assay
According to Luo et al. (2024), the pFveSCL23::GUS and pFveCXE15::GUS vectors containing FveSCL23/FveCXE15 promoter (1500 bp) were constructed. The related primers are shown in Supporting Information: Table S3. The bacterial suspensions of A. tumefaciens strains EHA105 containing the vectors mentioned above were mixed according to the requirements and then injected into N. benthamiana leaves. The GUS staining was performed with infected tobacco leaves according to the instruction of GUS stain kit (Coolaber, Beijing, China). According to Li, Xu, et al. (2017), the GUS fluorescence activity and GUS protein concentration of infected tobacco leaves were detected.
2.13 Yeast two-hybrid (Y2H) assay
The CDS of FveSCL23 was inserted into the pGADT7 vector to create the pGADT7-FveSCL23 fusion vector. The coding sequences of FveGAI, FveGAIP, FveRGA2, FveRGL1B and FveRGL1D were inserted into the pGBKT7 vector to create the pGBKT7-FveGAI, pGBKT7-FveGAIP, pGBKT7-FveRGA2, pGBKT7-FveRGL1B and pGBKT7-FveRGL1D vector. The related primers are shown in Supporting Information: Table S3. The pGADT7-FveDREB1B/pGADT7-FveSCL23 and pGBKT7-FveGAI/pGBKT7-FveGAIP/pGBKT7-FveRGA2/pGBKT7-FveRGL1B/pGBKT7-FveRGL1D vectors were co-transferred into Y2H yeasts and cultivated in SD/-Trp-Leu mediums. Subsequently, positive strains were cultivated in the SD/-Trp-Leu-His-Ade mediums with X-α-gal.
2.14 Pull-down assay
FveDREB1B and FveSCL23 coding sequences were linked to the pMAL-c2x vector with the MBP label to create the FveDREB1B-MBP and FveSCL23-MBP vector. FveGAIP and FveRGL1D CDS regions were linked to the pEGX4T vector with the GST label to create the FveGAIP-GST and FveRGL1D-GST vectors. The related primers are shown in Supporting Information: Table S3. The vectors mentioned above were transferred to Escherichia coli strain ‘BL21(DE3)’ to obtain proteins. Using IPTG (Isopropyl β-d-thiogalactoside), the proteins were induced, then purified, and the induced and purified proteins were detected by electrophoresis. The FveGAIP-GST and FveRGL1D-GST fusion proteins were kept immobilized on beads. The purified FveDREB1B-MBP/FveSCL23-MBP/MBP protein was incubated with FveGAIP-GST/FveRGL1D-GST protein fixed on beads. anti-GST antibody (TransGen, Beijing, China) and anti-MBP antibody (Cowin, Taizhou, Jiangsu Province, China) were used to detect proteins by immunoblotting, respectively.
2.15 BiFC assay
The CDS of FveDREB1B and FveSCL23 with the stop codon removed were linked to the pSPYNE vector containing the N-terminal of the sequence encoding YFP (nYFP) to create the FveDREB1B-nYFP and FveSCL23-nYFP vector. The coding sequences without the stop codon of FveGAIP and FveRGL1D were linked to the pSPYCE vector with the C-terminal of the sequence encoding YFP (cYFP) to create the FveGAIP-cYFP and FveRGL1D-cYFP vector. The bacterial suspensions of A. tumefaciens strains EHA105 containing the vectors mentioned above were mixed according to the requirements. Subsequently, they were injected into N. benthamiana leaves. After 2 days, confocal fluorescence microscopy (Leica DMi8 A, Wetzlar, Germany) was used to observe YFP fluorescence signals. The related primers are shown in Supporting Information: Table S3.
2.16 Statistical analysis
Using GraphPad Prism 9.5.1 software, statistical analyses were conducted by t test or one-way analysis of variance (ANOVA) combined with Tukey's post-hoc test (*p < 0.5, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: no significant difference). The results were reported as mean ± standard deviation (SD).
3 RESULTS
3.1 Cold stress significantly induces FveDREB1B expression
The low temperature in the solar greenhouse caused by the cold weather in winter has an adverse effect on the growth and development of strawberry plants. We inquired about the variation trend of outdoor temperature from November 2018 to February 2023 in Shenyang (123.43° E, 41.80° N) located in the north of China, and found that (Supporting Information: Figure S1a): every year, the lowest temperature is concentrated in December to January of the next year, the lowest value of extreme low temperature (daily minimum temperature) is −27°C, and the lowest value of average monthly low temperature (average monthly extreme low temperature) is −16°C, both of which appear in January 2021, meanwhile, the highest temperature during this period is only 9°C. In addition, we calculated the morning and noon temperatures in the solar greenhouse from December 2022 to January 2023. The line chart shows that the lowest temperature in the solar greenhouse in the morning is 4°C, and the lowest temperature in the noon is 6°C, both occurring in January 23, 2023 (Supporting Information: Figure S1b). It was found that the low temperature of 10°C is not conducive to the ripening of strawberry fruits, especially when the temperature drops to 4°C, it will seriously affect the colouring of strawberry fruit (Koehler et al., 2015; Mao et al., 2022). Therefore, the 4°C low temperature in the solar greenhouse in winter is still unfavourable to strawberry production, and improving the low temperature tolerance of strawberry plants has become one of the problems to be solved urgently in the development of strawberry industry.
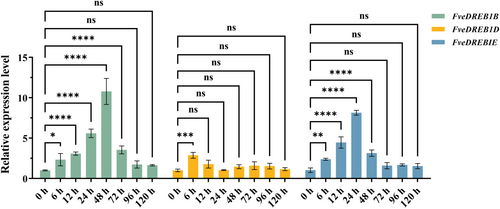
With reference to the cold-tolerant CBF1/2/3 (DREB1B/C/A) gene sequences in Arabidopsis (Gilmour et al., 2004; Thomashow & Torii, 2020) and based on the diploid woodland strawberry genome database (GDR), we found that there are three FveDREB1 genes in woodland strawberry: FvH4_6g18090 (named FveDREB1B in this study), FvH4_7g28950 (named FveDREB1D in this study) and FvH4_5g01440 (named FveDREB1E in this study). Subsequently, RT-qPCR was used to detect the expression of three FveDREB1 genes after different time of cold treatment at 4°C. The results showed that the expression of FveDREB1B reached its peak at 48 h, which was 10.8 times of that at 0 h; FveDREB1D was significantly expressed only after 6 h of cold treatment, and its expression was 2.9 times that of 0 h; the expression of FveDREB1E peaked at 24 h after cold treatment, 8.1 times that of 0 h (Figure 1). This indicates that among the three FveDREB1 genes, FveDREB1B is most significantly induced by cold treatment. Therefore, this study selectes FveDREB1B as the entry point to explore its mechanism of action on cold tolerance of woodland strawberry plants.
3.2 Characteristic analysis of FveDREB1B gene
Most of the strawberries planted in production are octoploid cultivated strawberries. Therefore, besides the diploid woodland strawberry ‘Yellow Wonder (YW)’ and ‘Hawai4 (H4)’, the coding region sequence of the DREB1B gene in octoploid cultivated strawberry ‘Yanli (YL)’ was also analysed. The results showed that the nucleotide sequences of FveDREB1B genes of ‘YW’ and ‘H4’ were completely consistent, with a total length of 624 bp, encoding 207 amino acids (Supporting Information: Figure S2). The nucleotide sequence comparison rate of DREB1B among ‘YW’, ‘H4’ and ‘YL’ was as high as 98.46%, and the amino acid sequence alignment rate was 97.93%, contained a conserved AP2 domain (Supporting Information: Figure S2), indicating that DREB1B was highly conserved among different ploidy strawberry resources.
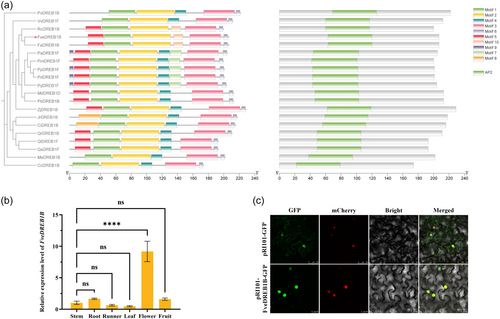
Phylogenetic analysis showed that FveDREB1B protein was most closely related to cultivated strawberry, followed by Rosa chinensis, and was also relatively close to other Rosaceae species such as Prunus avium, Prunus mume, Prunus persica, Prunus dulcis, Prunus yedoensis, Malus domestica and Pyrus × bretschneideri (Figure 2a). And FveDREB1B contained the same motif as FaDREB1B and RcDREB1B, and the AP2 domain was highly conserved in 20 species (Figure 2a). In addition, temporal and spatial expression results showed that FveDREB1B was significantly expressed only in the flower organs of woodland strawberry, while there was no difference in stems, roots, runners, leaves and fruits (Figure 2b). Subcellular localization results showed that the GFP fluorescence of FveDREB1B was only shown in the nucleus, so FveDREB1B belonged to the nuclear expression protein (Figure 2c).
3.3 FveDREB1B inhibits the beginning of reproductive growth of woodland strawberry
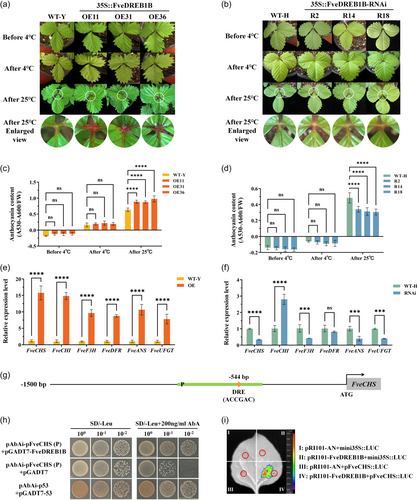
To investigate the biological function of FveDREB1B in various organs of woodland strawberry, we selected ‘YW’ without runners and ‘H4’ with runners as wild-type materials for transgenic experiments. FveDREB1B overexpressed lines (OE11, OE31, OE36) were obtained by overexpressing FveDREB1B in woodland strawberry ‘YW’ (WT-Y) (Figure 3a). Phenotypic observation and morphological investigation results showed that FveDREB1B overexpressed lines flower approximately 40 days later than WT-Y (Figure 3b,c). In addition, compared with WT-Y, the plant height, number of branches and leaves of FveDREB1B overexpressed lines were significantly decreased, while the petiole diameter was significantly increased, and the leaf colour was deeper, and the leaf surface spread was uneven (Figure 3b; Supporting Information: Table S4). Meanwhile, investigation results of tissue culture seedlings roots showed that the root length of FveDREB1B overexpressed lines was significantly shortened, about half of WT-Y (Supporting Information: Figure S3a,b), while the number of secondary roots was significantly higher than WT-Y (Supporting Information: Figure S3a,c).

Using RNAi technology, FveDREB1B silenced lines (R2, R14, R18) were obtained by silencing FveDREB1B in woodland strawberry ‘H4’ (WT-H) (Figure 3d). Phenotypic observation and morphological investigation results showed that FveDREB1B silenced lines flower approximately 20 days earlier than WT-H (Figure 3e,f). However, there were no significant differences in other phenotypes between FveDREB1B silenced lines and WT-H (Figure 3e; Supporting Information: Figure S4, Supporting Information: Table S5). Meanwhile, investigation results of tissue culture seedlings roots showed that there were also no significant differences in the root length and number of roots between FveDREB1B silenced lines and WT-H (Supporting Information: Figure S3d–f).
These results indicate that FveDREB1B inhibits the beginning of reproductive growth of woodland strawberry.
3.4 FveDREB1B improves cold tolerance of woodland strawberry
FveDREB1B overexpressed plants OE (OE11, OE31, OE36) and its control plant WT-Y, and FveDREB1B silenced plants RNAi (R2, R14, R18) and its control plant WT-H were cold treated at 4°C for 2 days, and physiological indexes were determined. After cold treatment, the electrolyte leakage rate, the contents of malondialdehyde (MDA) and hydrogen peroxide (H2O2) of OE plants were significantly lower than those of WT-Y (Figure 4a–c), while the proline (PRO) content and ascorbate peroxidase (APX) activity of OE plants were significantly higher than those of WT-Y (Figure 4d,e). In addition, DAB/NBT staining results showed that the colour of OE plant leaves after cold treatment was significantly lighter than that of WT-Y (Figure 4f,g).

On the contrary, after cold treatment, the electrolyte leakage rate, the contents of MDA and H2O2 of RNAi plants were significantly higher than those of WT-H (Figure 4a–c), while PRO content and APX activity of RNAi plants were significantly lower than those of WT-H (Figure 4d,e). In addition, DAB/NBT staining of RNAi plant leaves after cold treatment was significantly deeper than that of WT-H (Figure 4h,i).
These results indicate that after cold treatment at 4°C, FveDREB1B overexpressed plants accumulate more proline and enhanced the scavenging capacity of reactive oxygen, thus reducing the tissue damage and reactive oxygen content of plants. The opposite is true in FveDREB1B silenced plants. Hence, this suggests that FveDREB1B positively regulates cold tolerance of woodland strawberry.
3.5 RNA-sequencing analysis of FveDREB1B overexpressed plants after cold treatment
RNA-seq analysis was performed on FveDREB1B overexpressed plants OE and wild-type WT-Y plants treated at 4°C for 2 days to further clarify the mechanism of FveDREB1B improving cold tolerance of strawberry. Volcano plots showed that a total of 358 genes were differentially expressed in cold-treated FveDREB1B overexpressed plants, of which 277 genes were upregulated and 81 genes were downregulated (Figure 5a). Subsequently, GO classification analysis was performed on the 358 differentially expressed genes, and the results showed that these differentially expressed genes were involved in biological processes such as biological regulation, stimulus response, signal transduction, growth, and developmental process (Figure 5b). Since FveDREB1B positively regulates cold tolerance of woodland strawberry (Figure 4), 277 upregulated genes were selected for further KEGG pathway analysis. The KEGG bubble map showed that upregulated genes were most abundant in the plant hormone signal transduction pathway (ko04075) (Figure 5c). Hence, it is speculated that plant hormones might be participated in the process of FveDREB1B improving cold tolerance of woodland strawberry.
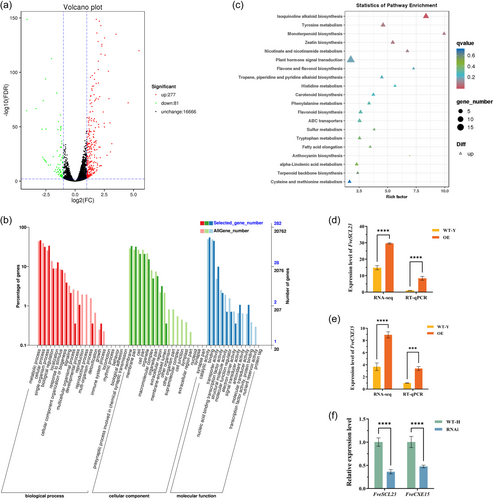
In Arabidopsis, cold stress reduces the level of active GA, thus promoting the regulation of AtCBF1 on cold tolerance of Arabidopsis (Achard et al., 2008). Therefore, we analysed the GA signalling pathway in the plant hormone signal transduction pathway (ko04075). It was found that two genes upregulated by FveDREB1B were enriched in GA signalling pathway: one is FveSCL23 belonging to SCR subfamily of GRAS family (Chen et al., 2019), which is enriched in the DELLA module of GA signalling pathway; the other is FveCXE15 belonging to carboxylesterase family, enriched in the GID1 module. Subsequently, the expression levels of these two genes in cold-treated FveDREB1B transgenic plants were analysed by RT-qPCR. Consistent with RNA-seq results, the expression levels of FveSCL23 and FveCXE15 were significantly upregulated in OE plants (Figure 5d,e), while the expression levels of FveSCL23 and FveCXE15 were significantly downregulated in RNAi plants (Figure 5f). Hence, this suggests that FveDREB1B promotes the expression of FveSCL23 and FveCXE15.
3.6 FveDREB1B positively regulates FveSCL23/FveCXE15 expression
By predicting and analyzing the promoters (1500 bp) of FveSCL23 and FveCXE15, it was found that both promoters contained the FveDREB1B binding site DRE (A/GCCGAC) element (Figure 6a). Subsequently, sequence fragments P1 and P2 containing DRE elements on promoters of FveSCL23 and FveCXE15 were selected for Y1H assay. The results showed that when the growth of pAbAi-pFveSCL23(P1)/pFveCXE15(P2)+pGADT7 yeast on SD/-Leu+50 ng mL−1 AbA selective medium was inhibited, pAbAi-pFveSCL23(P1)/pFveCXE15(P2)+pGADT7-FveDREB1B yeast was able to grow normally (Figure 6b). Meanwhile, the EMSA assay results showed that FveDREB1B-GST protein could be labelled by FveSCL23-Probe, and the band gradually weakened with the increase of competitors, while GST label could not be labelled by FveSCL23-Probe (Figure 6c). These results indicate that FveDREB1B directly binds to DRE elements on promoters of FveSCL23 and FveCXE15.
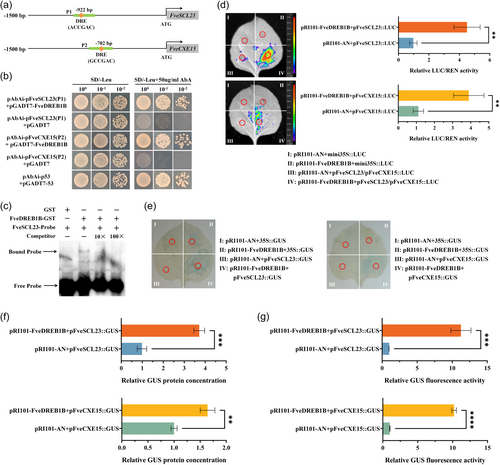
The LUC assay results showed that the tobacco leaves co-transferred by pRI101-FveDREB1B and pFveSCL23/pFveCXE15::LUC showed the brightest LUC fluorescence, and its LUC/REN fluorescence activity was significantly higher than that of pRI101-AN+pFveSCL23/pFveCXE15::LUC (Figure 6d). GUS staining assay obtained the same results: the tobacco leaves co-transferred by pRI101-FveDREB1B and pFveSCL23/pFveCXE15::GUS had the deepest GUS staining (Figure 6e); its GUS protein concentration (Figure 6f) and GUS fluorescence activity (Figure 6g) were significantly higher than those of pRI101-AN+pFveSCL23/pFveCXE15::GUS. Hence, FveDREB1B directly binds to FveSCL23 and FveCXE15 promoters to activate their expression.
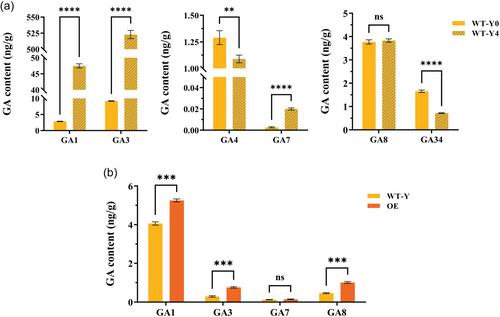
Since cold stress can induce FveDREB1B expression (Figure 1), and its downstream target genes are enriched in GA signalling pathway, we investigated the effects of cold stress and FveDREB1B expression levels on endogenous GA content in woodland strawberry plants. Firstly, wild-type strawberry plants WT-Y were cold-treated at 4°C for 2 days, and then the content of endogenous GA was detected. The results showed that after cold treatment, the contents of active GA1/GA3/GA7 and inactive GA8 increased, among them, the contents of active GA1/GA3/GA7 increased significantly, while the contents of active GA4 and inactive GA34 decreased significantly (Figure 7a). Subsequently, we also detected the endogenous GA contents of FveDREB1B overexpressed plants (OE) and its control plants (WT-Y). The results showed that the contents of active GA1/GA3/GA7 and inactive GA8 increased in OE plants, among them, the contents of active GA1/GA3 and inactive GA8 increased significantly, while the contents of active GA4 and inactive GA34 were not detected (Figure 7b). These results indicate that the effects of cold stress and the increase of FveDREB1B expression level on the endogenous GA content of woodland strawberry plants are basically consistent. Hence, FveDREB1B regulates the endogenous GA level of woodland strawberry under cold stress.
3.7 FveDREB1B and its downstream gene FveSCL23 interact with FveDELLA to improve cold tolerance of woodland strawberry
Cold-induced AtCBF1 enhanced cold tolerance of Arabidopsis by accumulating AtDELLA protein, while inhibiting its growth and development (Achard et al., 2008). This is very similar to the function of FveDREB1B in woodland strawberry (Figures 3 and 4; Supporting Information: Table S4). Since FveSCL23 activated by FveDREB1B is enriched in the DELLA module, we analysed the relationship between FveSCL23 and FveDELLA protein. According to the NCBI and GDR databases, nine genes encoding FveDELLA protein were identified in diploid woodland strawberry. They are FveGAI, FveGAI1A, FveGAI1B, FveGAIP, FveRGA2, FveRGL1A, FveRGL1B, FveRGL1C and FveRGL1D (Supporting Information: Table S2). We found that after cold treatment, the expression levels of FveGAI, FveGAIP, FveRGA2, FveRGL1B and FveRGL1D were significantly upregulated in FveDREB1B overexpressed plant OE (Figure 8a), and significantly downregulated in FveDREB1B silenced plant RNAi (Figure 8b), while the expression levels of FveGAI1A, FveGAI1B, FveRGL1A and FveRGL1C were not significantly different between FveDREB1B transgenic plants and control plants (Supporting Information: Figure S5). These results indicate that among the nine genes encoding the FveDELLA protein, only five genes, FveGAI, FveGAIP, FveRGA2, FveRGL1B and FveRGL1D, are induced by FveDREB1B under cold stress.
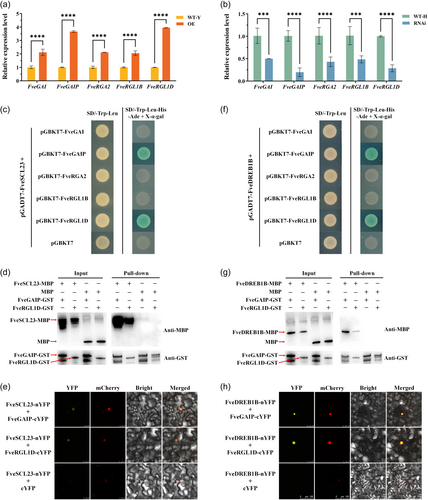
AtSCL3 maintains GA homeostasis in Arabidopsis by interacting with AtDELLA (Zhang et al., 2011). Therefore, we verified the interaction between FveSCL23 and the above five FveDELLA proteins. The Y2H results showed that on SD/-Trp-Leu-His-Ade+X-α-gal selective medium, only the strains transferred to pGBKT7-FveGAIP and pGBKT7-FveRGL1D grew well and were stained blue (Figure 8c). This indicates that among the five FveDELLA proteins, only FveGAIP and FveRGL1D interact with FveSCL23. Meanwhile, the pull-down assay demonstrated that FveSCL23 interacted with FveGAIP and FveRGL1D in vitro (Figure 8d). Moreover, the BiFC assay confirmed that FveSCL23 interacted with FveGAIP and FveRGL1D in nucleus (Figure 8e). These results indicate that FveSCL23 interacts with FveDELLA proteins.
Since the phenotype of FveDREB1B overexpressed woodland strawberry plants was very similar to that of Arabidopsis accumulating DELLA proteins (Achard et al., 2008), and FveDREB1B directly activated FveSCL23 expression (Figure 6), we further analysed the interaction between FveDREB1B and the above five FveDELLA proteins. The Y2H results showed that only FveGAIP and FveRGL1D interacted with FveDREB1B (Figure 8f). In addition, the pull-down and BiFC assays verified that FveDREB1B interacted with FveGAIP and FveRGL1D in vitro (Figure 8g) and in nucleus (Figure 8h). These results indicate that FveDREB1B interacts with FveDELLA proteins.
3.8 Anthocyanins are accumulated in the process of FveDREB1B enhancing the cold tolerance of woodland strawberry
Strawberry plants cultivated in solar greenhouses are subjected to cold stress in winter, and then climate warming increases the temperature in the greenhouse, thereby alleviating the effects of cold stress on strawberry plants. To simulate the actual situation of strawberry production, we placed FveDREB1B transgenic plants (OE11/31/36 and R2/14/18) and control plants (WT-Y and WT-H) at the low temperature of 4°C for 7 days, and then cultured them at room temperature 25°C for 7 days to observe their growth and development status. During cold treatment, both FveDREB1B transgenic plants and control plants stopped growing, and when temperature was restored to 25°C, their growth and development returned to normal (Supporting Information: Figure S6). After restoring growth at 25°C, the base of the three compound leaves of FveDREB1B overexpressed plants (OE11/31/36) showed purple-red, while the leaves of FveDREB1B silenced plants (R2/14/18) and control plants showed no change in colour (Figure 9a,b). In addition, after cold treatment, anthocyanin content in woodland strawberry leaves showed an increasing trend, meanwhile after strawberry plants restoring growth at 25°C, the anthocyanin content was significantly upregulated in leaves of FveDREB1B overexpressed plants and downregulated in leaves of FveDREB1B silenced plants (Figure 9c,d). However, there was no significant difference in leaf chlorophyll content between FveDREB1B transgenic plants and control plants (Supporting Information: Figure S7). Hence, woodland strawberry plants response to cold stress by accumulating anthocyanins in leaves, and FveDREB1B promotes anthocyanin accumulation.
In anthocyanin synthesis process, a series of enzymes involved in catalysis, including chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-β-hydroxylase (F3H), dihydroflavonol-4-reductase (DFR), anthocyanidin synthase (ANS), and UDP Glc-flavonoid-3-O glucosyltransferase (UFGT), and so forth (Wang, Xiao, et al., 2023). According to GDR databases, we identified the above 6 anthocyanin synthase genes (FveCHS, FveCHI, FveF3H, FveDFR, FveANS and FveUFGT) in diploid woodland strawberry. After strawberry plants restoring growth at 25°C, their expressions in leaves of FveDREB1B overexpressed plants were significantly upregulated (Figure 9e), while only FveCHS, FveF3H, FveANS and FveUFGT showed significantly downregulated expression levels in leaves of FveDREB1B silenced plants (Figure 9f). Therefore, among the 6 anthocyanin synthase genes, only the expressions of FveCHS, FveF3H, FveANS and FveUFGT are regulated by FveDREB1B.
Subsequently, the four gene promoters (1500 bp) were analysed and we found that only the FveCHS promoter contained DRE binding sites (Figure 9g). The Y1H results showed that FveDREB1B could directly bind to the DRE element on the FveCHS promoter (Figure 9h). Then, LUC assay results showed that FveDREB1B activated FveCHS expression by directly binding to its promoter (Figure 9i). These results indicate that FveDREB1B promotes anthocyanin accumulation in woodland strawberry leaves under cold stress by directly activating FveCHS expression and indirectly promoting the expression of FveF3H, FveANS and FveUFGT.
3.9 Profiles of FveDREB1B expression
Due to the abundant resources of Fragaria, there are different ploidy series (Nosrati, 2015). Therefore, we analysed the expression of DREB1B in 16 different ploidy strawberry resources (Supporting Information: Table S1). RT-qPCR showed that DREB1B expressions were higher in diploid Fragaria mandschurica (Fma), Fragaria nipponica (Fnip), Fragaria viridis (Fvi), tetraploid Fragaria orientalis (For), hexaploid Fragaria moschata (Fmos) and octoploid Yanli (YL), Fragaria virginiana (Fvir) than in Fragaria vesca (Fve) (Supporting Information: Figure S8). Among them, DREB1B expressions were significantly higher in Fma and Fnip (Supporting Information: Figure S8). In addition, DREB1B expressions were lower in diploid Fragaria nilgerrensis (Fnil) and octoploid Chulian (CL), Fragaria chiloensis (Fch) than in Fve (Supporting Information: Figure S8).
Combined with the statistics of different ploidy strawberry resources distribution areas (Lei et al., 2004), we analysed the distribution of 16 strawberry resources in higher latitudes and investigated their latitudes (Supporting Information: Table S1). The results showed that strawberry resources with higher DREB1B expressions were distributed in higher latitudes, such as Fma, Fnip, For, Fvi and Fmos, while strawberry resources with lower DREB1B expressions were distributed in lower latitudes, such as Fnil (Supporting Information: Figure S8; Supporting Information: Table S1). In general, regions at higher latitudes tend to have colder climates, which may mean that strawberry resources distributed at higher latitudes have stronger cold tolerance, while those distributed at lower latitudes have weaker cold tolerance.
4 DISCUSSION
4.1 FveDREB1B improves cold tolerance of woodland strawberry by stabilizing FveDELLA
Studies have shown that CBF/DREB1 is the core factor for plants' response to cold stress and transmit cold signals (Song et al., 2021). At present, the research on CBF/DREB1 regulation of plant cold tolerance mainly focuses on the regulatory effects of upstream factors of CBF/DREB1 on it, involving GA, BR, JA, ETH, SL and other hormonal pathways (Lantzouni et al., 2020; Li, Ye, et al., 2017; Qi et al., 2022; Shi et al., 2012; Wang, Li, et al., 2023), light signalling pathways, circadian pathways, and protein posttranslational modification (Ding et al., 2020). However, there are relatively few studies on how CBF/DREB1 regulates plant cold tolerance by regulating downstream genes. So far, only DELLA, anthocyanin and putrescine pathway have been found (Achard et al., 2008; Li et al. 2016, 2022). Among them, DELLA pathway has only been reported in Arabidopsis. Studies have shown that cold-induced AtCBF1 and AtCBF3 decreased the level of active GA by activating the expression of AtGA2ox3/6 and AtGA2ox7, thereby accumulating AtDELLA; meanwhile, AtCBF1 also accumulated AtDELLA by promoting AtRGL3 transcription, thereby inhibiting the growth and development of Arabidopsis and improving its cold tolerance (Achard et al., 2008; Zhou et al., 2017).
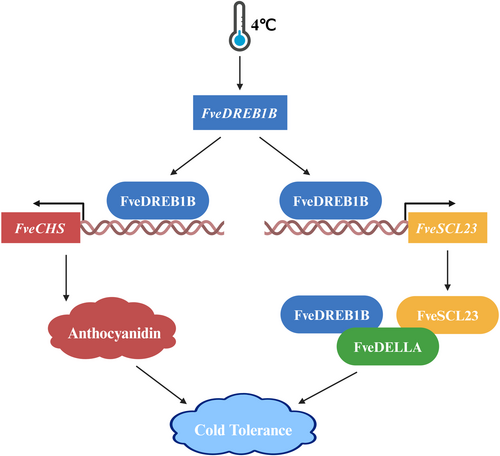
In this study, we found that cold-induced FveDREB1B directly activated FveSCL23 expression and promoted the interaction between FveSCL23 and FveDELLA; meanwhile, FveDREB1B can also directly interact with FveDELLA to jointly stabilize FveDELLA, thereby inhibiting the beginning of reproductive growth and enhancing cold tolerance of woodland strawberry (Figure 10). This is different from the mechanism by which AtCBF1 directly activates AtRGL3 expression to accumulate AtDELLA in Arabidopsis (Achard et al., 2008); and also different from the mechanism of AtCBFs to further accumulate AtDELLA by directly activating AtGA2ox expression and reducing the level of active GA (Achard et al., 2008; Zhou et al., 2017). Because we analysed the differentially expressed genes involved in GA biosynthesis in RNA-seq of FveDREB1B overexpressed plants after cold treatment, we found only one upregulated gene, FveGA2ox, and confirmed that FveDREB1B could indeed promote its expression (Supporting Information: Figure S9). However, FveGA2ox promoter does not contain FveDREB1B binding site DRE elements, which indicates that FveDREB1B promotes the expression of FveGA2ox indirectly rather than directly.
As a transcription factor of GRAS family, DELLA proteins were discovered, which may negatively regulate GA signalling pathway by directly regulating GA biosynthesis and GA receptor-related gene expression (Zentella et al., 2007). In this study, FveDELLA protein was stabilized by interacting with FveDREB1B and FveSCL23, which prevented it from playing a negative regulatory role in the GA signalling pathway. This may be the reason why the content of active GA1/GA3/GA7 increased under cold stress and FveDREB1B overexpression (Figure 7). As a key enzyme in the GA biosynthesis pathway, GA2ox promotes the conversion of active GA1 and GA4 into inactive GA8 and GA34, respectively (Hedden, 2020; Tang et al., 2022). As a result, the content of inactive GA8 also increased (Figure 7). Interestingly, the contents of active GA4 and inactive GA34 decreased under cold stress and FveDREB1B overexpression (Figure 7). It was found that the binding capacity of rice GA receptor GID1 to active GA4 was much greater than that of active GA1 and GA3 (Yoshida et al., 2018). We found that FveCXE15 activated by FveDREB1B was enriched in GID1 module of GA signalling pathway. Therefore, we speculated that the decrease of active GA4 content might be due to the binding with GID1, which led to the decrease of inactive GA34 content. However, whether FveCXE15 affects the binding of GID1 to active GA needs to be further verified.
Both FveCXE15 and GID1 belong to the CXE family, whose members are a class of enzymes capable of hydrolyzing esters (Li et al., 2024). CXEs mainly hydrolyze volatile esters and ester hormones to participate in the regulation of fruit flavour and plant growth and development. For example, transient expression of PpCXE1, MdCXE20 and PuCXE15 in peach, apple and ‘Nanguo’ pear (Pyrus ussuriensis) decreased the content of volatile esters in fruits (Cao et al., 2019; Li et al., 2023; Qi et al., 2023), while silencing of SlCXE1 and FaCXE1 in tomato and cultivated strawberry increased the content of volatile esters in fruits, thus affecting fruit flavour (Goulet et al., 2012; Martínez-Rivas et al., 2022); AtCXE15 bound to and hydrolyzed strigolactone (SL) and its analogues, thereby releasing the inhibitory effect of SLs on axillary bud growth (Xu et al., 2021), while the mutation of NtCXE22, the homologous gene of AtCXE15 in tobacco, reduced SLs content and shortened bud growth (Wang et al., 2022). In addition, CXEs are also involved in plant resistance to stress. For example, the overexpression of AtCXE8 increased the resistance of Arabidopsis to Botrytis cinerea (Lee et al., 2013), while the silence of GbCXE49 decreased the resistance of Gossypium barbadense to alkaline stress (Rui et al., 2022). Moreover, CXE family members are also involved in plant GA signal transduction, which is reflected in the binding of GID1 and active GA (Li et al., 2024). In this study, we found that FveDREB1B directly activated FveCXE15 expression under cold stress, and FveDREB1B improved the cold tolerance of woodland strawberry. Therefore, FveCXE15 may play a role in regulating the cold tolerance of woodland strawberry, but the specific mechanism remains to be revealed.
4.2 FveDREB1B also promoted the accumulation of anthocyanins in woodland strawberry leaves
Anthocyanins, one of the main plant pigments, exist in the form of anthocyanin glycosides in plants (An et al., 2023), which not only determine the colour of plant organs, but also play an important role in resistance to stress (Kim et al., 2017; Shin et al., 2016; Sun et al., 2017), especially cold stress (Wang et al., 2020; Xu et al., 2023). It was found that anthocyanins were accumulated in the process of CBF/DREB1 pathway enhancing cold tolerance of plants (Artlip et al., 2014; Gu et al., 2015; Wisniewski et al., 2011). At present, the molecular mechanism by which CBFs/DREB1s enhance plant cold tolerance by accumulating anthocyanins has only been reported in Arabidopsis. Studies have shown that cold-induced AtCBF1 can directly activate the expression of AtUGT79B2/B3 to increase anthocyanin content, thereby promoting ROS clearance and improving the cold tolerance of Arabidopsis (Li et al., 2016). However, the molecular mechanism by which anthocyanins regulate cold tolerance of strawberry through CBF/DREB1 pathway remains unclear.
In our study, we found that cold treatment could increase the anthocyanin content of woodland strawberry leaves, and after returning to room temperature 25°C culture, the anthocyanin content of FveDREB1B transgenic plants showed obvious differences, specifically as follows: in FveDREB1B overexpressed plants, the base of trifoliate compound leaves turned red (Figure 9a), the anthocyanin content of leaves increased more (Figure 9c), and anthocyanin structural genes expressions including FveUFGT, FveANS, FveDFR, FveF3H, FveCHI and FveCHS were significantly increased (Figure 9e); while in FveDREB1B silenced plants, the anthocyanin content of leaves increased less (Figure 9d), and expressions of FveUFGT, FveANS, FveF3H and FveCHS were significantly decreased (Figure 9f). These results indicate that under cold stress, FveDREB1B can accumulate anthocyanin in strawberry leaves by promoting the expression of some anthocyanin structural genes. Moreover, we found that only the FveCHS promoter contained the FveDREB1B binding site, while the FveF3H, FveANS and FveUFGT promoters did not (Figure 9g); meanwhile, we also verified that FveDREB1B activated FveCHS expression by directly binding to its promoter (Figure 9h,i). Therefore, FveDREB1B promoted anthocyanin accumulation by directly activating FveCHS expression after cold stress, which may further enhance the cold tolerance of woodland strawberry (Figure 10).
Interestingly, the expression of FveCHI was upregulated not only in FveDREB1B overexpressed plants, but also in FveDREB1B silenced plants (Figure 9e,f). It has been suggested that woodland strawberry fruits may respond to cold stress through the CBF/DREB1 pathway, which promotes the phosphorylation of FveMYB10, and then weakens the transcriptional activation of FveMYB10 on FveCHI (Mao et al., 2022). Therefore, we hypothesized that the low expression level of FveDREB1B in FveDREB1B silenced plants weakened the response of woodland strawberry to cold stress, which in turn weakened the promotion of FveMYB10 phosphorylation and promoted the transcriptional activation of FveMYB10 on FveCHI, thus leading to the upregulation of FveCHI expression in FveDREB1B silenced plants. However, the exact reason needs to be analysed by further experiments.
4.3 FveDREB1B was highly expressed in strawberry resources with strong cold tolerance
Diploid woodland strawberry is often studied as a model plant in the Rosaceae due to its small genome, while octoploid cultivated strawberry is the main species in production (Li et al., 2019). In this study, we compared the amino acid sequences of FveDREB1B in diploid woodland strawberry and FaDREB1B in octoploid cultivated strawberry, and found that they were highly consistent (Supporting Information: Figure S2) and contained the same motif elements and conserved AP2 domain (Figure 2a). The phylogenetic tree showed that they have the closest relationship, followed by the other Rosaceae species (Figure 2a). This suggests that the pathway by which FveDREB1B regulates the cold tolerance of diploid woodland strawberry revealed in this study is also applicable to octoploid cultivated strawberry. At the same time, the FveDREB1B pathway also provides a new idea for the study of the regulatory mechanism of cold tolerance of other Rosaceae fruit trees, such as sweet cherry, plum, peach, apple and white pear, which will help to improve the overwintering and cold resistance of Rosaceae deciduous fruit trees, and also increase the possibility of their northward planting.
Wild strawberry resources are abundant, including different ploidy resources, such as diploid, tetraploid, hexaploid and octoploid (Lei et al., 2004). Among them, low-ploidy wild germplasm contains abundant genetic resources with unique quality, seasonal fruit, shallow dormancy, resistance to stress, pests and diseases and other excellent traits, which can be used as important materials for the improvement of cultivated F. ananassa (Lei et al., 2004). In this study, we detected the expression levels of DREB1B in different ploidy strawberry resources and analysed the latitudes of their distribution regions. We found that Fma, Fnip, For, Fvi and Fmos expressing higher levels of DREB1B were all distributed at higher latitudes, while Fnil expressing lower levels of DREB1B were distributed at lower latitudes (Supporting Information: Figure S8; Supporting Information: Table S1). In view of the higher latitudes have more cold climate conditions, we suspected that Fma, Fnip, For, Fvi and Fmos might have grater cold tolerance, and Fnil might have poorer cold tolerance. In addition, previous research has proved that For is extremely cold resistant, while Fnil is not cold resistant, and it is easy to freeze to death in winter in northeast China (Lei et al., 2004). The cold tolerance of the above six strawberry resources is consistent with DREB1B expression in them. Therefore, it is a viable method to analyse the expression level of DREB1B in the screening of cold-resistant strawberry germplasm resources and the identification of hybrid progeny, and even in other Rosaceae plants.
ACKNOWLEDGEMENTS
This research was supported by the Liaoning Key Agricultural Project (2023JH1/10200003); the Basic Scientific Research Program of Colleges and Universities of Liaoning Province Education Department (JYTMS20231292); and the Shenyang Science and Technology Mission Program (23-410-2-04).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data involved in this study are available upon reasonable request by the corresponding author.




