Stomatal dynamics in Alloteropsis semialata arise from the evolving interplay between photosynthetic physiology, stomatal size and biochemistry
Abstract
C4 plants are expected to have faster stomatal movements than C3 species because they tend to have smaller guard cells. However, little is known about how the evolution of C4 photosynthesis influences stomatal dynamics in relation to guard cell size and environmental factors. We studied photosynthetically diverse populations of the grass Alloteropsis semialata, showing that the origin of C4 photosynthesis in this species was associated with a shortening of stomatal guard and subsidiary cells. However, for a given cell size, C4 and C3–C4 intermediate individuals had similar or slower light-induced stomatal opening speeds than C3 individuals. Conversely, when exposed to decreasing light, stomata in C4 plants closed as fast as those in non-C4 plants. Polyploid formation in some C4 plants led to larger stomatal cells and was associated with slower stomatal opening. Conversely, diversification of C4 diploid plants into wetter environments was associated with an acceleration of stomatal opening. Overall, there was significant relationship between light-saturated photosynthesis and stomatal opening speed in the C4 plants, implying that photosynthetic energy production was limiting for stomatal opening. Stomatal dynamics in this wild grass therefore arise from the evolving interplay between photosynthetic physiology and the size and biochemical function of stomatal complexes.
1 INTRODUCTION
Gas exchange is fundamental for plant growth and yield, such that carbon gain comes at the cost of significant water loss (Brestic et al., 2018; Matthews et al., 2017). Enhancing photosynthesis while reducing water loss has therefore become an important target for crop improvement (Lawson & Blatt, 2014; Long et al., 2006). Several strategies are being applied to enhance carbon fixation, for example: the optimisation of photosynthetic enzymes (e.g., Rubisco, ATP synthase and phosphoribulokinase) and the Calvin–Benson–Bassham cycle, introducing the C4 and non-plant carbon concentrating mechanisms into C3 plants, improving mesophyll conductance to CO2 and optimising responses to fluctuating light conditions (Kaiser et al., 2019; Orr et al., 2017). However, the balance between carbon gain and water loss largely depends on stomatal behaviour, and this has been less well studied than carbon fixation. Therefore, to increase leaf water-use efficiency (WUE), more attention is now being paid to the physiological responses of stomatal conductance (gs) to environmental conditions (Matthews et al., 2020; Ozeki et al., 2022; Vialet-Chabrand et al., 2017).
Stomatal aperture is regulated via internal signals in response to environmental cues (light, CO2), to ensure that leaves balance gaseous diffusion (Lawson & Blatt, 2014). The speeds of stomatal opening and closing depend on several factors, including direct effects like stomatal size and morphology (Faralli et al., 2019; Lawson & Blatt, 2014), and indirect influences such as plant water status (Durand et al., 2020; Ozeki et al., 2022) and photosynthetic pathway (McAusland et al., 2016; Ozeki et al., 2022). Many previous studies have shown that both the size and density of stomata are important, since they combine to influence the rate of stomatal movements and set the maximum value of gs (Faralli et al., 2019; Lawson & Blatt, 2014; Vialet-Chabrand et al., 2017). In particular, smaller guard cells have a high surface-area-to-volume ratio, causing them to change osmotic pressure via the active transport of ions across the cell membrane more rapidly than larger cells. They also have smaller pores to shut and less distance to move. In combination, these factors enable them to close faster than larger guard cells in response to changing environmental cues (Faralli et al., 2019; Raven, 2014).
Previous work has shown that C4 plants in general have evolved smaller stomata for a given stomatal density (SD; Table S1) than C3 plants, leading to a lower maximum value for gs (Taylor et al., 2012). This adaptation arises because C4 photosynthesis increases the CO2 concentration around the CO2-fixing enzyme Rubisco (Bräutigam & Gowik, 2016), which enables C4 plants to maintain carbon fixation at a lower diffusive conductance (and therefore a higher WUE) than C3 plants. Smaller stomata at a higher density in C4 crops are associated with rapid stomatal movements (McAusland et al., 2016), which are faster than those in C3 species (Ozeki et al., 2022). However, under high nitrogen conditions the difference is explained by stomatal size and density, such that stomatal speed follows a common relationship with stomatal size or density in C4 and C3 crops (Ozeki et al., 2022). The effect of photosynthetic pathway acts in addition to the rapid environmental responses of dumbbell-shaped guard cells typical of grasses and some other monocots (McAusland et al., 2016). Both the size and shape of guard cells may therefore influence the range of possible speeds in C4 and C3 species.
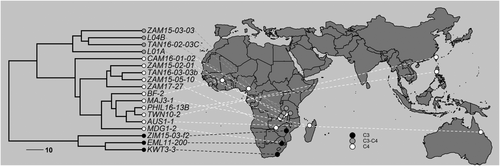
We have used the grass Alloteropsis semialata as a model for investigating the evolution of photosynthetic physiology in C3 and C4 plants, since it shows a high level of intraspecific variation in photosynthesis, including intermediate forms (Lundgren et al., 2016). As a grass (Poaceae), its guard cells are of the dumbbell-shaped type, and guard cell size varies in relation to polyploidy (Linder et al., 2018). These features make A. semialata a good study system for investigating how stomatal function changes with photosynthetic pathway and guard cell size. Its large geographical distribution across Africa, Asia and Oceania offers the additional opportunity to investigate whether stomatal function adapts to local climatic conditions. In this paper, we use populations of A. semialata sampled from across its geographical range (Figure 1; Table S2) to investigate how natural variation in photosynthetic type, cell size and habitat conditions influence dynamic stomatal responses to changing light conditions. We test the hypotheses that: (i) C4 pathway evolution gives rise to smaller guard cells in C4 diploid populations, which have faster stomatal responses to light than in the C3 type because of their smaller size; (ii) the larger guard cells of polyploids are slower responding than the smaller guard cells of diploids; and (iii) warm and dry environmental conditions select for stomata with a water-conserving strategy of slow opening and fast closure.
2 MATERIALS AND METHODS
2.1 Plant material and bioclimatic variables
Plants of A. semialata with various photosynthetic types (C3, C3–C4 and C4) and ploidy levels (diploid, hexaploid and dodecaploid) from 18 geographic origins (Figure 1; Table S2) were collected in this study (Bianconi et al., 2020; Lundgren et al., 2016). There were 11 populations with three replicates, four populations with two replicates, two populations with one plant, and one population with five replicates, according to the collection of plants. A total 48 individuals were involved in this study. Plants were grown in plastic pots (sized 1 L) containing John Innes No. 2 compost (John Innes Manufacturers Association) and fertilised once a month with Scotts Evergreen Lawn Food (The Scotts Company). Experiments were carried out in 2021.
The values of bioclimatic variables including annual precipitation were extracted from the WorldClim database (Alenazi et al., 2023; Fick & Hijmans, 2017) based on the geographic coordinates of collection locations of populations, using the Raster package (Alenazi et al., 2023; Hijmans, 2023) in R software (http://www.r-project.org/, version 4.2.1).
2.2 Phylogeny and genome sizes
For molecular dating, 100 sets of five randomly chosen Benchmarking Universal Single-Copy Orthologs (BUSCO) genes were selected and analysed to produce a set of trees that represented the various histories existing within the genomes. A reference-based method (Dunning, Moreno-Villena, et al., 2019; Olofsson et al., 2016, 2019), with the chromosome-scale genome of an Australian individual of A. semialata (AUS1-1; Dunning, Olofsson, et al., 2019) as a reference was used to generate consensus sequences for each BUSCO. Full details of molecular dating are provided in Raimondeau et al. (2023). A time-calibrated phylogenetic tree for A. semialata was generated based on nuclear genomes under a coalescence model by Bayesian inference as implemented in *Beast2 v.2.6.4 (Bouckaert et al., 2019; Raimondeau et al., 2023). The DNA substitution model was set to a GTR + G as its reliable across most genes (Abadi et al., 2019), and the relaxed molecular clock with a log-normal distribution of rates was applied. Each individual was set as a different species to obtain a phylogenetic tree that indicates the relationships among accessions. The specific approach of generating a summary tree and mapping the median ages on nodes of the highest credibility tree is provided in Bouckaert (2010) and Raimondeau et al. (2023).
Genome sizes of some A. semialata individuals were estimated using the Sysmex Partec Cyflow SL3 flow cytometry, following the method with minor modifications described by Doležel et al. (2007) and Bianconi et al. (2020). Ebihara with 3% PVP was applied to the fresh samples, which were analysed by a flow cytometer fitted with a 100 mW green solid-state laser (Cobalt Samba). According to the internal calibration standards, for diploid populations, Petroselinum crispum ‘Champion Moss Curled’ (4.5 pg/2 C) was used, while for populations with C-values more than three times larger than any diploids, Pisum sativum ‘Ctirad’ (9.09 pg/2 C) was used (Bianconi et al., 2020). Data for the remaining A. semialata individuals were retrieved from previous publications (Bianconi et al., 2020; Olofsson et al., 2016, 2021). Ploidy levels were assigned based on the estimates of genome size for individuals with known chromosome numbers and genome size (Bianconi et al., 2020).
2.3 Stomatal traits
Stomatal impressions were taken from two surfaces of the same leaves measured in gas exchange experiments. Dental putty (President Plus-light body; Coltène/Whaledent Ltd.) impressions were produced following the methods of Weyers and Johansen (1985) and Dunn et al. (2019), and nail polish peels made from impressions were transferred onto microscope slides. Guard cell length (GCL), guard cell width (GCW), subsidiary cell length (SCL), subsidiary cell width (SCW) and SD were measured using Image Pro-Plus 6.0 (Media Cybernetics). Guard cell surface area (GCSA) and guard cell volume (GCV) were estimated by assuming that guard cells are shaped like capsules, that is, cylindrical with hemispherical ends (Raven, 2014). Full details are provided by Raven (2014). GCSA to volume ratio (GCSA:GCV) was calculated as GCSV/GCV.
2.4 Leaf gas exchange
All photosynthetic parameters were measured using the LI-COR 6400XT portable gas-exchange system with a 6400-40 fluorometer head unit (LI-COR). Gas exchange measurements were carried out on the youngest fully expanded leaf of well-watered plants. The LI-COR cuvette conditions during gas exchange measurements were controlled as follows: a CO2 concentration of 400 µmol mol−1, a leaf temperature of 24 ± 1°C, a leaf vapour pressure deficit in the range 1.4–1.9 kPa, and a flow rate of 500 μmol s−1.
The responses of net CO2 assimilation (A) and stomatal conductance (gs) to a step-change in photosynthetic photon flux density (PPFD) were carried out as described using the method of McAusland et al. (2016), with the following changes to the protocol: (i) leaves were equilibrated to a PPFD of 100 μmol m−2 s−1 until both A and gs were at a steady state; (ii) PPFD was then increased to 1000 μmol m−2 s−1 for 1 h until a new steady-state was reached with A and gs recorded every 30 s, before returning to 100 μmol m−2 s−1 for a further 1 h to reach the final steady state.
The same leaf was used during the same day to record the relationship between steady-state gs and irradiance. Measurements were recorded every 30 s starting from low irradiance to high irradiance levels (0, 50, 100, 200, 400, 800, 1200, 1600, 2000 μmol m−2 s−1). Each irradiance level was provided for at least 30 min to reach a steady-state value of gs (Ng & Jarvis, 1980).
2.5 Modelling stomatal responses to a fluctuating environment
2.6 Statistical analysis
R software (http://www.r-project.org/, version 4.2.1) was used to statistically analyse the data and generate graphs. Tests of significant differences were identified using a non-parametric test (specifically the Kruskal–Wallis test) to determine the significance of the differences in means among groups (photosynthetic types plus ploidy levels). Phylogenetic generalised least squares (PGLS) models were performed to test for correlations while considering evolutionary relationships. Values of Pagel's lambda from zero to one represented the strength of the phylogenetic signal. A summary function in R was then run for each model to determine the adjusted multiple correlation coefficients (r2) and statistical significances of covariates between variables (p < 0.05).
3 RESULTS
3.1 Stomatal characteristics
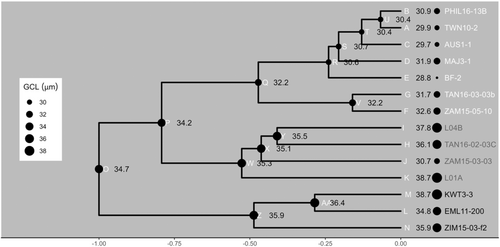
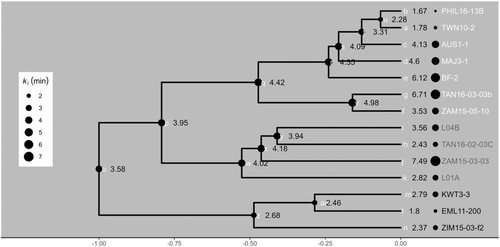
The non-parametric Kruskal–Wallis test showed that C4 diploid grasses had significant shorter (p < 0.05) GCLs than either non-C4 diploid populations or C4 hexaploid and dodecaploid populations (Figure 2a). The analysis of ancestral state reconstructions for GCL in all diploids also showed that the common ancestor of C4 A. semialata had shorter GCLs than those of C3–C4 or C3 plants (Figure 2a; Figure 3 node Q vs. nodes W and Z), indicating that cell shortening coincided with the origin of C4 photosynthesis. However, there were no significant differences in GCW, GCV, GCSA to volume ratio (GCSA:GCV) and SD among diploids (Figure 2). GCL and GCW became larger as the ploidy levels increased in C4 plants (Figures 2 and 3). Specifically, among C4 grasses, there were significant positive associations (p < 0.05) between genome size and guard cell size (Figure 4a,b), which is consistent with previous studies (Beaulieu et al., 2008; Jordan et al., 2015; Lomax et al., 2009). However, for a given genome size, C3 and C3–C4 plants had longer guard cells with a similar width to C4 plants (Figures 2 and 3). GCL was therefore similar, but GCW was greater, in C4 hexaploids compared with C3 and C3–C4 diploids (Figure 2a,b). As a consequence, GCV was lowest in C4 diploids, followed by C3–C4 and C3 diploids, C4 hexaploids, and C4 dodecaploids (Figure 2c). However, C3 and C4 diploids had a significantly greater (p < 0.05) ratio of GCSA:GCV than hexaploids and dodecaploids (Figure 2d).
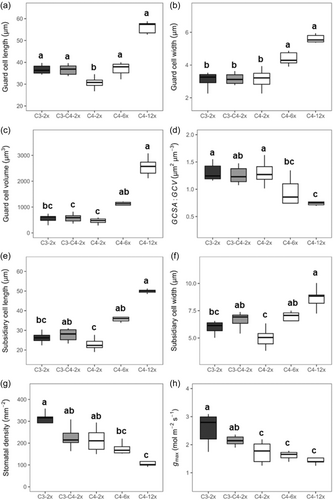
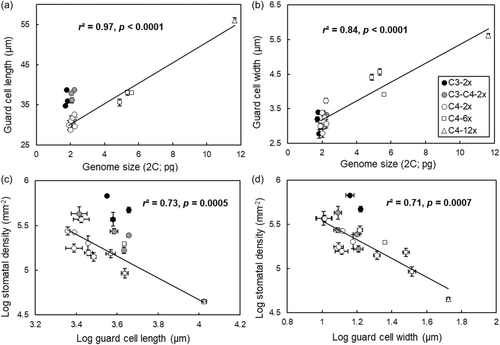
SCLs and widths in C4 diploids were both significantly lower (p < 0.05) than in C3–C4 diploids, C4 hexaploids and dodecaploids (Figure 2e,f). SD in C4 and non-C4 diploids was significantly higher (p < 0.05) than that in dodecaploids (Figure 2g). However, there were no significant differences in SD among the diploids with different photosynthetic types (Figure 2g). Calculated gmax based on stomatal size and density was significantly lower (p < 0.05) in C4 populations than in C3 and C3–C4 populations (Figure 2h). Compared to C3 diploids, the gmax value was reduced by 40% in C4 dodecaploids (Figure 2h). There was an offset in the log-log relationships between guard cell size and SD for C4 plants in comparison with non-C4 plants, particularly when guard cell size was measured via cell length (Figure 4c). However, the equivalent offset for the relationship with GCW was still able to distinguish C3 from the combination of C3–C4 and C4 types (Figure 4d).
3.2 Dynamic responses of photosynthesis and stomatal conductance to light
C3 and C4 individuals showed different stomatal responses to a step change in the light environment, especially in dynamic responses of gs (Figures 5 and S2–S4). For the dynamic responses of photosynthetic rate, the initial value of net CO2 assimilation rate (A0) at the initial PPFD of 100 µmol m−2 s−1 showed no significant variation, such that A0 was lower than 5 µmol m−2 s−1 for all groups (Figure 5a). The rapid rise in net leaf CO2 assimilation rate (A) in response to an increase in PPFD to 1000 µmol m−2 s−1 occurred within the first 10 min in all groups. However, it took double the time for A to reach a maximum steady state (As) in the C4 dodecaploids than in the others. As expected, C4 plants had higher As under high PPFD than C3 and C3–C4 intermediates plants (Figure 5a).
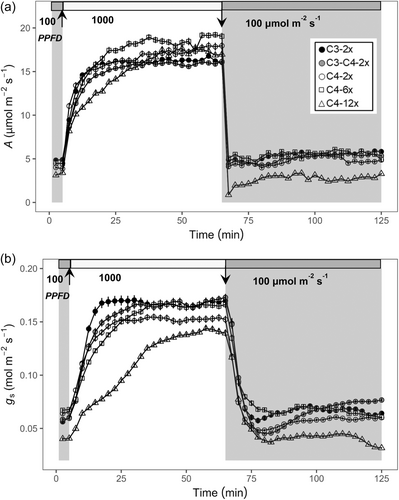
The stomatal opening phase was characterised by an increase in gs to a maximum steady-state value (Gs). The maximum rate of stomatal opening (Slmax_i) of C3 plants was the steepest, followed by C3–C4 intermediate plants, C4 diploid plants, C4 hexaploid plants, and C4 dodecaploid plants (Figure 5b; Table S3). In particular, the maximum slopes of C4 hexaploids and dodecaploids were significantly (p < 0.05) lower than those of C3 diploids (Table S3). The rates of stomatal opening also differed among C4 plants with differing ploidy levels (Figure 5b; Table S3). As expected, C4 diploids had a significant (p < 0.05) higher Slmax_i than C4 dodecaploids (Table S3). Fitted model parameters (for details, see Figure S1) describing the stomatal opening response showed significant differences (p < 0.05) in non-C4 plants compared to C4 dodecaploid plants (Table S3). In particular, C3 and C3–C4 diploids had significantly lower (p < 0.05) values of the opening time constant (ki), representing faster stomatal opening than dodecaploids (Table S3). To isolate the effects of photosynthetic pathway evolution from those of polyploidy, which only occurs in the C4 types, we made an ancestral state reconstruction of ki for the diploids only. This showed that the common ancestor of C4 A. semialata had a similar stomatal opening speed to that of C3–C4 plants (Figure 6 Node q vs. Node w; Table S3). In contrast, the common ancestor of C4 and C3–C4 A. semialata had a slower stomatal opening speed than that of C3 plants (Figure 6 Node p vs. Node z; Table S3).
On the other hand, stomatal closure in response to a decrease in PPFD from 1000 to 100 µmol m−2 s−1 followed a similar pattern in all groups (Figures 5 and S5), despite C4 dodecaploids displaying the lowest value of final steady-state A and gs (Figure 5). Compared to the speed of stomatal responses to increasing light conditions, the speed of stomatal closing was substantially faster. Nevertheless, its rapidity was still an order of magnitude slower than that of A. An almost immediate decrease of A to a new target of steady-state was established before gs reaching the final steady-state value.
3.3 Factors explaining variation in stomatal opening speed
In general, PGLS analysis showed relationships between cell size and parameters of stomatal opening responses in C4 plants (Figure 7). There was a significant (p < 0.05) positive relationship between the opening time constant (ki) and GCL, SCL or GCV across the C4 plants (Figure 7). Larger guard cells, subsidiary cells, and higher GCV were characterised by higher values of ki, corresponding to slower stomatal opening speed (Figure 7). For a given GCL, C4 hexaploid plants had a slower stomatal opening speed than C3 diploid plants (Figure 7a).
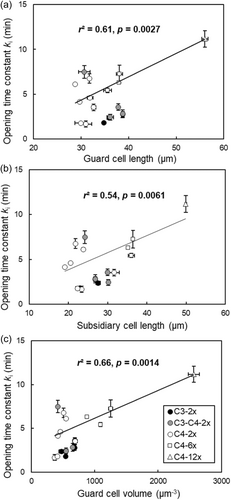
Besides the effects of guard cell size and photosynthetic pathway, local climates played an additional role in stomatal behaviour. Figures 6 and 8a showed significant variation (p < 0.05) among C4 diploids in the values of ki, which ranged fourfold from 1.7 to 6.8 min. To isolate the effects of environment from those of photosynthetic type and polyploidy, we therefore analysed the relationship of ki in C4 diploids to climate. Crucially, there was a significant (p < 0.05) negative relationship between ki and annual precipitation among the seven populations of C4 diploid plants (Figure 8a). This means that the speed of stomatal opening was faster in C4 diploids that have migrated into wetter environments. The opening lag time (λi) for stomatal opening was also significantly (p < 0.05) influenced by annual precipitation (Figure 8b). However, unlike the rapidity of stomatal opening, C4 diploids in the wetter environments had longer lag times in their responses to increasing light (Figure 8b).

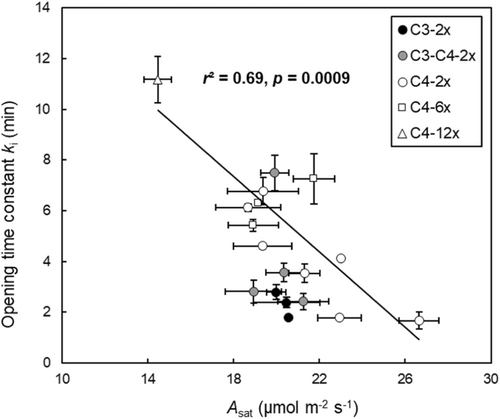
Finally, regressions of the light-saturated photosynthetic rate (Asat) versus opening time constant ki for A. semialata in C4 type showed that populations with higher values of Asat had a faster stomatal opening speed (Figure 9). In addition, C4 plants had slower opening stomata (higher ki) than C3 plants for a given photosynthetic rate (Figure 9).
4 DISCUSSION
4.1 Guard cells shortened following the evolution of C4 photosynthesis in diploids
The common ancestor of C4 A. semialata had shorter guard cells than C3–C4 or C3 plants (Figures 2a and 3), implying that the initial evolution of C4 photosynthesis led to a shortening of guard cells before diversification in this species. This finding, in conjunction with previous work showing that smaller guard cells for a given density is a general property of C4 grass stomata (Taylor et al., 2012), suggests that the size of guard cells is adjusted after the evolution of C4 photosynthesis, and indicates a close link between photosynthetic physiology and guard cell size. One of the potential explanations for this would be that the rapid light-induction of C4 photosynthesis requires faster stomatal opening, which is facilitated by smaller guard cells.
There is no coincident change in GCW during C4 evolution in A. semialata (Figures 2b and S6). Width changes after polyploid formation in C4 A. semialata show that these are possible if all cell types across the leaf are enlarged. However, within a ploidy level, width may be limited in these grasses by the development of stomatal complexes within ordered rows of cells (i.e., cell files), such that their proximity to adjacent rows constrains their width (McKown & Bergmann, 2020). GCW may be further limited by association with subsidiary cells, whose shape and signalling responses are highly coordinated with those of guard cells (Gray et al., 2020; McKown & Bergmann, 2018). However, SCW did decrease after the evolution of C4 photosynthesis (Figure 2f), showing that width changes are possible within stomatal complexes. This may be important, because subsidiary cells are thought to adjust the range of pore apertures and stomatal responsiveness (Raissig et al., 2017). In fact, subsidiary cells may be important regulators of the speeds of stomatal opening and closure in grasses (Chen et al., 2017; Raissig et al., 2017).
SD, on the other hand, shows considerable overlap among diploid populations of A. semialata. Even though the C3 populations have significantly higher SD than C4 hexaploid and dodecaploid populations (Figure 2g), two of the C3 populations have values that overlap with the C4 range (Figure 4c), showing that this trait is not directly associated with photosynthetic type in the same way as guard cell size (Figures S7–S9). More generally, variation among other species in stomatal size and density is inversely related, such that C4 species tend to have lower stomatal densities than C3 plants (McAusland et al., 2016; Taylor et al., 2012). Our data imply that this general difference in density arises as a secondary adaptation after the change in guard cell size.
Among the C4 populations of A. semialata that differ in ploidy levels, larger guard cells have a slower stomatal response to increasing light, which is consistent with previous studies of cell size effects on stomatal dynamics (Drake et al., 2013; Franks & Beerling, 2009; Hetherington & Woodward, 2003; Lawson & Blatt, 2014). The size relationship arises from a lower surface-area-to-volume ratio in larger guard cells, which limits the capacity of ion pumping and solute transport across the plasma membrane to increase cellular concentrations of osmoticants (Lawson & Blatt, 2014; Raven, 2014). Polyploid formation and its association with larger cells therefore leads directly to slower stomata. However, cell size is not the only determinant of stomatal opening and closing speeds, and other factors including guard cell shape, mechanical constraints and biochemical characteristics also play critical roles (Franks & Farquhar, 2007; McAusland et al., 2016; Ozeki et al., 2022; Raven, 2014).
If all else were equal, the evolution of shorter guard cells in C4 diploid populations of A. semialata would therefore be expected to increase both opening and closing speeds in response to fluctuating light. However, our data indicate that this is not the case, implicating further changes in stomatal biology during the transition to C4 photosynthesis.
4.2 Transition to C3–C4 intermediate physiology initially slows stomatal opening
Our data show that some of the C4 diploids have a similar stomatal opening speed to those of C3 and C3–C4 plants, such that there is no overall difference in speed among diploids with different photosynthetic types (Figure 6; Table S3). However, there is considerable variation in opening speed among the diploid C4 plants, despite limited variation in GCL or GCV (Figures 2a,d and 6). The evidence therefore shows a marked diversification of stomatal function in A. semialata following the evolution of C4 photosynthesis. But did the first C3–C4 or C4 plants in this species have stomata that opened at a slower or similar speed to those of their C3 sisters?
Ancestral state reconstruction of the opening time constant among diploids shows that the common ancestor of C4 and C3–C4 plants in our study had a slower opening speed than that of the C3 plants (Figures 6 and S10). Subsequent diversification within the C4 clade has been associated with mean annual rainfall (Figure 8). Ancestral state reconstruction shows that C4 physiology emerged once in A. semialata in a relatively dry climate (Figure 8). In summary, this evidence therefore indicates that the initial divergence between populations using C3 photosynthesis, from those in which carbon fixation occurred partially (C3–C4) or wholly (C4) via PEPC, was associated with a change in the stomatal opening speed (Figure 6). Subsequent diversification among C4 populations as they migrated across the paleo-tropics led to the repeated evolution of faster stomata in wetter climate regions (Figure 8a).
A potential mechanism underlying these changes in stomatal opening rate is implied by the relationship of ki to Asat. The best current models assume that stomatal opening speed is biochemically limited by the ATP supply from cyclic and noncyclic photosynthetic electron transport (Lawson & Blatt, 2014; Matthews et al., 2020; Ng & Jarvis, 1980; Raven, 2014). Bellasio et al. (2017) developed a dynamic hydro-mechanical and biochemical model which showed that a lag in ATP production may mechanistically account for both the lag phase of stomatal response to increasing light, and stomatal opening speed. A lowering of photosynthetic capacity and/or disruption of guard cell ATP supply during the emergence of C3–C4 and C4 photosynthesis are therefore plausible explanations for our observation of slower stomatal opening. The observation that the initial, maximum rates of stomatal opening are similar in C4 and non-C4 plants (Figures 5, S5, and S11; Table S3) support this explanation, implying that opening is limited by the supply of energy or solutes, rather than the ion pumping capacity of guard cells. An alternative explanation could be that the blue or red light sensitivity of stomatal opening are disrupted by C4 pathway evolution. However, new evidence suggests that these mechanisms are conserved across C3 and C4 types of A. semialata (Bernardo et al., 2023).
C4 photosynthesis is unlikely to be very efficient when it first evolves. In A. semialata, the process arises through a small number of changes to anatomy and biochemistry (Dunning, Moreno-Villena, et al., 2019; Lundgren et al., 2019). Lateral gene transfer subsequently contributes to more rapid improvements in the catalytic efficiency of key C4 enzymes, including PEPC (Phansopa et al., 2020). At the same time, leaf gas exchange is also likely optimised through the regulation of SD, and the formation and patterning of mesophyll airspace (Lundgren et al., 2019; Sugano et al., 2010). Our data imply that biochemical integration of stomatal function with C4 photosynthetic metabolism may be an important aspect of that optimisation process.
4.3 Adaptation to wetter climates accelerates stomatal opening speed in C4 plants
Guard cell size and photosynthetic type are the dominant effects on stomatal opening speed in A. semialata. However, within the C4 diploid type, the speed of stomatal responses to increasing light conditions differs significantly among populations (Figures 6 and 7), implying secondary adaptation of stomatal opening speed during diversification (Drake et al., 2013; Raven, 2014). This observation could be explained partly in the context of environmental characteristics across the geographical distribution of this species, with the stomatal opening time constant significantly related to annual precipitation in C4 diploids (Figure 8a). This shows that when A. semialata C4 plants have migrated into wetter habitats, their stomatal opening adapted by becoming faster. However, we also observed that the opening lag time was also significantly correlated with annual precipitation (Figure 8b), such that a longer lag time is coupled to a faster opening speed when C4 diploids migrate into wetter environments.
Xiong et al. (2018) showed that a terrestrial herb (Gossypium hirsutum) had slower stomatal opening than a wet habitat species (Centella asiatica). Slow stomatal opening during shade-sun transitions may transiently limit CO2 assimilation, if water is sufficient. The observed change in opening speed may serve as an adaptation to overcome this limitation in environmental contexts where water conservation is not critical. Conversely, the water-conserving effects of stomata that are not fully open may be more important for plant performance in drier habitats than transient CO2-limitation.
5 CONCLUSIONS
Our analysis suggests that the origin of C4 photosynthesis led to a reduction in GCL, but this shortening of guard cells in C4 plants occurred after a slowing of stomatal opening associated with the initial establishment of C3–C4 intermediate and then C4 metabolism. This result is inconsistent with the hypothesis that smaller guard cells arising from C4 pathway evolution have faster responses to light than in C3 plants, and shows that the picture is more nuanced than implied by previous work in crops. The association of stomatal opening speed with the rate of photosynthesis implies a disruption to guard cell energy supply during the establishment of C4 physiology. Stomatal opening was further slowed after polyploid formation, presumably by the enlargement of guard cells and the associated reduction in GCV. However, stomatal opening was accelerated in diploids by adaptation to wet climate conditions and the optimisation of C4 photosynthesis during the diversification of this species. Our work provides explanations for the diversity of stomatal function across the range of a single species, demonstrating the importance of an interplay between physiological innovation, cell structure and function in the evolutionary dynamics of stomatal behaviour.
ACKNOWLEDGEMENTS
We thank the China Scholarship Council (CSC) for financial support to Yanmin Zhou. We also thank Pascal-Antoine Christin for providing plant materials and a phylogenetic tree of A. semialata species, Matheus Bianconi for R code to extract the parameters of climate, Sahr Mian for genome size measurements, and Julie Gray and Pascal-Antoine Christin for their constructive comments on the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in Zenodo at https://zenodo.org/records/12743587




