Rice CRYPTOCHROME-INTERACTING BASIC HELIX-LOOP-HELIX 1-LIKE interacts with OsCRY2 and promotes flowering by upregulating Early heading date 1
Abstract
Flowering time is a crucial adaptive response to seasonal variation in plants and is regulated by environmental cues such as photoperiod and temperature. In this study, we demonstrated the regulatory function of rice CRYPTOCHROME-INTERACTING BASIC HELIX-LOOP-HELIX 1-LIKE (OsCIBL1) in flowering time. Overexpression of OsCIB1L promoted flowering, whereas the oscib1l knockout mutation did not alter flowering time independent of photoperiodic conditions. Cryptochromes (CRYs) are blue light photoreceptors that enable plants to sense photoperiodic changes. OsCIBL1 interacted with OsCRY2, a member of the rice CRY family (OsCRY1a, OsCRY1b, and OsCRY2), and bound to the Early heading date 1 (Ehd1) promoter, activating the rice-specific Ehd1-Heading date 3a/RICE FLOWERING LOCUS T 1 pathway for flowering induction. Dual-luciferase reporter assays showed that the OsCIBL1-OsCRY2 complex required blue light to induce Ehd1 transcription. Natural alleles resulting from nonsynonymous single nucleotide polymorphisms in OsCIB1L and OsCRY2 may contribute to the adaptive expansion of rice cultivation areas. These results expand our understanding of the molecular mechanisms controlling rice flowering and highlight the importance of blue light-responsive genes in the geographic distribution of rice.
1 INTRODUCTION
Flowering, an essential process for plant reproduction and survival, is influenced by both internal genetic factors and external cues such as photoperiod and temperature. Understanding flowering mechanisms is essential for successful plant reproduction in a world facing climate change. Changes in flowering time caused by fluctuations in temperature and day length can threaten the yield of food crops that support more than half of the world's population (Craufurd & Wheeler, 2009; Tun et al., 2021). A thorough understanding of flowering regulation is crucial for achieving agricultural success and protecting food security as the climate becomes increasingly unpredictable.
Photoperiod is a critical environmental cue that allows plants to perceive and adapt to environmental changes. Flowering plants are primarily classified into three categories based on their response to day length: long-day (LD) plants, which flower during longer day lengths; short-day (SD) plants, which respond to shorter day lengths; and day-neutral plants, which flower regardless of the photoperiod (Thomas & Vince-prue, 1997). Plants sense the photoperiod by detecting light signals through their photoreceptors. At least five classes of photoreceptors have been identified in higher plants: phytochromes, which perceive red/far-red light; cryptochromes (CRYs); phototropins; F-box proteins, which detect blue/UV-A light; and UVR8, which senses UV-B light (Bae & Choi, 2008).
CRYs are conserved across various evolutionary lineages ranging from microbes to plants and animals (Chaves et al., 2011; Mei & Dvornyk, 2015). Two AtCRYs (AtCRY1 and AtCRY2) have been identified in the facultative LD plant Arabidopsis thaliana. AtCRY1 primarily mediates de-etiolation under blue light, whereas AtCRY2 is involved in regulating photoperiodic flowering (Ahmad & Cashmore, 1993; Liu et al., 2008). AtCRY2 photoexcited by blue light stabilises the transcriptional activator CONSTANS (CO) and activates the basic helix-loop-helix (bHLH) transcription factor CRYPTOCHROME-INTERACTING bHLH1 (CIB1), thereby accelerating the transcription of the florigen gene FLOWERING LOCUS T (FT) (Liu et al., 2008, 2013, 2018; Wang et al., 2016). Three different CRYs, i.e., OsCRY1a, OsCRY1b, and OsCRY2, have been characterised in rice (Oryza sativa), a facultative SD plant (Matsumoto et al., 2003; Zhang et al., 2006). These photoreceptors play a critical role in mediating the plant response to blue light by suppressing the elongation of leaf sheaths and blades. However, only OsCRY2 has been implicated in promoting the flowering time in rice (Hirose et al., 2006; Singh et al., 2023a, 2023b). Reducing OsCRY2 expression in antisense transgenic plants resulted in delayed flowering under both SD and LD conditions. In contrast, transgenic Arabidopsis plants overexpressing OsiCRY2, the CRY2 homologue in indica rice, exhibit early flowering (Singh et al., 2023a). Recent research has also shown that OsCRY2 interacts with OsFBO10, which is orthologous to Arabidopsis FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (AtFKF1), and is involved in the regulation of photoperiodic flowering in rice. However, further studies are required to explore the detailed mechanisms underlying this interaction (Singh et al., 2023b).
CIBs play an important role in inducing flowering in response to blue light. For instance, Arabidopsis CIB (AtCIBs) promote flowering in conjunction with photo-excited AtCRY2 in Arabidopsis (Liu et al., 2008, 2013, 2018; Wang et al., 2016). Four members of the AtCIB gene family (AtCIB1, AtCIB2, AtCIB4, and AtCIB5) exhibit functional redundancies in regulating AtCRY2-dependent flowering (Liu et al., 2008, 2013). Although recombinant AtCIB1 protein binds to G-box sequences (CACGTG), heterodimerization with CIB-related proteins in vivo can recognise the E-box motif (CANNTG) (Liu et al., 2013). The AtCIB1-AtCRY2-CO complex accelerates FT transcription by directly binding to the E-box motif in the promoter region (Liu et al., 2018). The interaction between GmCIB1 and GmCRY2 regulates flowering and leaf senescence in soybeans (Glycine max) (Meng et al., 2013; Yang et al., 2015). AcCIB2 in pineapple (Ananas comosus) modulates flowering time and responds to abiotic stressors (Aslam et al., 2020).
Rice has a unique flowering pathway mediated by a B-type response regulator, Early heading date 1 (Ehd1). Ehd1 acts as an activator of rice florigens Heading date 3a (Hd3a) and RICE FLOWERING LOCUS T 1 (RFT1) under both LD and SD conditions (Doi et al., 2004). However, only a few floral regulators are involved in the blue light-induced activation of Ehd1 transcription. OsGI-dependent circadian regulation induces Ehd1 in response to blue light during the morning, regardless of the photoperiod (Itoh et al., 2010). Rice EARLY FLOWERING 3 (OsELF3-1) facilitates Ehd1 expression in response to blue light under SD conditions (Zhao et al., 2012). In addition, rice FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (OsFKF1) enhanced Ehd1 expression in response to blue light, thereby activating the floral initiation pathway (Han et al., 2015).
In our previous study, OsbHLH079 was identified as a key regulator of leaf angle and senescence in rice (Kim et al., 2023; Seo et al., 2020). In this study, we have delineated a functional parallelism between OsbHLH079 and its Arabidopsis counterpart, AtCIB1. Therefore, we named this rice as CRYPTOCHROME-INTERACTING BASIC HELIX-LOOP-HELIX 1-LIKE (OsCIB1L). Our results showed that OsCIB1L forms a complex with OsCRY2, regardless of light conditions. Overexpression of OsCIB1L resulted in early flowering, independent of the photoperiod, whereas oscib1l knockout mutants did not show any changes in flowering time. Furthermore, we discovered that OsCIB1L binds directly to the Ehd1 promoter containing the E-box motif, leading to activation of the Ehd1-Hd3a/RFT1 pathway. Dual-luciferase reporter assays showed that co-expression of OsCIB1L and OsCRY2, especially under blue light conditions, enhanced the induction of Ehd1 expression. In addition, the analysis of nonsynonymous single nucleotide polymorphisms (SNPs) in OsCIB1L and OsCRY2 implied that natural variations in these genes may contribute to the adaptive expansion of rice cultivation areas. These findings improve our understanding of how rice regulates flowering in response to blue light and highlight the importance of blue light-responsive genes in influencing the geographic distribution of rice.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Genetic information on the rice T-DNA insertion and transgenic plants has been described in our previous reports (Kim et al., 2023; Seo et al., 2020). The activation-tagged T-DNA insertion line, oscib1l-D (PFG_3A-01275), was obtained from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu/cgi-bin/RiceGE) (Jeon et al., 2000; Jeong et al., 2002). Transgenic plants overexpressing OsCIB1L (OsCIB1L-OE5 and OE12) were generated by inserting the full-length coding region of OsCIB1L into the pMDC32 vector containing the 35S promoter (Curtis & Grossniklaus, 2003; Kim et al., 2023; Seo et al., 2020). Genome-edited transgenic plants (oscib1l-1 and oscib1l-2) were generated using pOs-sgRNA entry and a pH-Ubi-cas9-7 destination vector containing a Cas9 expression cassette (Kim et al., 2023; Miao et al., 2013; Seo et al., 2020). The parental japonica cultivar “Dongjin” (hereafter referred to as WT) and transgenic plants were grown in a paddy field located in Suwon, South Korea (37° N latitude) under natural LD (NLD) conditions (>14 h sunlight/day). The rice plants were grown in growth chambers under LD (14.5 h light/9.5 h dark) or SD (10 h light/14 h dark) conditions. The growth chambers were maintained at 30°C during the day and 24°C during the night. The growth chambers utilised light-emitting diodes (LEDs) with an average photon flux density of around 300 µmol m−2s−1 as a light source. The heading date, also referred to as the flowering time, was determined as the duration between the sowing date and the emergence of the first panicle in the main culm.
2.2 Reverse transcription and quantitative PCR (RT-qPCR)
Rice leaves were collected every 3 h from 70-day-old plants grown under LD and 30-day-old plants grown under SD conditions to analyse the diurnal expression of flowering-time genes. The expression of flowering-time genes was measured weekly at zeitgeber (ZT) 1 in rice leaves under both LD and SD conditions. Total RNA was extracted from rice leaves using an MG RNA Extraction Kit following the manufacturer's instructions. Total RNA (2 μg) was used in a 25-µL volume with M-MLV reverse transcriptase and oligo(dT)15 primer and diluted with 75-µL water to synthesise first-strand cDNA. For qPCR reactions, 2 µL of the diluted first-strand cDNA mixture, 10 µL of 2 X Gotaq PCR Mix, and 2 µL of 10 pM primer pairs were prepared in a 20-µL volume. qPCR was performed on a LightCycler 2.0 instrument under the following conditions: 95°C for 2 min, followed by 42 cycles of 95°C for 5 s, 59°C for 15 s, and 72°C for 10 s, using a comparative critical threshold value (Caldana et al., 2007; Nolan et al., 2006; Wong & Medrano, 2005). OsUBQ5 was used as an internal control (Jain et al., 2006). Primer specificity was confirmed by amplification of a single sharp melting curve peak. The primers for the analysis of flowering time gene expression and their respective primers are listed in Table S1. This analysis was conducted using independent samples obtained from different tissues and, each experiment was repeated at least three times.
2.3 Bimolecular fluorescence complementation (BiFC) assays
The pCR8/GW/TOPO vector was used to clone full-length OsCIB1L and OsCRY2 cDNAs. These cDNAs were then subcloned into the BiFC Gateway vectors pSAT4-DEST-nEYFP-C1 (pE3136) and pSAT5-DEST-cEYFP-C1 (pE3130) (Citovsky et al., 2006), using Gateway LR Clonase II Enzyme Mix (Invitrogen). The two recombinant plasmids encoding the nYFP and cYFP fusions were mixed in equal parts and introduced into onion (Allium cepa) epidermal cell layers using a DNA particle delivery system. Transformed onion epidermal cells were incubated on MS plates at 25°C in the dark for 20 h. Yellow fluorescence was detected using a confocal laser scanning microscope. We subcloned the full-length coding sequences of OsCIB1L and OsCRY2 into pAM-PAT-35S:YFPn and pAM-PAT-35S:YFPc (Lefebvre et al., 2010), respectively, using the Gateway LR Clonase II Enzyme Mix to investigate the interaction between OsCIB1 and OsCRY2 in rice protoplasts. Recombinant plasmids encoding the nYFP and cYFP fusions were mixed at a 1:1 (w/w) ratio and transformed into rice protoplasts isolated from suspension-cultured rice cells using PEG-mediated transformation (Yoo et al., 2007). Fluorescence signals were observed using a Carl Zeiss Axioskop 2 confocal microscope and the ZEN image acquisition software (blue edition). YFP fluorescence was detected at an excitation wavelength of 513 nm and an emission wavelength range of 505–530 nm.
2.4 Co-immunoprecipitation (Co-IP) assays
For co-IP assays, we cloned OsCIB1L, OsCRY1a, OsCRY1b, and OsCRY2 into pGA3817 and pGA3818 vectors (Kim et al., 2009), respectively, resulting in pUbi:OsOsCIB1L-Myc, pUbi:OsCRY1a-HA, pUbi:OsCRY1b-HA, and pUbi:OsCRY2-HA. The fusion constructs were transfected into protoplasts isolated from rice suspension cells (Toriyama & Hinata, 1985) using polyethylene glycol (PEG)-mediated transfection (Yoo et al., 2007). The transfected protoplasts were then suspended in a protoplast incubation solution and kept in the dark. Following a 16-h incubation at room temperature, the transfected protoplasts were harvested and used for co-IP analysis. The protoplasts were then resuspended in IP buffer containing 75-mM NaCl, 50-mM Tris-HCl, pH 7.5, 5-mM EDTA, 1% Triton X-100, 1-mM dithiothreitol, 1-mM phenylmethanesulfonyl fluoride, 2-mM NaF, 20-µM MG132 and a 1:100 dilution of the complete Protease inhibitor cocktail. The samples were briefly vortexed and then centrifuged at 13 000 rpm for 5 min at 4°C. The supernatants were immunoprecipitated using 3 µL of anti-Myc agarose beads. Before the addition of anti-Myc agarose beads, 10% of the extract was set aside as the input control sample. The remainder of the extract was incubated with anti-Myc agarose beads at 4°C for 2 h. After three washes with a washing buffer, the protein-bound agarose beads were eluted using 20 µL of the IP buffer. The eluates were separated by electrophoresis on a 10% sodium dodecyl sulphate-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. Membranes were incubated with horseradish peroxidase-conjugated anti-Myc and anti-HA monoclonal antibodies. Proteins bound to the antibodies were detected using an ECL system, according to the manufacturer's instructions.
2.5 Yeast one-hybrid (Y1H) assays
The Y1H assays were performed according to the Yeast Protocol Handbook. The full-length cDNA of OsCIB1L was amplified through RT-PCR using gene-specific primers to generate the Gal4 activation domain (AD) constructs (Table S1) and cloned into the pGADT7 vector as prey. For the bait, several DNA fragments of putative target genes of OsCIB1L (Ehd1, DTH8, RFT1, and Hd3a) were amplified through genomic PCR using gene-specific primers (Table S1). These products were then inserted into the pLacZi vector for use as bait. The YM4271 yeast cell strain was used to introduce each bait–prey pair. The β-galactosidase (β-Gal) activity was measured by liquid assay using chlorophenol red β-d-galactopyranoside (CPRG). All procedures were performed in accordance with the yeast protocol handbook. The total activity was divided by the optical cell density to normalise the β-Gal activities. These experiments were repeated multiple times, each time with independent clones obtained from different yeast transformants.
2.6 Yeast two-hybrid (Y2H) assays
The Y2H assays were performed according to the Yeast Protocol Handbook. The full-length coding sequences of OsCIB1L and OsCRY2 were amplified from the first-strand WT cDNA library using gene-specific primers (Table S1) to generate Gal4 AD constructs. The OsbHLH140, OsbHLH085, and OsbHLH092 cDNAs were amplified from the first-strand WT cDNA library using gene-specific primers (Table S1) to generate the GAL4 DNA-binding domain (BD) constructs. The pair of constructs were co-transformed into yeast strain AH109 and the transformed colonies were cultured on selective media at 30°C for 2–3 days. The experiments were repeated multiple times, each time using independent clones obtained from different yeast transformants.
2.7 Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed according to previously described protocols with minor modifications (Haring et al., 2007; Saleh et al., 2008). Briefly, 1 g of rice leaves from transgenic plants expressing HA and OsCIB1L-HA was fixed with 1% (w/v) formaldehyde for 20 min under vacuum at room temperature and ground in liquid nitrogen. The nuclei were isolated, and the chromatin complexes were sonicated using Bioruptor II. An anti-Myc polyclonal antibody and protein A agarose beads were used for immunoprecipitation. After reverse cross-linking and protein digestion, DNA was purified using a QIAquick PCR Purification Kit. Finally, qPCR was performed using a LightCycler 2.0 instrument. OsUBQ5 served as the internal control. The gene-specific primers used are listed in Table S1.
2.8 Luciferase activity assays in rice protoplasts
The plasmid carrying the LUC reporter gene was constructed by inserting a fragment from the Ehd1 promoter region [−2351 to +19 bp from the transcription start site (TSS)] into the pJD301 vector that contains the LUC reporter gene at the C-terminus. The effector plasmid was generated by cloning the full-length coding sequence of OsCIB1L upstream of a sequence encoding a MYC-tag into the pGA3817 vector (Kim et al., 2009). The pEGB35S:Renilla:Tnos (GB0109) plasmid was used as the internal control. The reporter (2 μg), effector (4 μg), and internal control (1 μg) plasmids were cotransfected into rice protoplasts using PEG-mediated transfection (Yoo et al., 2007). Transfected protoplasts were suspended and incubated in the dark. LUC activity in each cell lysate was measured using a dual-luciferase reporter assay kit.
2.9 Phylogenetic analysis
To examine the phylogenetic relationship among AtCIB1 homologues in rice, we obtained the full-length protein sequences from the NCBI through Basic Local Alignment Search Tool analysis. The phylogenetic tree was constructed using MEGA X software (Kumar et al., 2018) by the maximum-likelihood method.
2.10 Identification of SNPs
We searched for SNPs in the coding sequences of OsCIB1L in a publicly available database to investigate the natural variations in OsCIB1L in cultivated rice. SNPs were analysed using data from the 3 K Rice Genome Project from the International Rice Genebank Collection Information System (IRGCIS) database (Mansueto et al., 2017; https://snp-seek.irri.org/). The japonica Nipponbare allele was used as the reference genome for SNP analysis. Flowering time data associated with the SNPs were obtained from the IRGCIS database.
2.11 Statistical analysis
All experiments were performed in triplicate. Data are expressed as means with standard deviation. Two-sided Student's t-tests were conducted to determine the statistical significance of differences between means with p-values < 0.05 being considered significant. One-way analysis of variance (ANOVA) with Duncan's least significant range test was used to test the statistical significance of the differences between more than two populations. All central values depicted in the figures represent the means of biological or experimental replicates. The box plots represent the distribution of flowering times (Spitzer et al., 2014; Williamson et al., 1989). In the box plots, the centre lines display the medians, and the X symbols indicate the mean values. The box limits indicate the 25th and 75th percentiles, as determined using the R software. The whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. The outliers are represented as dots above or below the whiskers in the box plot. Statistical analyses were conducted using the RStudio software (https://posit.co/products/open-source/rstudio/) (R version 3.6.1).
3 RESULTS
3.1 Overexpression of OsCIB1L causes photoperiod-insensitive early flowering in rice
Previous studies have demonstrated the possible roles of OsCIB1L (originally termed OsbHLH079) in plant development, particularly in the regulation of leaf angle and senescence (Kim et al., 2023; Seo et al., 2020). In this study, we examined the flowering time of transgenic rice plants overexpressing OsCIB1L in a paddy field in Suwon, South Korea (37° N latitude), under NLD conditions (14-h sunlight/day). The transcript levels of OsCIB1L were significantly higher in the plants generated by activation-tagged T-DNA insertion (oscib1l-D) or by ectopic expression of 35S:OsCIB1L (OsCIB1L-OE5 and OE12) than in their parental japonica cultivar “Dongjin” (WT) (Figure 1a). The rice plants overexpressing OsCIB1L exhibited earlier flowering (oscib1l-D, 105 days to heading [DTH]; OsCIB1L-OE5, 108 DTH; OsCIB1L-OE12, 105 DTH) than the WT (111 DTH) under NLD conditions (Figure 1b–d). Furthermore, oscib1l-D flowered approximately 20 and 10 days earlier (LD, 98 DTH; SD, 54 DTH) than WT (LD, 119 DTH; SD, 64 DTH) under both LD and SD conditions (Figure 1e,f). These results implied that OsCIB1L promotes flowering under both LD and SD conditions.
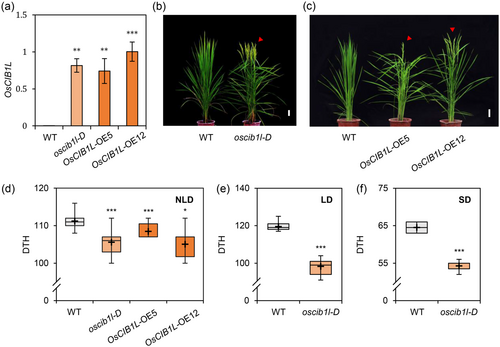
We measured the flowering times of the oscib1l-1 and oscib1l-2 mutants, which were generated through CRISPR/Cas9-mediated targeted mutagenesis, as previously reported (Kim et al., 2023) (Supporting Information S2: Figure S1a,b). The oscib1l mutants did not show any significant changes in flowering time compared to the WT plants. This suggested that the function of OsCIB1L in flowering may be redundant with other OsbHLH proteins (Supporting Information S2: Figure S1c,d). We identified six OsbHLHs with high homology to AtCIB1 using the NCBI database to explore the existence of redundant genes. The phylogenetic tree revealed that OsCIB1L and AtCIB1 are part of the same clade (Supporting Information S2: Figure S2). These included OsbHLH090, OsbHLH140, OsbHLH093, OsbHLH085, OsbHLH092, and OsbHLH089. We conducted a Y2H analysis to determine whether OsCIB1L physically interacts with OsbHLH proteins. Three of these (OsbHLH90, OsbHLH093, and OsbHLH089) were excluded because of their self-activation. Further, Y2H analysis revealed that OsCIB1L interacts with OsbHLH140, OsbHLH085, and OsbHLH092 (Supporting Information S2, Figure S3a). Notably, OsbHLH092 interacted with both OsCRY2 and OsCIB1L. In addition, we measured the transcript levels of three redundant genes (OsbHLH140, OsbHLH085, and OsbHLH092) in oscib1l mutants. However, there was no significant difference in the expression of these genes between the WT and oscib1l mutants (Supporting Information S2: Figure S3b). These findings demonstrated that OsCIB1L and its homologues contribute to photoperiod-insensitive early flowering in rice.
3.2 OsCIB1L interacts with OsCRY2
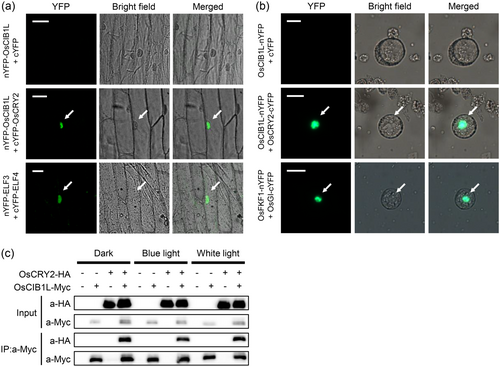
Comparative genome-wide analysis of bHLH between rice and Arabidopsis revealed that OsCIB1L is a homologue of AtCIB1 (Li et al., 2006; Seo et al., 2020). It has been hypothesised that OsCIB1L interacts with OsCRY2 to regulate flowering time, whereas AtCIB1 interacts with CRY2 to initiate flowering (Liu et al., 2008; Liu et al., 2013). BiFC assays were performed using onion (Allium cepa) epidermal cells to test this hypothesis. Cotransfection with nYFP-OsCIB1L and cYFP-OsCRY2 plasmids resulted in the reconstitution of YFP fluorescence in the nucleus (Figure 2a). This result was confirmed by transient expression of BiFC vectors in rice protoplasts isolated from suspension-cultured rice cells (Figure 2b). In addition, we assessed the diurnal expression patterns of OsCIB1L and OsCRY2 under both LD and SD conditions. The results indicated that the transcript levels of OsCIB1L and OsCRY2 were synchronised in LD and SD, with peaks between ZT1 and ZT4 (Supporting Information S2: Figure S4). These results suggested the possibility of a physical interaction between OsCIB1L and OsCRY2. Because the AtCIB1-AtCRY2 complex assembles in a blue-light-dependent manner, we conducted co-IP assays in rice protoplasts under different light conditions, including in the dark and under blue and white light. This revealed that the interaction between OsCIB1L and OsCRY2 was light-independent (Figure 2c). The rice genome harbours three cryptochrome genes, OsCRY1a, OsCRY1b, and OsCRY2, which are upregulated in response to blue light (Hirose et al., 2006). We investigated whether OsCIB1L interacted with OsCRY1a and/or OsCRY1b. co-IP assays revealed no interaction between OsCIB1L and either OsCRY1a or OsCRY1b (Supporting Information S2: Figure S5). Collectively, these results suggest that OsCIB1L interacts exclusively with OsCRY2 to form a complex under both light and dark conditions.
3.3 OsCIB1L promotes flowering through the Ehd1-Hd3a/RFT1 pathway
We examined the diurnal expression patterns of flowering time genes in both WT and oscib1l-D mutants to investigate the regulatory role of OsCIB1L in the floral induction pathway. RT-qPCR revealed higher transcript levels of the rice florigen genes Hd3a (Kojima et al., 2002) and RFT1 (Komiya et al., 2008) in oscib1l-D than in the WT, particularly around ZT1, under both LD and SD conditions (Figure 3a–d). Next, we analysed the expression of Early heading date 1 (Ehd1) (Doi et al., 2004) and Heading date 1 (Hd1) (Yano et al., 2000), which act as upstream regulators of florigens. Transcript levels of Ehd1 were significantly higher in oscib1l-D mutants near dawn (ZT1 in LDs and ZT22 in SDs) (Figure 3e,f). In contrast, the expression of Hd1 was similar in the WT and oscib1l-D (Figure 3g,h). These findings suggest that OsCIB1L is involved in the Ehd1-Hd3a/RFT1 flowering pathway.
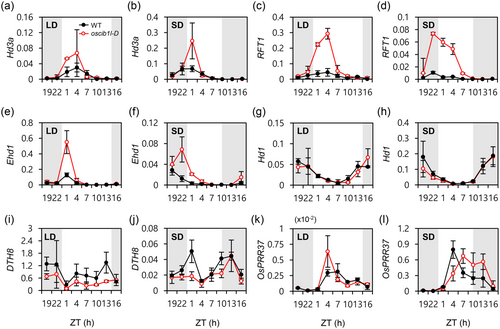
We monitored several upstream regulators of Ehd1, including positive regulators such as Ehd2, Ehd3, Ehd4, and OsMADS51 (Gao et al., 2013; Kim et al., 2007; Matsubara et al., 2008; Matsubara et al., 2011), and negative regulators such as Ghd7 (for grain number, plant height, and heading date), PSEUDO-RESPONSE REGULATOR 37 (OsPRR37) and DAYS TO HEADING 8 (DTH8) (Koo et al., 2013; Wei et al., 2010; Xue et al., 2008; Yan et al., 2011; Yan et al., 2013), to understand how OsCIB1L affects the Ehd1-mediated pathway. Our results showed a slight decrease in DTH8 mRNA levels in oscib1l-D plants compared to WT plants under both LD and SD conditions, as determined by RT-qPCR (Figure 3i,j). The transcript levels of the other regulators displayed similar patterns in WT and oscib1l-D under the same conditions (Figure 3k,l, Supporting Information S2: Figure S6a–j).
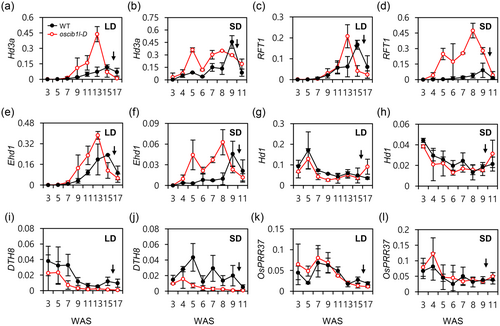
We measured the weekly expression patterns of flowering time genes in WT and oscib1l-D plants under LD and SD conditions until heading to further confirm the effects of OsCIB1L on flower induction. We examined the transcript levels of Hd3a, RFT1, Hd1, Ehd1, OsPRR37, and DTH8 in fully developed leaves harvested at ZT1. The oscib1l-D plants exhibited significantly higher expression levels of Hd3a, RFT1, and Ehd1 (Figure 4a–f) and lower expression levels of DTH8 compared to that of the WT (Figure 4i,j). Furthermore, the weekly expression of Hd1 and OsPRR37 in oscib1l-D was similar to that in the WT (Figure 4g,h,k,l). These findings suggested that OsCIB1L promotes flowering by inducing the Ehd1-Hd3a/RFT1 pathway by inhibiting DTH8 expression.
3.4 OsCIB1L binds directly to the promoter of Ehd1
AtCIB1 recognises G-box (CACGTG) and E-box motifs (CANNTG) (Liu et al., 2008; Liu et al., 2013). We performed Y1H assays to determine whether OsCIB1L binds to the promoter regions of DTH8, Ehd1, Hd3a, and RFT1 (Figure 5a). However, it did not bind to the promoter regions of DTH8, Hd3a, or RFT1, despite the presence of several E-box sequences. We found that OsCIB1L bound directly to a specific region of the Ehd1 promoter (pEhd1-2) containing the E-box motif (Figure 5b). We conducted a detailed sequence analysis of the E-box motifs present in the promoters of DTH8, Hd3a, and RFT1. Among the different types of E-box motifs (Supporting Information S3: Table S4), only the pEhd1-2 region harboured a CACCTG sequence. Therefore, OsCIB1L likely binds to the pEhd1-2 region of CACCTG. ChIP assays further confirmed that OsCIB1L binds to the Ehd1 promoter region (P2) with the E-box motif but not to other sections of the Ehd1 or DTH8 promoters (Figure 5c). These results indicated that OsCIB1L binds directly to the Ehd1 promoter, promoting the expression of Ehd1.
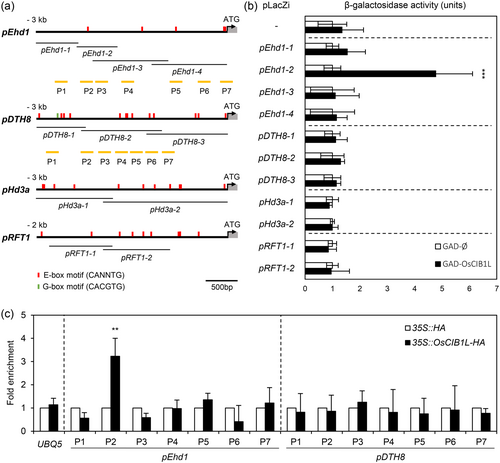
3.5 OsCIB1L and OsCRY2 upregulate Ehd1 in response to blue light
We conducted a dual-luciferase reporter assay using rice protoplasts to investigate whether OsCIB1L induced Ehd1 expression. The Ehd1 promoter (2-kb upstream of the TSS) was fused to the firefly luciferase reporter (LUC) gene (pEhd1:LUC; Figure 6a). Protoplasts transfected with the pEhd1:LUC plasmid exhibited a significant increase in LUC activity when cotransfected with the pUbi:OsCIB1L-Myc plasmid, compared to those transfected with the control pUbi:Myc vector (Figure 6b). This suggested that OsCIB1L functions as a transcriptional activator of Ehd1 by directly binding to its promoter.
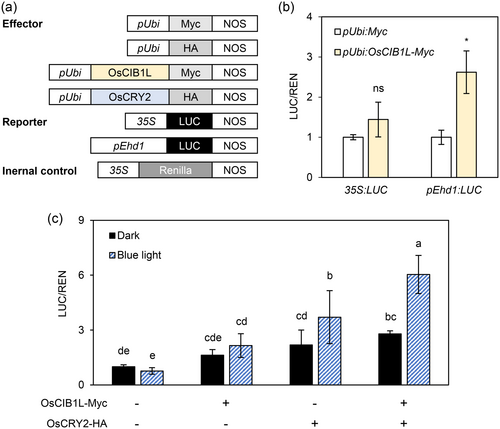
We assessed LUC activity in rice protoplasts containing various combinations of recombinant plasmids to determine whether the cotransfection of OsCIB1L and OsCRY2 enhanced Ehd1 expression in response to blue light (Figure 6c). Rice protoplasts were incubated under dark or blue light conditions. Under dark conditions, LUC activity driven by the pEhd1:LUC plasmid was significantly higher in rice protoplasts cotransfected with both pUbi:OsCIB1L-Myc and pUbi:OsCRY2-HA plasmids than in those infected with empty plasmids. Under blue light, rice protoplasts infected with either one or both plasmids showed elevated LUC activity compared to those infected with empty plasmids. Rice protoplasts incubated under blue light showed a significant increase in LUC activity compared to those incubated in the dark (Figure 6c). These results suggested that the OsCIB1L-OsCRY2 complex activates Ehd1 transcription in the presence of blue light.
3.6 Genetic diversity in OsCIB1L and OsCRY2 contributes to the expansion of rice cultivation regions
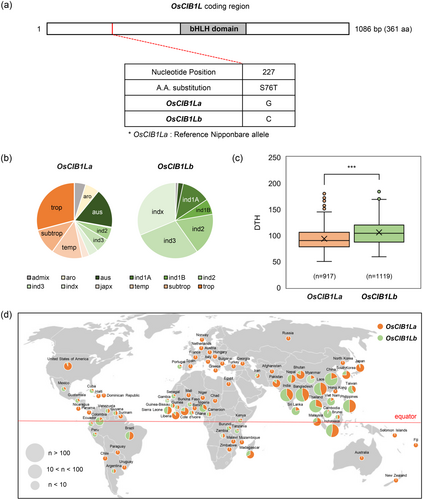
Rice varieties carry natural heading-related alleles that increase their regional adaptation (Lee et al., 2022). We examined SNPs in their coding sequences and flowering times across a data set of O. sativa varieties from IRGCIS (Mansueto et al., 2017) to investigate the potential natural variation in OsCIB1L among cultivated rice varieties. Among the 10 SNPs in 2036 rice varieties, one nonsynonymous SNP resulted in an amino acid substitution (Ser76Thr) (Supporting Information S2: Figure S7). This polymorphism distinguished two distinct haplotypes: OsCIB1La and OsCIB1Lb (Supporting Information S3: Table S2, Figure 7a). OsCIB1La and OsCIB1Lb were predominantly observed in japonica and indica, respectively (Figure 7b). We then examined the association between each OsCIB1L haplotype and flowering in 2,036 rice varieties obtained from the IRGCIS database. The results showed that rice varieties carrying the OsCIB1La haplotype exhibited earlier flowering (average 93 DTH) than those carrying the OsCIB1Lb haplotype (average 106 DTH) (Figure 7c). In addition, we surveyed the geographical distribution of OsCIB1L haplotypes. The Venn diagram, depicting a world map, revealed that rice varieties carrying the OsCIB1La haplotype were distributed across a wide range of regions, from low to high latitudes. In contrast, individuals carrying the OsCIB1Lb haplotype were more likely to be confined to the lower latitudinal zones around the equator (Figure 7d). These findings suggested that the genetic variation in OsCIB1L contributes to the global cultivation of rice.
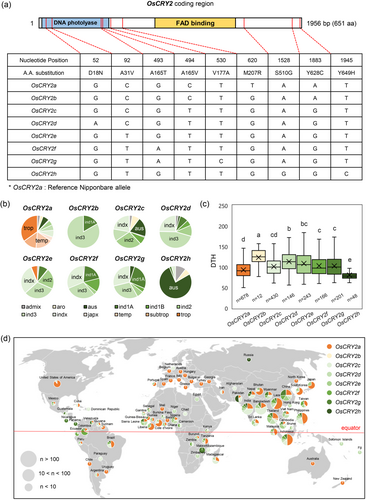
Having established the association between OsCIB1L and OsCRY2 in modulating flowering time, we investigated the effect of allelic variations in OsCRY2 on flowering. Our analysis identified eight nonsynonymous SNPs classified into nine haplotypes (OsCRY2a-h) (Supporting Information S3: Table S3, Figure 8a). The OsCRY2a haplotype is prevalent in most japonica varieties that exhibit early flowering. In contrast, the OsCRY2b-g haplotypes were mainly found in indica varieties, which exhibited delayed flowering compared with rice varieties harbouring the OsCRY2a haplotype (Figure 8b,c). The aus-type cultivars carrying the OsCRY2h haplotype exhibited extremely early flowering. Geographic distribution analysis showed that rice varieties carrying the OsCRY2a or OsCRY2h haplotypes were distributed across a wider range of regions, from low to high latitudes, compared to those carrying the OsCRY2b-g haplotypes, which were narrowly distributed in lower latitudinal zones around the equator (Figure 8d). It is widely accepted that Oryza rufipogon is the ancestor of cultivated rice (Londo et al., 2006). We also analysed SNPs in the coding regions of O. rufipogon CIB1L (OrCIB1L) and CRY2 (OrCRY2) using data from the Oryza Genome Database. Several SNPs were identified in O. rufipogon, including nine SNPs in OrCIB1L and 12 SNPs in OrCRY2. A small number of these SNPs, i.e., one in OsCIB1L and seven SNPs in OsCRY2, have been domesticated in O. sativa (Supporting Information S2: Figure S8). These findings suggest that natural allelic variations in OsCIB1L and OsCRY2 affect rice cultivation across different latitudes.
4 DISCUSSION
4.1 Rice has conserved blue light-mediated floral regulatory mechanisms
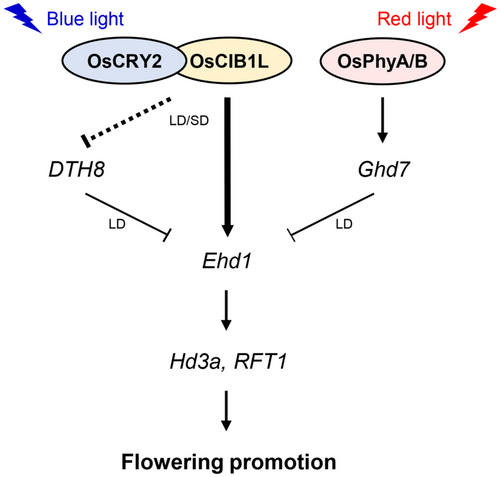
In the present study, we discovered that OsCIB1L forms a complex with OsCRY2 in rice, enhancing Ehd1 expression by binding to its E-box motif-containing promoter, which is accelerated by blue light. These regulatory mechanisms are specific to rice and differ from those observed in Arabidopsis. First, the AtCIB1-AtCRY2 complex in Arabidopsis directly binds to the FT promoter. However, the OsCIB1L-OsCRY2 complex associates with the Ehd1 promoter, activating the Ehd1-Hd3a/RFT1 pathway rather than the RFT1 or Hd3a promoter (Liu et al., 2008, 2013). Second, whereas AtCIB1 and GmCIB1 interacted exclusively with their respective CRY2 partners under blue light, OsCIB1L interacted with OsCRY2 under both light and dark conditions (Liu et al., 2008; Meng et al., 2013). However, the OsCIB1L-OsCRY2 complex depended on blue light to activate Ehd1 transcription (Figure 6). CRYs undergo conformational changes upon photoexcitation by blue light, facilitating interactions with other proteins and orchestrating the regulation of target gene expression (Palayam et al., 2021; Yu et al., 2007; Yu et al., 2009). For example, AtCRY1 and AtCRY2 can form dimers with CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) E3 ubiquitin ligase under both blue light and dark conditions. However, their interaction affinity increased only upon exposure to blue light (Ponnu et al., 2019; Wang et al., 2001; Yang et al., 2001). Similarly, it has been speculated that the activity of the OsCIB1L-OsCRY2 complex is induced upon exposure to blue light. This activation might enhance its binding affinity to the Ehd1 promoter or its interaction with other photoexcited proteins, thereby activating the Ehd1-Hd3a/RFT1 pathway. Third, the timing of the OsCIB1L-OsCRY2 complex in rice differed from that of the AtCIB1-AtCRY2 complex. The upregulation of florigens during the evening hours is important for controlling photoperiodic flowering under LD conditions in Arabidopsis. Thus, the AtCRY2-AtCIB1-CO complex forms in the late afternoon to activate FT transcription (Liu et al., 2018). In contrast, the morning expression of the florigen is emphasised in rice. Phytochromes, induced by red light, suppress Ehd1 expression by activating Ghd7 in the morning under LD conditions (Figure 9; Itoh et al., 2010; Nemoto et al., 2016). In this study, the OsCIB1L-OsCRY2 complex was observed in the morning when Ehd1 expression was upregulated (Figure 3, Supporting Information S2: Figure S4). In particular, in WT plants, the expression of OsCIB1L increased earlier under SD conditions compared to LD conditions (Supporting Information S2: Figure S4), subsequently leading to the early activation of the Ehd1-Hd3a/RFT1 pathway under SD conditions (Figure 3). Therefore, it was assumed that OsCIB1L fine-tuned Ehd1 expression in conjunction with phytochrome-induced inhibitory mechanisms during the morning (Figure 9).
The GmCIB1–GmCRY2a complex is involved in the senescence of soybean leaves. GmCIB1 physically interacts with GmCRY2a under blue light. However, photoexcited GmCRY2a inhibited the binding of GmCIB to the E-box of its target genes (Meng et al., 2013). This suggested that higher plants have evolutionarily conserved blue light-mediated regulatory mechanisms specific to plant species.
4.2 CIB proteins may exhibit redundancy in promoting rice flowering
The atcib1 atcib5 double mutant in Arabidopsis exhibited slightly earlier flowering than the wild type, whereas the atcib1 single mutant showed no change in flowering time (Liu et al., 2008). These genetic studies indicated that AtCIB1 shares functional redundancy with AtCIB2, AtCIB4, and AtCIB5 in the promotion FT expression (Liu et al., 2008, 2013). Moreover, bHLH proteins, such as AtCIB2, AtCIB4, and AtCIB5 interact with both AtCIB1 and AtCRY2 (Liu et al., 2013). In addition, AtCIB1 binds to both the canonical E-box (CACGTG, referred to as the G-box) and the noncanonical E-box (CANNTG). However, the binding affinity to the noncanonical E-box increased when AtCIB1 forms a heterodimer with other AtCIBs compared to the homodimerization of AtCIB1 (Liu et al., 2013). These results indicated that AtCIBs have redundant functions in the regulation of AtCRY2-dependent flowering.
Overexpression of OsCIB1L in rice leads to early flowering, whereas the oscib1l knockout had the same flowering time as the WT (Figure 1, Supporting Information S2: Figure S1). OsCIB1L physically interacted with OsbHLH140, OsbHLH085, and OsbHLH092. OsbHLH092 also interacted with OsCRY2 (Supporting Information S2: Figure S3a). Although genetic and molecular analyses are necessary to determine the functional redundancy of OsCIB1L, it is likely that OsCIB1L and its homologues form a complex with OsCRY2, facilitating blue-light-dependent Ehd1 activation and promoting flowering.
4.3 Natural variations in OsCIB1L and OsCRY2 could potentially enhance adaptability to diverse environmental regions
Photoperiod sensitivity is the most important factor affecting global rice production. In high-altitude regions, the rice cultivation period is limited by seasonal temperatures ranging from late spring to early autumn. For optimal development, rice must flower during the longer daylight conditions in summer, ensuring sufficient time for grain filling before the onset of cold weather (Fujino et al., 2019). Rice varieties cultivated in the northern regions flower early with weak or no photoperiod sensitivity, allowing rice production under NLD conditions in a short summer period (Fujino & Sekiguchi, 2005; Wei et al., 2008). Flowering plants often exhibit allelic variations in key genes that regulate flowering, which can significantly influence their ability to adapt to different environments. Utilising these natural genetic variations is pivotal in crop breeding to achieve stable yields through natural and artificial selection processes (Lin et al., 2021). The adaptation of rice to high-latitude regions has been facilitated by loss-of-function alleles in several flowering repressors such as Hd1, OsPRR37, Ghd7, and DTH8 (Fujino et al., 2013; Gao et al., 2014; Goretti et al., 2017; Takahashi et al., 2009; Zhang et al., 2015). These alleles confer photoperiod insensitivity and promote early flowering, which is essential for adaptation to the northernmost regions. In addition, strong alleles of positive floral regulators, such as OsCCA1, DTH2, and Ehd4, promote early flowering, which is advantageous for growth in high-latitude environments (Gao et al., 2013; Lee et al., 2022; Wu et al., 2013). Notably, our discovery of a single nonsynonymous SNP in OsCIB1L, resulting in two distinct haplotypes, supports this idea. Rice varieties carrying the japonica-type OsCIB1La allele displayed notably earlier flowering, which was more prevalent at higher latitudes. This indicated its potential as a strong functional variant for photoperiod-insensitive early flowering.
Previous studies have also demonstrated the impact of natural variations in Arabidopsis CRY2 on flowering and temperature sensitivity. El-Din El-Assal et al. (2001), Sanchez-Bermejo et al. (2015), and Wang et al. (2019) showed that a single amino acid change in CRY2 in Arabidopsis accessions from the tropical Cape Verde Islands (Cvi Arabidopsis) resulted in diminished temperature sensitivity and early flowering under SD conditions. These phenotypes were associated with consistently high CRY2 protein levels in the Cvi accession in contrast to the rhythmic expression observed in the Landsberg erecta (Ler) strain under SDs (El-Din El-Assal et al., 2001). Building on these findings, we explored the natural variations in OsCRY2 in rice and identified eight nonsynonymous SNPs and nine distinct haplotypes. These haplotypes exhibited a geographical distribution pattern between japonica and indica varieties that was linked to differences in flowering time. In a previous study, it was observed that OsCRY2 antisense transgenics exhibited delayed flowering compared to the WT (Hirose et al., 2006). This suggests that the japonica-type OsCRY2 allele (OsCRY2a) is functional for early flowering. In addition, the OsCRY2h haplotype, predominantly found in aus-type cultivars, is associated with extremely early flowering. These aus-type rice varieties, originating in India and Bangladesh, are known for their rapid life cycles (Fan et al., 2020; Kovach et al., 2009; Vaughan et al., 2008). Genetic variation in OsCRY2 has significantly contributed to the environmental adaptability of rice cultivation, as evidenced by its frequent discovery in high-altitude regions such as Pakistan, Nepal, and parts of Russia. Furthermore, aus-type varieties are known for their tolerance to extreme environmental conditions such as heat and drought stress. Therefore, the OsCRY2h haplotype holds great promise for future rice breeding programmes, particularly in the context of climate change (Casartelli et al., 2018; Goel et al., 2019; Liao et al., 2022).
Allelic variations in floral regulators have been shown to play a significant role in the distribution of cultivated rice, O. sativa, and its wild ancestor, O. rufipogon (Jing et al., 2018; Lee et al., 2022; Matsubara et al., 2012). For instance, rice varieties carrying strong functional alleles of OsCCA1, a positive floral regulator, exhibit photoperiod-insensitive early flowering, contributing to the northward expansion of rice cultivation. All OsCCA1 SNPs in O. sativa descended from those in O. rufipogon (Lee et al., 2022). Among the 9 SNPs in OrCIB1L and 12 SNPs in OrCRY2, 1 OrCIB1L SNP and 7 OrCRY2 SNPs were inherited from O. sativa (Supporting Information S2: Figure S8). This implied that the existing natural alleles of OsCIB1L and OsCRY2 in cultivated rice likely originated from the allelic pool of OrCIB1L and OrCRY2 in wild O. rufipogon. During the domestication and regional adaptation of cultivated rice, natural or artificial processes selected natural alleles of OsCIB1L and OsCRY2 that confer reduced photoperiod sensitivity.
ACKNOWLEDGEMENTS
We appreciate Dr. Gynheung An for providing the seeds of T-DNA mutants. This work was supported by the National Research Foundation of Korea (NRF) grants (2022R1A2C1091553 to Nam-Chon Paek), the Next-Generation BioGreen 21 Programme (PJ01365601 to Hyeryung Yoon), Rural Development Administration, Republic of Korea, and the Incheon National University Research Grant (20210311 to Kiyoon Kang).
ACCESSION NUMBERS
The gene sequences referenced in this article are available in the Rice Annotation Project Database and Resource (http://rice.plantbiology.msu.edu) under the following accession numbers: OsCIB1L, Os02g47660; OsCRY2, Os02g41550; OsbHLH090, Os01g68700; OsbHLH140, Os03g51910; OsbHLH093, Os04g28280; OsbHLH085, Os09g29830; OsbHLH092, Os09g32510; OsbHLH089, Os11g25560; OsCRY1a, Os02g36380; OsCRY1b, Os04g37920; Hd3a, Os06g06320; RFT1, Os06g06300; Hd1, Os06g16370; Ehd1, Os10g32600; OsPRR37, Os07g49460; DTH8, Os08g07740; Ehd2, Os10g28330; Ehd3, Os08g01420; Ehd4, Os03g02160; OsMADS51, Os01g69850.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




