Heat waves induce milkweed resistance to a specialist herbivore via increased toxicity and reduced nutrient content
Abstract
Over the last decade, a large effort has been made to understand how extreme climate events disrupt species interactions. Yet, it is unclear how these events affect plants and herbivores directly, via metabolic changes, and indirectly, via their subsequent altered interaction. We exposed common milkweed (Asclepias syriaca) and monarch caterpillars (Danaus plexippus) to control (26:14°C, day:night) or heat wave (HW) conditions (36:24°C, day:night) for 4 days and then moved each organism to a new control or HW partner to disentangle the direct and indirect effects of heat exposure on each organism. We found that the HW directly benefited plants in terms of growth and defence expression (increased latex exudation and total cardenolides) and insect her'bivores through faster larval development. Conversely, indirect HW effects caused both plant latex and total cardenolides to decrease after subsequent herbivory. Nonetheless, increasing trends of more toxic cardenolides and lower leaf nutritional quality after herbivory by HW caterpillars likely led to reduced plant damage compared to controls. Our findings reveal that indirect impacts of HWs may play a greater role in shaping plant-herbivore interactions via changes in key physiological traits, providing valuable understanding of how ecological interactions may proceed in a changing world.
1 INTRODUCTION
Climate change is altering patterns of temperature and precipitation in ways that influence plants (Hamann et al., 2021b), insects (Harvey et al., 2022), and their interactions (Chidawanyika et al., 2019; Hamann et al., 2021a; Root et al., 2003). A large body of work has now shown that gradual warming can influence plants and insects through changes in their growth rate, development, and phenology with cascading ecological impacts on the community and on agriculture (de Manincor et al., 2023; Deutsch et al., 2018; Kingsolver et al., 2013; Thakur, 2020). The frequency and intensity of extreme climate events, however, are also increasing with climate change (Lange et al., 2020; Laufkötter et al., 2020), and studies suggest they are likely to have major ecological consequences (Harvey et al., 2022; Smith, 2011; Thakur et al., 2022). For example, climate extremes, such as heat waves (HW, brief periods of abnormally high temperatures), may lead to rapid population declines within a season (Marchand et al., 2006; Seifert et al., 2015), with large-scale and long-term consequences for ecological communities (De Boeck et al., 2011; Piessens et al., 2009; Vasseur et al., 2014).
Most studies of the impacts of HWs, and climate change in general, on plant-insect interactions have examined the direct effects of heat on the physiology and performance of plants and insects separately (Colinet et al., 2015; Hamann et al., 2021b). This approach has provided a valuable, yet limited, understanding of how organismal responses to climate change may cascade to species interactions. A smaller number of studies have examined the effects of heat on plants and insects simultaneously while interacting (e.g., de Sassi & Tylianakis, 2012; Guyer et al., 2021; Havko et al., 2020b; Kharouba & Yang, 2021), revealing the ecological outcome but hampering our ability to tease apart the relative importance of the direct and indirect effects of heat on interacting organisms. As a result, a major gap in our understanding of the impacts of climate change on plant-insect interactions is the extent to which outcomes are determined by the direct effects of climate on the individual species versus the indirect effects through climate-derived changes in traits central to their interactions. One promising tool for disentangling the direct and indirect effects of extreme events on plant-insect interactions is reciprocal transplant experiments. These experiments have been the gold-standard approach for teasing apart genotype by environment interactions in plant local adaptation (Kawecki & Ebert, 2004), but they could also help teasing apart the direct and indirect effects of extreme events by pairing climate-stressed organisms with interacting species that have experienced control or similar stressful conditions.
Despite over 25 years of research on the effects of climate change on plant-herbivore interactions, the underlying mechanisms of organismal responses to climate change and how those responses shape interactions still remain poorly understood. Previous studies have shown that plant chemical investment (as a proxy for defence) and tissue quality may either increase (Couture et al., 2015; Dyer et al., 2013; Kivimäenpää et al., 2016) or decrease (Guyer et al., 2021; Jamieson et al., 2015; Zhao et al., 2016) in direct response to climate warming. In addition, plant responses to herbivory after warming were not only variable, but also showed distinct effects on herbivores (Cope et al., 2023; Guyer et al., 2021; Jamieson et al., 2015; Kuczyk et al., 2021). This inconsistency among studies may be due, in part, to a major functional gap. Plant traits often assumed as defensive also play other ecological roles apart from defence (e.g., many terpenoid and phenolic compounds) (Moore et al., 2014), and may therefore function as weak predictors of herbivore resistance. Also, changes in plant primary metabolism often alter tissue nutritional quality, impacting herbivory, but are often overlooked in the herbivory literature (but see e.g. Couture et al., 2015; Guyer et al., 2021; Wetzel et al., 2016). Thus, a stronger focus on how facets of climate change alter the underlying mechanisms of plant-herbivore interactions through functionally defensive plant chemistry, tissue quality relevant for herbivores and insect physiology is key to predict the fate of plant-herbivore interactions in the future.
Here we studied the direct and indirect effects of HWs on the specialised interaction between the monarch butterfly (Danaus plexippus) and its main host, common milkweed (Asclepias syriaca), by tracking both organismal performance and the traits relevant to their interaction. Milkweeds contain cardiac glycosides (i.e., cardenolides), which have an exclusively defensive function against herbivores, whereas monarchs evolved a suite of counteradaptations to circumvent those defences (Agrawal, 2017). Unlike more generalised systems, milkweed defences and monarch counterdefences are well matched due to their shared evolutionary history (Agrawal et al., 2021; Agrawal et al., 2024; López-Goldar et al., 2022), making them an ideal system to test hypotheses about the impacts of HWs on plant and insect traits that are strongly tied to their interaction (Agrawal, 2005). Briefly, we exposed common milkweed and monarch caterpillars to control (26:14°C, day:night) or simulated HW conditions (+10°C, 36:24°C, day:night) for 4 days in controlled conditions. Then, we reciprocally transplanted plants and caterpillars by reassigning each individual to a new partner from the same or different heat treatment for another 4 days in control conditions.
Previous work showed that elevated temperatures had an overall positive direct effect on plant and monarch performance separately, but effects of temperature on their performance during the interaction were positive or negative for the plant or the herbivore contingent on the milkweed species (Couture et al., 2015; Faldyn et al., 2018; Kharouba & Yang, 2021). These contrasting findings may be because elevated temperatures from average climate projections have likely had positive effects on some milkweed species (e.g., tropical milkweed) but not on others, with distinct consequences for the interaction. By using HW events—rather than average temperature increases—both plants and insects are expected to likely experience greater stress (Thakur et al., 2022). On the other hand, and more importantly, in these studies both organisms have been simultaneously tested in control (plant-insect: control-control) or elevated temperatures while interacting (heat-heat). Exploring interactions between plants and insects in a factorial design (e.g., control-heat and heat-control for plant-insect combinations) will be particularly informative to tease apart the relative importance of direct and indirect effects of drivers of climate change on species interactions.
We hypothesised that HW conditions affect plants and herbivores both directly via heat-induced physiological changes and indirectly via their subsequently altered interaction. Specifically, we aimed to answer the following questions: 1) How do HWs directly impact plants and their insect herbivores? We predicted that heat would directly benefit insect growth rate but would impose a stress on plant growth and defence; 2) How do indirect effects of HWs influence the outcome of plant-herbivore interactions? We predicted that the faster insect growth by heat would outweigh the defensive responses of both control and heat-stressed plants, whereas control insects encounter a window of opportunity for increasing their performance in heat-stressed compared to control plants.
2 METHODS
2.1 Plant and insect material
Common milkweed seeds were collected in the field at the Kellogg Biological Station (Hickory Corners; 42.41010932, −85.39122989) in November 2021 and stored at 4°C. In September 2023, seeds were surface sterilized with 10% bleach, rinsed, nicked, and left in the dark at 29°C for 5 days. Germinated seedlings were planted in a mix of 25% perlite, 75% Lamberts soil in 10 cm plastic pots, and grown in a growth chamber with 14 h daylight and 26:14°C day:night (control conditions) with a cover lid to keep high relative humidity. After a week, lid was removed and plants were randomly relocated every week between two identical growth chambers set at the same control conditions until they reached 8 weeks old. Plants were fertilized once with slow release fertilizer (Osmocote Pro® 19-5-8) 7 days after planting, once with dilute fertilizer [Peters Professional® N:P:K 20:20:20, 120 ppm N (μg·g−1)] applied at Day 15, and were watered as needed. Monarch eggs were collected from a colony of wild-caught butterflies from the same area as above and maintained at 25°C in the lab.
Our plant and insect material mimicked the natural phenology of milkweed-monarch interactions expected in nature for the region and occurs during a time when heat waves are likely. We also avoided potential plant flowering responses interfering in our study since common milkweed does not flower after one or 2 years when grown from seed.
2.2 Heat wave and herbivory treatments
We randomly assigned 102 8-week-old plants across two growth chambers (51 plants each) initially set to control conditions (see above). Before starting the experiment, we measured plant heights from the lowest leaf node carrying the cotyledons to the highest node carrying the newest emerging leaves. Then, one monarch neonate caterpillar was put on each of the 26 plants, randomized in each growth chamber. The remaining 25 plants were not challenged with the herbivore. Immediately after, plants from both chambers were watered to field capacity to avoid confounding effects of drought due to heat conditions. Subsequently, one growth chamber was set to +10°C heat wave (HW) temperatures (36:24°C day:night with a 3-h ramp at each transition) for 4 days, while the other chamber was left at control conditions. We started the HW treatment at 19:00 h to reduce the potential for sudden heat shock.
After the 4-day heat treatment, both chambers were returned to control conditions. Monarch caterpillars were removed from the plants, weighed, and were returned later (see below). Six control and six HW caterpillars were frozen at −80° C after weighing for subsequent related work (López-Goldar et al. unpublished). Plant height was measured as above on all individuals after the HW treatment (Day-4 height). Then, damaged plants from each growth chamber were examined for herbivore damage whereas undamaged ones remained untouched to use later (see below). Herbivory damage (mm2 of leaf area removed) was visually estimated using calibrated area templates for all leaves, and separated by new, young, and old leaves (Supporting Information S1: Figure S1). Latex of damaged plants was collected from cutting the petioles of the two youngest, fully expanded leaves (Supporting Information S1: Figure S1) in pre-weighed microtubes and immediately weighed. Then, the two leaves were then flash-frozen in liquid N and stored at −80°C until they were freeze-dried for chemical analysis of cardenolides (see below).
The remaining undamaged plants and caterpillars were randomly shuffled to create all four possible plant-insect treatment combinations (n = 10 each): control insect on a control plant, HW insect on a control plant, control insect on a HW plant, and HW insect on a HW plant. We let the plants and insects interact for 4 additional days. For both first and second 4-day periods, we checked the caterpillars every day. During the second 4-day period, some caterpillars went missing from their host plant, therefore slightly reducing sample sizes (n = 6–8). After this period, monarch caterpillars were weighed, final plant height (Day 8) and herbivore damage were measured as above, and we collected latex and the youngest fully expanded leaves for cardenolide analysis from all plants as above.
2.3 Plant cardenolide analysis by HPLC
Cardenolides were extracted, detected, identified and quantified as in López-Goldar and Agrawal (2023) with slight modifications. Cardenolides were extracted from each leaf sample by adding 1 mL of 100% methanol (spiked with 20 μg of digitoxin (Sigma #D5878) as internal standard in our case) to 50 mg of ground material and 20 FastPrep beads. Samples were extracted by agitation on a FastPrep-24 homogenizer twice for 45 s at 6.5 m s−1, and then centrifuged at 14000 rpm for 12 min. Supernatants were dried down in a vacuum concentrator at 35°C, resuspended in 250 μL of 16:16:68 (%) methanol:acetonitrile:water (vol:vol:vol), and filtered using 0.45 μm hydrophilic membranes.
We detected and quantified leaf cardenolides using an Agilent 1100 HPLC coupled to a diode array detector and a Gemini C18 reversed-phase column (3 um, 150 mm x 4.6 mm column, Phenomenex, Torrance, CA). We injected 15 μL of each sample into the HPLC at a constant flow of 0.7 mL min−1 with a gradient of acetonitrile (A) and water as follows: 0–2 min at 16% A (84% B); 2–25 min from 16% to 70% A; 25–30 min from 70% to 95% A; 30–35 min at 95% A; followed by 10 min reconditioning back at 16% A. Peaks were recorded at 218 nm and absorbance spectra were measured between 200 and 400 nm. Cardenolides were identified from a characteristic single absorption maximum between 214 and 222 nm in the peak's UV spectrum, corresponding to the unsaturated lactone ring indicative of cardenolides (including the internal standard digitoxin). Individual cardenolide concentrations in each sample were estimated by using the peak area and known concentration of the internal standard on a dry mass basis of leaf tissue (mg g−1 d.w.).
For each sample, we calculated the number of distinct cardenolide peaks (cardenolide richness), the total concentration of cardenolides, and the cardenolide non-polarity. We calculated cardenolide non-polarity using P = sum(piTi), where Ti is the retention time of the ith cardenolide weighted by its proportion (pi) within a sample, following (Rasmann & Agrawal, 2011). Higher non-polarity values represent greater proportion of less polar compounds, which generally have greater toxicity against milkweed herbivore specialists (Agrawal et al., 2021; Agrawal et al., 2022). These compounds have shown higher affinity and inhibit more strongly the enzymatic activity of their physiological target, the Na + /K + -ATPase (i.e., sodium pump enzyme), of adapted insects like the monarch (Agrawal & Hastings, 2023; Agrawal et al., 2021; Agrawal et al., 2022). We excluded cardenolides not present in at least 25% of the total samples from the analyses (9 of 23 compounds) due to lack of representation across treatment combinations.
2.4 Plant nutrient analysis
We extracted amino acids from each leaf sample by adding 400 μL of deionized water containing 13C,15N stable isotope-labeled amino acid internal standards (Millipore Sigma, Burlington, MA) at 100 µM to 20 mg of ground dry material. Incubation at 90°C for 5 min, followed by rapid cooling on ice, was performed before centrifugation at 13,000 g for 5 min. Extracted free amino acids were then mixed with an equal volume of 20 mM perfluoroheptanoic acid (PFHA) in water for improved chromatographic separation. Detection and quantification were carried out at the Michigan State University Mass Spectrometry Facility using Ultrahigh Pressure Liquid Chromatography (UPLC) coupled to a triple quadrupole mass analyser (Waters Quattro Micro), equipped with an Acquity UPLC HSS T3 column (2.1 × 100 mm, 1.7 µm particle size, Waters). We injected 10 μL of each sample into the UPLC at a constant flow of 0.3 mL min−1 with a gradient of 10 mM PFHA (A) and acetonitrile as follows: 0–1 min at 100% A (0% B); 1–8 min from 100% to 35% A; 8–8.01 min from 35% to 10% A; 8.01-9 min at 10% A; 9–9.01 min from 10% to 100% A; 9.01–13 min at 100% A. Metabolites were ionized by electrospray ionization in positive ion mode with a capillary voltage of 1.0 kV. Source temperature was 120°C, desolvation temperature was 350°C and desolvation and cone gas flows were 800 L/h and 40 L/h, respectively.
MS/MS data were obtained using a multiple reaction monitoring method (Supporting Information S1: Tables S1–S3) and processed using the TargetLynx tool in MassLynx software from Waters. We estimated amino acid concentrations from peak areas normalized to labeled internal standards using Quanlynx software (Waters) on a leaf dry mass basis (mmol·mg−1 dw leaf tissue). Quality control measures included avoiding multiple freeze/thaw cycles of standards, and aliquots of the standard curve samples (100 µL each) were used only once. We estimated total amino acid concentration and total dietary essential amino acid concentration for insects from the individual concentrations of the 20 free amino acids quantified. Essential amino acids for animals are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine (Wu & Li, 2022).
2.5 Statistical analyses
We used linear models in PROC MIXED (SAS v9.4) to examine the effects of the treatments on plant and insect responses 1) immediately after the 4-day HW treatment and 2) for the subsequent 4 days after the organisms were reciprocally transplanted and returned to control conditions.
For the analysis of the first 4 days, we examined the effects of HW, Herbivory (damaged or no herbivore), and their interaction (fixed) on plant relative growth rate (using Day-4 and Day-0 heights of all plants). Plant relative growth rate was estimated as ln(final height)—ln(initial height), divided by the 4 days of the duration of the HW. We also examined the effect of the HW treatment on insect mass, latex exudation, total cardenolide concentration, cardenolide polarity, total and essential amino acids only in damaged plants by insects. Heterogeneous variance models for factor HW were used for insect mass, cardenolide polarity, total and essential amino acids. Plant damage was partitioned in a repeated measures model, in which HW, Leaf age (new, young, old) and their interaction were fixed factors, and the same individual plant was included as subject. Leaf age was the repeated measure and, since closer leaves (e.g., new and young; young and old) are expected to be more similar than distant leaves (e.g., new and old) in the plant, an autoregressive variance structure was fit using the ‘TYPE = AR(1)’ option in the REPEATED statement. Variables were log- or square root-transformed to meet assumptions of normality using Shapiro-Wilk tests. Day-0 plant height showed no differences for any factor (HW: F1, 95 = 0.13, p = 0.717; Herbivory: F1, 95 = 0.03, p = 0.866; HW × Herbivory: F1, 95 = 0.04, p = 0.842), indicating that treatment groups were similar in height before the experiment.
For the analysis of Days 4–8 (post HW treatment and reciprocal transplant), we examined the effects of plant treatment (control or HW), caterpillar treatment (no herbivore, control caterpillar, or HW caterpillar) and their interaction on plant relative growth rate (for the 4 days post HW), exuded fresh latex, individual and total cardenolide concentrations, cardenolide polarity, total and essential amino acids, and insect mass (difference between Day 8 mass and Day 4 mass). Plant damage was partitioned as above in a repeated measures model, in which plant, caterpillar, leaf age and their two- and three-way interactions as fixed factors, and plant individual as subject. Heterogeneous variance models for factor HW were used for individual cardenolides 7.4 and 16.2. Variables were log-, square root- or cubic root-transformed to meet normality assumptions using Shapiro-Wilk tests.
All analyses were followed by specific contrast tests using Fisher's LSD to compare differences between the levels of each factor.
2.6 Multivariate analysis of plant chemistry
We analysed the multivariate changes in plant cardenolide chemistry due to the heat treatment and herbivory by the different caterpillars (control and HW) after Days 4–8 post HW treatment using permutational multivariate analysis of variance (PERMANOVA) in R v4.3.0 [R Core Team, (Oksanen et al., 2022)]. We first transformed the cardenolide matrix into frequencies and used Bray-Curtis dissimilarity. We then tested the effects of the HW, caterpillar treatment and their interaction on the plant using PERMANOVA with 999 permutations (adonis2). To further understand specific differences between the effects of the HW, the different caterpillars, and their interaction on the plant chemistry, we conducted a post-hoc multivariate pairwise comparisons for each of the factors tested [pairwise. adonis in package pairwiseAdonis, (Martínez-Arbizu, 2017)].
2.7 Path analysis
We conducted a path analysis to test the hypothesis that the effects of plant and insect HW treatments on insect damage were mediated indirectly through the effects of heat on changes in plant and insect traits using PROC CALIS and the RAM statement in SAS 9.4. We restricted the analysis to the second 4-day period, where plants and insects previously exposed to heat treatment were factorially combined to infer indirect effects of heat on their interaction through changes in their traits. We excluded caterpillar mass from the analysis since it did not change during the interaction with the plant in this period (see Results). Thus, we used heat treatment for the plant and insect as extrinsic variables, and plant total cardenolides, plant essential amino acids and insect damage as intrinsic variables, and all were transformed as above to achieve normality prior the analysis.
3 RESULTS
3.1 Plant and insect responses during the 4-day heat wave treatment
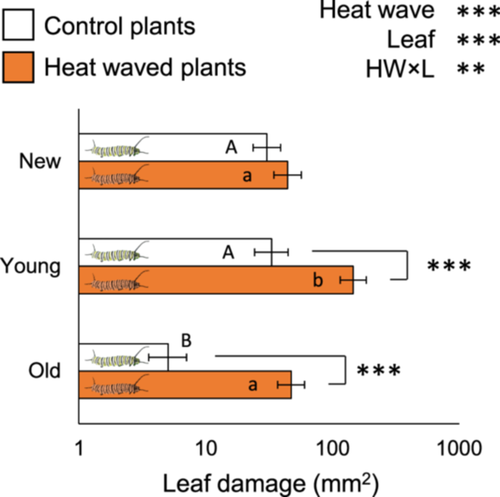
The HW directly caused monarch caterpillars to grow larger through greater leaf consumption. At the end of the 4-day HW, monarch caterpillars under HW conditions had consumed 3.8-fold more leaf tissue (F1, 47 = 47.76, p < 0.001) and grew 5.8-fold larger (F1, 45 = 128.6, p < 0.001) compared to controls. In addition to overall increased consumption, our HW treatment caused caterpillars to shift patterns of leaf feeding within plants (HW×Leaf age interaction, F2, 70 = 6.1, p = 0.004). Compared to control caterpillars, HW caterpillars ate 9.3-fold more from old leaves (p < 0.001) and 4.4-fold more from young leaves (p < 0.001) but ate similar amounts from new leaves (p = 0.289) (Figure 1).
The HW directly favored plant growth, but only in absence of herbivory. Plant relative growth rate depended on an interaction between the HW and herbivory treatments (HW × Herbivory, F1,86 = 15.96, p < 0.001) (Supporting Information S1: Figure S2a). In the absence of herbivory, HW conditions increased plant relative growth rate by 216% (p < 0.001), but for plants exposed to herbivory, the HW increased plant relative growth rate by only 13% (p = 0.374), suggesting that herbivory antagonizes the growth response of the plants to high temperature. Plants exposed to herbivores in control and HW treatments did not differ in their latex exudation (F1, 50 = 0.41, p = 0.526), cardenolide concentrations (F1, 48 = 0, p = 0.987), cardenolide polarity (F1, 48 = 0.09, p = 0.770), total or essential amino acids (F1, 48 = 1.86, p = 0.179 and F1, 48 = 2.28, p = 0.138, respectively) at the end of the 4-day period, suggesting that the effect of heat on insect growth and consumption was not indirectly mediated through changes in plant defences or tissue nutritional quality during the HW treatment.
3.2 Plant and insect responses 4 days after the heat wave treatment
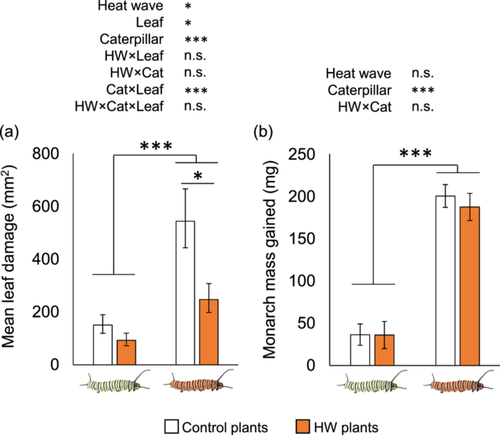
Damage caused, but not insect performance, was indirectly impacted by prior heat exposure of the plant and the insect. In the 4 days after the end of the HW treatment and after reciprocally reassigning plants and caterpillars, HW caterpillars consumed 3.1-fold more leaf tissue (F1, 24 = 24.6, p < 0.001) and grew 5.3-fold more (F1, 21 = 115.07, p < 0.001) than controls (Figure 2a). However, there was no overall effect of the past HW status of the plant on insect size (F1, 21 = 0.32, p = 0.651), nor was there a Plant×Caterpillar type interaction (F1, 21 = 0.18, p = 0.673) (Figure 2b). Leaf damage was on average greater in controls compared to HW plants (F1, 24 = 7.79, p = 0.01). Although feeding patterns across leaves were similar between Plant heat treatments for each Caterpillar type (Plant × Caterpillar × Leaf, F1, 24 = 0.64, p = 0.538), HW caterpillars fed 20-fold more on old leaves compared to controls in both plant heat treatments (p < 0.001; Supporting Information S1: Figure S3).
Plant responses to herbivory were trait-specific and indirectly influenced by the prior heat treatment experienced by the plant and the insect, especially plant defensive traits. The strong, direct effect of the HW on the relative growth rate of undamaged plants during the 4-day heat treatment did not continue into Days 4–8 (HW, F1, 38 = 1.06, p = 0.309), and was not affected by the herbivory treatment (HW × Herbivory, F2, 38 = 0.41, p = 0.668) (Supporting Information S1: Figure S2b).
In contrast to the lack of differences in growth, we saw strong interactive effects of the past plant and caterpillar heat treatments on plant defensive and nutritive traits. Latex exudation increased in control plants but decreased in HW plants upon herbivory, with larger effects for the larger, HW caterpillars than the smaller, control caterpillars (Plant×Caterpillar interaction F2, 41 = 3.72, p = 0.033). Specifically, latex exudation was 1.45-fold greater in undamaged HW plants compared to undamaged controls (although not significant, p = 0.092), similar between HW and control plants upon herbivory by control caterpillars, and 50% lower in damaged HW plants compared to damaged controls upon herbivory by HW caterpillars (p = 0.041) (Figure 3a). Latex exudation did not change overall between plant (F1, 41 = 0.25, p = 0.621) or caterpillar heat treatments (F2, 41 = 0.63, p = 0.538).
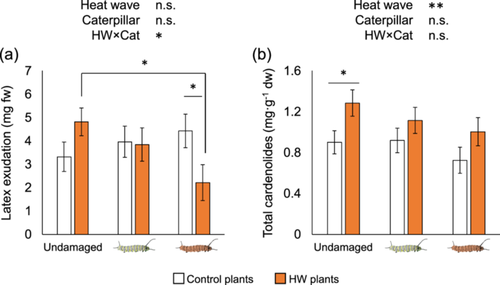
The multivariate patterns of plant cardenolide composition differed between control and HW plants (F1, 38 = 2.03, p = 0.025) and marginally by caterpillar treatment (F2, 38 = 1.57, p = 0.051), but not by their interaction (F2, 38 = 0.5214, p = 0.976). Feeding by the larger HW caterpillars caused strong changes in the multivariate cardenolide composition relative to plants with control caterpillars (p = 0.02) and undamaged plants (p = 0.04). In contrast, no changes were observed from feeding by control caterpillars compared to undamaged plants (p = 0.895), indicating that plants are more sensitive to damage caused by HW caterpillars. The effect of the HW on the multivariate cardenolide chemistry was reflected in an overall increase of total cardenolide concentrations in HW plants (Plant, F1, 38 = 7.71, p = 0.009), in which undamaged HW plants showed significant 1.43-fold greater concentrations than undamaged control plants (p = 0.031). We observed a decreasing trend of cardenolide concentrations in HW plants upon herbivory in a damage-dependent manner (Figure 3b) similarly as it occurred with latex exudation for HW plants, in which a 22% reduction was found in plants damaged by the larger, HW caterpillars compared to undamaged plants, although this was not significant (p = 0.145).
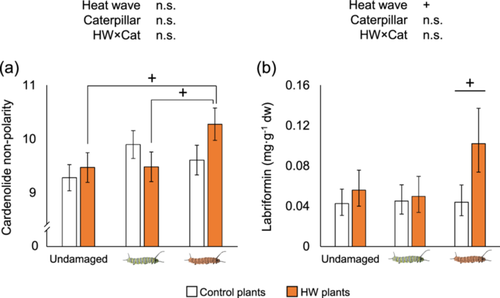
Cardenolide non-polarity did not vary due to plant (F1, 39 = 0.91, p = 0.347) or caterpillar treatments (F2, 39 = 1.39, p = 0.261), and we found no overall plant×caterpillar interaction (F2, 39 = 2.55, p = 0.091). Pairwise comparisons, however, suggest increasing trends of cardenolide non-polarity (i.e., increase the relative proportion of less polar, more toxic compounds) by 8% in HW plants after herbivory by HW caterpillars compared to undamaged HW plants (p = 0.056) and by 6% compared to HW plants damaged by control caterpillars (6%, p = 0.059) (Figure 4a). This trend seems to be explained by shifts in the chemical profile of HW plants after herbivory by HW caterpillars, for which we observed a decrease in some individual early eluting, polar toxins and an increase of late eluting, less polar compounds, respectively (Supporting Information S1: Figure S4). Among the less polar, more toxic compounds, the cardenolide labriformin (retention time 15.9, Supporting Information S1: Figure S4) showed a 130% increase in HW plants compared to control plants in response to HW caterpillars (p = 0.078, Figure 4b).
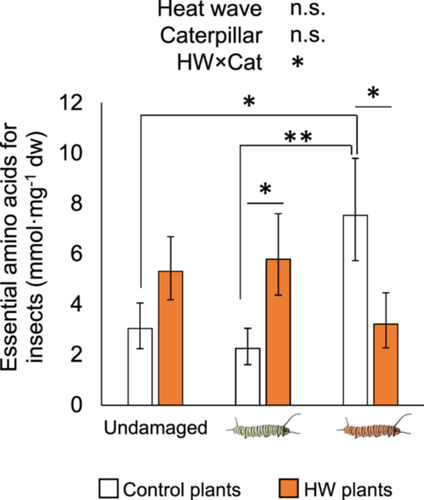
Essential amino acids in leaf tissue depended on an interaction between the past plant and caterpillar heat treatments (F2, 40 = 5.09, p = 0.011), though they did not change with plant (F1, 40 = 0.73, p = 0.397) or caterpillar treatments by themselves (F1, 40 = 0.56, p = 0.578). Specifically, a significant increase of 157% for essential amino acids in HW versus control damaged plants was found in response to small control caterpillars, whereas a significant 133% increase in control versus HW damaged plants was observed in response to large HW caterpillars (Figure 5). In contrast, total amino acids did not change due to plant (F1, 40 = 0.04, p = 0.837) or caterpillar heat treatments (F2, 40 = 0.80, p = 0.455), nor plant×caterpillar interaction (F2, 40 = 2.91, p = 0.066). This suggests that changes in plant tissue quality in response to herbivory after a HW event seem more relevant for those essential nutrients that insects can obtain only from their diets.
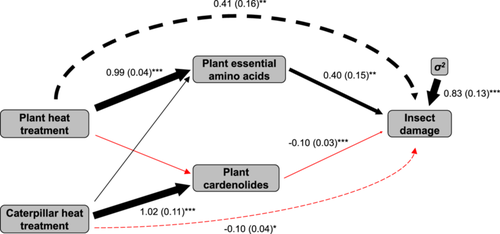
Our path analysis revealed that insect damage was indirectly mediated by the effects of the prior heat treatment experienced by both the plant and insect on plant traits (Figure 6). Plant heat treatment had a significant direct positive effect on plant essential amino acids (but not on cardenolides), which in turn had a significant positive effect on insect damage. Caterpillar heat treatment had a significant direct positive effect on plant cardenolides (but not on plant essential amino acids), which had a significant negative effect on insect damage. Plant heat treatment had a significant indirect positive effect on insect damage mediated by plant essential amino acids, whereas caterpillar heat treatment had an indirect negative effect on insect damage mediated by plant total cardenolides (Figure 6).
4 DISCUSSION
Despite progress over the last decade in understanding how extreme climate events may disrupt species interactions and community ecology (De Boeck et al., 2011; Descombes et al., 2020; Thompson et al., 2013; Wernberg et al., 2012), the underlying mechanisms of HW-mediated disruption of plant-herbivore interactions are poorly understood (but see Havko et al., 2020a).
Here we show that the impacts of a HW on a plant-insect interaction may be weakly predicted from the direct effects of heat on either organism alone. Instead, organismal performance and interaction outcomes depended more on the indirect effects of heat on each organism acting through physiological changes that influence the interaction in a trait-specific manner. Moreover, the extreme event did not alter the overall outcome of the interaction even though traits mediating the interaction showed important changes. Below we explain the consequences of the direct and indirect effects of a HW on each organism separately and during the interaction, and their ecological implications contingent on which organism has experienced the extreme event, inferred from our reciprocal transplant approach.
4.1 Heat waves have immediate positive effects on plants and herbivores separately but may favor the insect during the interaction
Our heat treatment had direct, immediate, and positive effects on caterpillar growth, which contrasts with previous findings of declines in monarch performance while developing at constant temperatures above 33°C (Zalucki, 1982). Although our experiment involved temperatures above that threshold, related work on monarch larvae reported that fluctuating day-night temperatures, rather than more constant ones, typically result in faster development and greater insect performance (Nail et al., 2015; York & Oberhauser, 2002), supporting our findings in a similar environmental context. In this sense, high but fluctuating temperatures may allow nighttime recovery, thus preventing heat stress damage from accumulating over time (Colinet et al., 2015). Importantly, our temperature manipulation reflects the pattern of many natural HWs (Smith, 2011).
The HW also had direct positive effects on plant growth and defences in undamaged plants. Our findings are in line with the general notion that plant vegetative growth is accelerated during moderate heat, improving plant competitive ability (Pincebourde et al., 2017; Zvereva & Kozlov, 2006), but can also occur as a phenological adaptation to escape mortality before extreme drought conditions occur later in the summer in plant species inhabiting more stressful environments (Popovic & Lowry, 2020; Rafferty et al., 2020). Likewise, recent evidence from model systems, like Arabidopsis and tomato, suggests that jasmonate-related defence responses are upregulated under elevated temperatures due to stabilization of the jasmonate receptor by heat shock proteins (Havko et al., 2020a; Wang et al., 2016). Interestingly, plant growth responses were transient and decayed over time to control levels by the end (Day 8) of the experiment compared to defensive traits, suggesting that a single HW event may exert short-term or long-term responses contingent on the particular plant trait investigated.
While HWs directly enhanced both insect development and plant growth and defence when examined separately, we hypothesise insects may have been favored in the plant-herbivore interaction during the first 4 days of the heat wave. Specifically, the faster insect feeding rate during heat, which caused much more damage on the plants and eventually led to greater insect mass through faster development, was not mediated by changes in plant defence or nutrient levels compared to damaged plants in control temperature conditions. Conversely, HW plants that experienced herbivory had their growth rate reduced to similar levels as damaged control plants. These results may indicate that HWs can cause insects to overcome plant defences more easily through faster feeding and development, causing substantial damage in a few days (Havko et al., 2020b), which concomitantly antagonized with the increased plant competitive ability initially induced by elevated temperature (Havko et al., 2020a). This may increase the chance for caterpillars to avoid potential induced defense responses deployed later from plants that could otherwise negatively affect monarchs, especially if they are in earlier stages of development. Therefore, HWs of only 4 days may negatively impact plant populations due to their strong, direct and positive effects on the development and feeding rate of herbivores. Long-term, even a single HW within a season could likely cause a disproportionate increase in the insect herbivore biomass compared to that of plants and natural enemies, with cascading consequences for the entire community (de Sassi & Tylianakis, 2012). Conversely, timing of heat waves may also result in reduced population densities due to increased monarch mortality when occurring during critical insect stages like egg development or pupation (James, 2016).
4.2 Heat waves indirectly reduce herbivory by inducing more toxic cardenolides and decreasing tissue nutrient quality
During the 4-day period after the HW event, we observed that latex exudation and total cardenolide concentrations tended to increase upon herbivory or heat alone but were impaired by both HW and herbivory, especially by HW caterpillars, which were significantly larger and did significantly more damage than control caterpillars. One potential explanation for this result is that induction of plant defences may be limited in response to multiple stresses (Couture et al., 2015; Hamann et al., 2021a; Havko et al., 2020a) compared to single stressors (e.g., Couture et al., 2015; Faldyn et al., 2018; Rasmann & Agrawal, 2011). This suggests that an increase in the frequency and intensity of HWs may make the world a more stressful place for common milkweed, with higher heat stress and consumption from herbivores. From the herbivore's perspective, our study focused on the initial larval stages because they are among the most critical phases for survival while interacting with the host plant (Zalucki & Malcolm, 1999; Zalucki et al., 2001). Nonetheless, faster consumption, development and greater mass during larval stages may not be always reflected in greater survival during pupation, adult mass or fecundity (Veyrat et al., 2016; York & Oberhauser, 2002). Therefore, future studies should include other stages of development to provide a complete picture of the impacts of extreme events directly on insect larval performance and adult fecundity, and indirectly through host plant traits.
Surprisingly, despite the HW-mediated reductions in plant defences on damaged plants, consumption by HW caterpillars was lower on HW plants than on control plants. We hypothesise that this is indirectly mediated by the increase in induction of more toxic cardenolides by HW plants and a decrease in tissue nutritional quality. Specifically, HW plants damaged by HW caterpillars increased the concentrations of labriformin by 130% compared to controls, which has been reported as one of the most toxic compounds in common milkweed and is not sequestered by specialist herbivores (Agrawal et al., 2022; Brower et al., 1984). Also, damaged HW plants had significantly lower amounts of essential dietary amino acids compared to damaged control plants by HW caterpillars. Our path analysis also supported that the effects of heat on the plant and on the insect indirectly affected the damage caused by the insect through changes in these plant traits. Therefore, plants seem to reduce herbivore damage when both experience a HW through increases in plant tissue toxicity and reducing its nutritive quality.
Although changes in defensive cardenolides and tissue quality of HW plants upon herbivory can explain the lower damage caused by HW caterpillars compared to controls, it does not explain the similar performance of herbivores. We hypothesise that several physiological mechanisms, directly or indirectly related to plant resistance to herbivores, are at play, with implications for herbivore performance. First, the multivariate changes toward more toxic cardenolide composition in HW plants upon herbivory by large HW caterpillars likely allowed milkweed to save resources while defending in stressful environments. This is possible since recent evidence revealed that most cardenolides are often positively correlated among them in different milkweed species, but not the nitrogen-containing toxic compounds (Agrawal et al., 2021; López-Goldar & Agrawal, 2023; López-Goldar et al., 2022), suggesting independence in their biosynthetic pathways in response to environmental conditions. Second, it is possible that HW plants may have reallocated nutrients to other tissues, either to reduce nutrient losses due to herbivory or to prevent increased risk of mortality due to combined HW and herbivory stresses. It has been reported that plants can upregulate amino acid transporters in tissues under stressful environments to mobilize and store nutrients in other, better protected tissues as a bet-hedging strategy for the future (Wan et al., 2017). This strategy may be common in this and other milkweed species since they resprout from the rhizome-like root structure next year after the winter period. Therefore, these two physiological mechanisms not only allowed the plant to save resources under the combination of heat and herbivory, but may have also prevented larger, HW caterpillars to cause massive damage and gain more mass despite the reductions in defensive latex exudation and total cardenolide concentrations.
4.3 The ecological implications of heat waves for plant-herbivore interactions
Plants and insects adjust their physiology and phenology to climate variation (Colinet et al., 2015; Hamann et al., 2021b), but they also need to adjust these environmental-mediated changes when they interact. Just as plants prime their defences after detecting herbivore cues to help them deploy faster, stronger, and more efficient defence responses to subsequent herbivore attacks (Martinez-Medina et al., 2016), we speculate that plants also adjust their defences in response to a HW to match the faster growth and higher consumption of insect herbivores during elevated temperatures. In our study, specifically, plants that experienced the same temperature treatment as its herbivore deployed a more efficient response against the massive damage from larger caterpillars through induction of more toxic defences and while maintaining a reduced tissue nutrient quality, despite the overall reduction of more quantitative defences. This suggests that ‘less is more’, in which the increased relative proportion of most toxic compounds, and likely not total concentrations, may be a better predictor of resistance (Mirzaei et al., 2020), and that plant nutrient variability also plays an crucial role in reducing the negative impacts of herbivores on plants (Wetzel et al., 2016). Other components of plant defence, such as volatile organic compounds (VOCs, not measured in this study), can be affected by temperature and influence herbivory directly or through interactions with third trophic levels (Kergunteuil et al., 2019; Kivimäenpää et al., 2016; Sentis et al., 2013). Differences in herbivore-induced VOCs across populations have been reported in milkweeds (Wason et al., 2013), and these compounds were related to parasitoid attraction to aphids (Meier & Hunter, 2019; Wason & Hunter, 2014). Nonetheless, to date, it is unclear the implications of VOCs on monarch feeding or host selection by caterpillars, although female oviposition may be influenced (Jones & Agrawal, 2019; Kessler & Baldwin, 2001). Therefore, examining multiple functional axes of plant and insect physiology in direct or indirect response to extreme events can deepen our understanding of the ecological consequences of climate change in plant-herbivore interactions.
At larger scales, by the time a HW event occurs, some plants within a community may be already experiencing herbivory, while others may be attacked in the future or not be damaged at all. We hypothesise that this will create a heterogeneous mosaic of plant phenotypes in the population, with potential cascading consequences not only for plant-herbivore interactions, but also for pollinators, mediated by changes in floral phenology and display (de Manincor et al., 2023). These heat-mediated changes in the plant-insect dynamics can also be reflected in shifts between generalists and specialists (Ali & Agrawal, 2012). For instance, plants with greater levels of heat-induced defences may be better defended against more generalist herbivores that are less tolerant to plant toxins. In addition, resource availability may also modify plant physiological responses to heat (Heckathorn et al., 1996). For example, plants inhabiting nutrient-poor environments (e.g., low nitrogen) may show constrained induction of nitrogen-containing compounds (like some toxic cardenolides in milkweeds, or glucosinolates in Brassicaceae) under heat stress upon herbivory. Finally, milkweeds are generally sun-loving species (Coverdale & Agrawal, 2021), and many of them show some resistance to drought stress (which may concomitantly occur with heat waves) due to the presence of adaptive leaf traits such as trichomes and waxes, like common milkweed (Agrawal et al., 2009). Nonetheless, recent work showed that monarch caterpillars had higher survival on milkweed species with greater expression of drought-resistance traits, suggesting an evolutionary tradeoff in cross-resistance to drought and herbivory stresses between species (Carvajal Acosta et al., 2022).
Climate change studies have been increasing in importance, and moving plants between multiple chambers during experiments is a typical approach to avoid biases due to chamber-specific effects. In our study, we only had access to two chambers because of space constraints, and the lack of differences in plant height and the low variation between the assigned treatments before moving the plants for the 4-day heat treatment indicates minimal differences between chambers. Despite that, since heat waves involve strong temperature increases of short duration, as in our study, moving plants and insects between chambers may have interrupted the effect of the treatment and likely increased undesired variation between treatments more than the intrinsic differences between chambers. Thus, considering alternative experimental approaches contingent on the climate change factors explored is key to accurately simulate environmental scenarios to continue advancing the field.
A final implication of our work is the importance of disentangling the direct and indirect effects of climate events on organisms and their interactions. If we had only examined the direct effects of heat on the plant and the herbivore separately, we would have wrongly concluded that extreme heat did not alter this plant-herbivore interaction. Likewise, by only exploring the overall effect of heat on the outcome of the interaction, our interpretation that insect herbivores seem to outweigh plant defences and impair plant competitive ability during heat would be also biased. Therefore, by teasing apart the direct and indirect effects of climate change events on species interactions, here we provide evidence that indirect effects—rather than direct—on each organism's physiology are crucial to understand how plant-herbivore interactions may proceed in a changing world.
ACKNOWLEDGEMENTS
We thank the Wetzel lab group for comments on the manuscript. We thank Michael Kalwajtys for his help with sampling, and the Michigan State University Mass Spectrometry Facility for the amino acid analysis. This research was supported by the Plant Resilience Institute at Michigan State University (PRI-MSU), the Montana State University College of Agriculture, the Montana Agricultural Experiment Station, and U.S. NSF grant (IOS-2209762) to Anurag A. Agrawal.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Zenodo at https://zenodo.org/records/10488528.




