Sextuple knockouts of a highly conserved and coexpressed AUXIN/INDOLE-3-ACETIC ACID gene set confer shade avoidance-like responses in Arabidopsis
Abstract
AUXIN/INDOLE-3-ACETIC ACIDs are transcriptional repressors for auxin signalling. Aux/IAAs of Arabidopsis thaliana display some functional redundancy. The IAA3/SHY2 clade (IAA1, IAA2, IAA3 and IAA4) show strong sequence similarity, but no higher-order mutants have been reported. Here, through CRISPR/Cas9 genome editing, we generated loss-of-function iaa1/2/3/4 mutants. The quadruple mutants only exhibited a weak phenotype. Thus, we additionally knocked out IAA7/AXR2 and IAA16, which are coexpressed with IAA1/2/3/4. Remarkably, under white light control conditions, the iaa1/2/3/4/7/16 mutants exhibited a shade avoidance-like phenotype with over-elongated hypocotyls and petioles and hyponastic leaves. The sextuple mutants were highly sensitive to low light intensity, and the hypocotyl cells of the mutants were excessively elongated. Transcriptome profiling and qRT-PCR analyses revealed that the sextuple mutation upregulated IAA19/MSG2 and IAA29, two shared shade/auxin signalling targets. Besides, genes encoding cell wall-remodelling proteins and shade-responsive transcription regulators were upregulated. Using dual-luciferase reporter assays, we verified that IAA2/IAA7 targeted the promoters of cell wall-remodelling genes to inhibit their transcription. Our work indicates that the IAA1/2/3/4/7/16 gene set is required for the optimal integration of auxin and shade signalling. The mutants generated here should be valuable for exploring the complex interactions among signal sensors, transcription activators and transcription repressors during hormone/environmental responses.
1 INTRODUCTION
Light is essential in plant growth and development. As a sessile organism, plants have evolved the ability to modify their growth behaviour in response to light regime changes. Specifically, plants increase their stem and petiole heights and adjust the angle and structure of their foliage to acquire more sunlight when sheltered by neighbouring vegetation, a phenomenon known as shade avoidance response (SAR) (Casal & Fankhauser, 2023; Franklin, 2008; Smith & Whitelam, 1997). Plant canopies absorb red (R), blue (B) and UV-B light but reflect and transmit far-red (FR) light. In Arabidopsis, cryptochromes 1/2 (CRY1/2) and UV-RESISTANCE 8 (UV8) mediate the perception of B and UV-B lights, respectively, while the R:FR ratio is detected by the phytochrome photoreceptors (Fiorucci & Fankhauser, 2017; Franklin & Whitelam, 2005; Martinez-Garcia & Rodriguez-Concepcion, 2023). Among the five members (phyA to phyE), phyB is the core player of SAR. Under a high R:FR ratio, phyB is active and relocates in the nucleus to repress the function of phytochrome interacting factors (PIFs), a clade of typical basic helix-loop-helix (bHLH) family transcription factors. Under a low R:FR ratio, phyB becomes inactive to release the PIFs inhibition, leading to transcriptional reprogramming (de Wit et al., 2016). Low levels of B light and an absence of UV-B light also participate in SAR (Hersch et al., 2014; Krahmer & Fankhauser, 2024).
To fine-tune SAR, PIFs induce the expression of long hypocotyl in far-red light 1 (HFR1), phytochrome rapidly regulated 1 (PAR1/2) and PIF 3-like 1 (PIL1), which encode atypical bHLH transcription repressors of PIFs (Buti et al., 2020). In addition, the shade signal is integrated with multiple phytohormone signals (Ballaré & Pierik, 2017; Iglesias et al., 2018; Korver et al., 2018; Krahmer & Fankhauser, 2024) to eventually develop a shade avoidance syndrome (SAS). Notably, specific members of the YUCCA family auxin biosynthesis genes, YUC2/5/8/9, are directly induced by PIF1/3/4/5 in blades to supply hypocotyls and petioles with newly synthesised auxin (Casal & Fankhauser, 2023; Kohnen et al., 2016). As another feature of transcriptional control, certain members of class-II homeodomain-leucine zipper (HD-ZIP) transcription factor genes, such as ATHB2, ATHB4 and HAT2, are rapidly upregulated (Sawa et al., 2002; Sorin et al., 2009). Recent studies suggest that three members of class I teosinte branched 1-cycloidea-PCF transcription factors, TCP5/13/17, are required for shade-induced hypocotyl elongation (Hur et al., 2024; Zhou et al., 2018).
Auxin regulates numerous processes, including photomorphogenesis, phototropism and SAR. A classical auxin nuclear signalling machinery comprises three components: transport inhibitor response 1/auxin signalling f-boxes (TIR1/AFBs; auxin receptors), auxin/indole-3-acetic acids (Aux/IAAs; transcription repressors) and auxin response factors (ARFs; transcription factors) (Carrillo-Carrasco et al., 2023; Caumon & Vernoux, 2023; Leyser, 2018). Canonical Aux/IAA proteins have four domains (I to IV) (Figueiredo & Strader, 2022; Reed, 2001). At low auxin concentrations, Aux/IAAs bind to ARFs through domains III/IV and recruit the corepressor topless/TPL-related proteins (TPL/TPRs) through domain I to inhibit the transcription activity of ARFs. When the auxin concentrations increase, Aux/IAAs are rapidly ubiquitinated by TIR1/AFBs and degraded by the 26 S proteasome, whereas ARFs are released to activate the transcription of downstream genes, including Aux/IAAs, forming a negative feedback loop (Carrillo-Carrasco et al., 2023; Caumon & Vernoux, 2023; Leyser, 2018; Liscum & Reed, 2002). The domain II of Aux/IAAs is recognised by TIR1/AFBs; consequently, mutations of key amino acid residues in this domain lead to the stabilisation of Aux/IAAs and gain-of-function phenotypes in corresponding mutants.
The Arabidopsis genome has 29 Aux/IAA genes. Gain-of-function mutants have been reported in 14 of 24 canonical Aux/IAAs, including IAA1/auxin resistant 5 (AXR5), IAA3/short hypocotyl 2 (SHY2), IAA6/SHY1, IAA7/AXR2, IAA8, IAA12/bodenlos (BDL), IAA13, IAA14/solitary root (SLR), IAA15, IAA16, IAA17/AXR3, IAA18/CRANE/POTENT, IAA19/massugu 2 (MSG2) and IAA28/IAA-alanine resistant 2 (IAR2) (Israeli et al., 2020; Liscum & Reed, 2002; Nagpal et al., 2000; Reed et al., 2018; Tatematsu et al., 2004; Tian and Reed, 1999). Domain II mutants iaa6/shy1 and iaa3/shy2 were identified by screening the suppressor hy2 (long hypocotyl 2), which has a defect in encoding an enzyme required for the biosynthesis of phytochrome chromophore (Kim et al., 1996). Domain II mutants iaa7/axr2 and iaa17/axr3 also exhibited short hypocotyls, and the mutations could alleviate the long hypocotyl phenotype of the phyB mutant (Nagpal et al., 2000). Additionally, shade-induced hypocotyl elongation was severely reduced in these mutants (Li, Li, et al., 2021; Li, Zhang, et al., 2021; Procko et al., 2016; Xi et al., 2021). These gain-of-function phenotypes indicate the role of Aux/IAAs in cell expansion regulation but must be interpreted with caution since they can be misleading regarding the mutations' hypermorphic and ectopic effects (Israeli et al., 2020; Liscum & Reed, 2002).
Loss-of-function mutation is an alternative approach to studying the functions of Aux/IAAs. Early studies using ethyl methanesulfonate mutants have reported that the loss-of-function mutants iaa3/shy2-31 and iaa7/axr2-5 exhibited slightly elongated hypocotyl (Nagpal et al., 2000; Tian & Reed, 1999). Overvoorde et al. (2005) identified insertion mutants in 12 Arabidopsis Aux/IAAs, including iaa4, iaa5, iaa6, iaa8, iaa9, iaa11, iaa12, iaa14, iaa17, iaa19, iaa31 and iaa33. Strikingly, single, double or triple loss-of-function mutants lack a visible phenotype in growth and development compared to the wild-type (WT), indicating a high degree of functional overlap and compensation among the Aux/IAA family members (Overvoorde et al., 2005). Knocking down four Aux/IAAs (IAA7/8/17/19) using artificial microRNA promotes hypocotyl elongation and rescues the short hypocotyl phenotypes of the iaa7/axr2-1 gain-of-function mutant (Reed et al., 2018). As reported recently, a few loss-of-function aux/iaa mutants exhibited defects under stress conditions, including the iaa5/6/19 triple mutant under drought or shade conditions (Nguyen et al., 2023; Salehin et al., 2019; Shani et al., 2017), the iaa15 mutant under high osmotic pressure (Kim et al., 2022), and the iaa11 mutant under UV-AB stress (Mielecki et al., 2022). Nevertheless, generating higher-order loss-of-function mutants to unravel the fine-tuned developmental outcomes and robustness of Aux/IAAs is a pressing demand for the plant science community (Israeli et al., 2020; Ori, 2019).
The IAA3/SHY2 clade of Arabidopsis have four members, IAA1, IAA2, IAA3 and IAA4. They have a unique feature in the cis-regulatory auxin-responsive element AuxREs (Lieberman-Lazarovich et al., 2019; Remington et al., 2004). The TGTCCC pattern of AuxREs in these genes is deeply conserved across angiosperms, but its biological significance remains unclear (Cherenkov et al., 2018; Lieberman-Lazarovich et al., 2019; Yocca & Edger, 2022). Here, we generated loss-of-function iaa1/2/3/4 mutants using the CRISPR/Cas9 genome editing technique. The quadruple mutant seedlings only exhibited a weak phenotype. We continued knocking out IAA7/AXR2 and IAA16, which are coexpressed with IAA1/2/3/4. Remarkably, under white light photoperiodic conditions, the sextuple iaa1/2/3/4/7/16 mutants exhibited a SAS-like phenotype and they were highly sensitive to low light intensity. Transcriptome profiling and qRT-PCR analyses revealed that the sextuple mutation upregulated genes encoding cell wall-remodelling proteins and shade-responsive transcription regulators. Our work indicates that the IAA1/2/3/4/7/16 gene set is required for the optimal integration of auxin and shade signalling. The higher-order mutants generated here should be valuable for the plant biology community to explore the complex interactions among signal sensors, transcription activators and transcription repressors during hormone and environmental responses.
2 MATERIALS AND METHODS
2.1 Plant materials, growth conditions and shade/auxin treatment
WT Arabidopsis thaliana Columbia-0 (Col-0), 35 S::PHYB (CS8037), and phyB-8 (CS6216) were obtained from the Arabidopsis Biological Resource Centre.
Arabidopsis seeds were spread on Gamborg's B5 medium with 1% (w/v) sucrose and 0.8% or 1% (w/v) agar for horizontal and vertical growth, respectively. After 2 days of stratification at 4°C, plates were placed in a growth chamber for cultivation at 23°C, 80 μmol m−2 s−1 irradiance, and 16 h light/8 h dark. Horizontal growth and vertical growth have their own advantages and disadvantages for phenotypic characterisation. Arabidopsis seedlings under vertical growth had longer hypocotyls and roots than these under horizontal growth. Without specific statement, seedlings were under horizontal growth.
For deep shade treatment, 5-day-old seedlings were cultivated in a chamber with low R:FR ratio illumination (R:FR = 0.1) and low light intensity (20 μmol m−2 s−1) for four more days. To evaluate the effects of light intensity on seedling growth, 8-day-seedlings grown on different light intensities from 60 to 100 μmol m−2 s−1 irradiation were sampled. For different R:FR ratio treatment, 5-day-old seedlings were transferred to chambers with R:FR ratios of 0.1 or 0.4 (80 μmol m−2 s−1 irradiation) and cultivated for 8, 16 and 32 h. To test auxin sensitivity of hypocotyls, 6-day-old seedlings were immersed in a liquid B5 medium containing 0, 0.1, 0.5, 2.5, 12.5 and 25 μM 1-naphthylacetic acid (NAA) and grown for 8 h.
2.2 Generation of aux/iaa mutants
Using the CRISPR/Cas9 genome editing technique described previously (Qiu et al., 2020), we individually acquired iaa1, iaa2, iaa3 and iaa4 single mutants. The iaa1/2/3/4 quadruple mutants were generated through three rounds of crossing and screening. To generate the iaa1/2/3/4/7/16 sextuple mutants, we genome-edited IAA7 and IAA16 in the iaa1/2/3/4 quadruple mutant background. The iaa1/2/3/4/7/16 sextuple mutant was also backcrossed with the WT Col-0 to ensure that the mutated forms of the corresponding genes were the same as in the sextuple mutant. However, the tight linkage between IAA2 (AT3G23030) and IAA7 (AT3G23050) makes obtaining the iaa7 and iaa7/16 mutants impossible. Thus, only the iaa2/7, iaa2/7/16 and iaa16 mutants were generated here.
2.3 Gene expression analysis
Hypocotyls, blades, or shoot parts of Arabidopsis seedlings were isolated for RNA extraction. The genotype, age and treatment of each sample were indicated in the text and figure legends. Samples were immediately placed in liquid nitrogen after cutting. Total RNA was extracted using an Easy Plant RNA Rapid Extraction Kit (Easy-Do), then reverse-transcribed to cDNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO). qRT-PCR was performed in CFX Opus 96 (Bio-Rad) using SYBR Green reagent (Takara). The relative expression levels of genes were calculated based on the method using ubiquitin extension protein 1 (UBQ1) or tubulin beta chain 2 (TUB2) as standard genes (Qiu et al., 2020). Primers for qRT-PCR are listed in Supporting Information: Table 2.
2.4 Microscopy
Scanning electron microscopy (SEM) was used to observe the surface morphology of Arabidopsis hypocotyls. Light microscopy was used to determine the width of hypocotyls and the microstructure of cross-sections, following previously reported methods (Desnos et al., 1996; Qiu et al., 2020). Nine-day-old seedlings were observed.
2.5 RNA-seq and bioinformatics analysis
Hypocotyls of 7-day-old WT and iaa1/2/3/4/7/16 #1 seedlings were isolated for RNA-seq. Each sample contained three biological replicates that were sent to BIOTREE for RNA-seq for library construction, Illumina HiSeq sequencing and bioinformatics analysis, including a quality check of the raw reads, alignment of raw reads to the Arabidopsis genome, assembly of gene expression from aligned reads, identification of DEGs and enrichment analysis. Genes with a |log2 (Fold Change)| ≥ 1 and q < 0.05 were considered DEGs. The data have been deposited on NCBI (National Centre for Biotechnology Information, https://www.ncbi.nlm.nih.gov/geo/) with accession numbers PRJNA1039520. Heatmaps were constructed using OECloud tools (https://cloud.oebiotech.com).
2.6 Dual-luciferase assay
To generate luciferase effector constructs, 35 S::3 × FLAG-IAA2, 35 S::3 × FLAG-IAA7, 35 S::3 × FLAG-IAA16, and the coding sequences of IAA2, IAA7 and IAA16 were cloned into the pCAMBIA1300-35S-FLAG-N vector. To generate luciferase reporter constructs, XTH16pro::LUC, XTH17pro::LUC, EXPL2pro::LUC, and EXPL3pro::LUC, and the 1–2 kb sequences in the promoter regions of XTH16, XTH17, EXPL2 and EXPL3 were cloned into the pGreenII0800-LUC vector. Primers for construct generation are listed in Supporting Information S1: Table 2. Effector and reporter constructs were transformed into Agrobacterium tumefaciens GV3101 or EHA105 (with psoup), respectively. Tobacco (Nicotiana benthamiana) leaves were infiltrated with mixed Agrobacterium. After 3 days of cultivation, infiltrated leaves were collected for the detection of Firefly luciferase using a live imaging system (NightShade LB 985, BERTHOLD) or quantitative detection using a dual-luciferase reporter assay kit (Promega, E1910).
2.7 Generation of complementary lines
To generate complementary lines, plants of the sextuple mutant were transformed with Agrobacterium carrying the 35 S::3 × FLAG-IAA2 or 35 S::3 × FLAG-IAA7 construct using a vacuum infiltration method. Transgenic plants were selected by a protocol described previously (Huang et al., 2023).
2.8 Statistical analysis
For the phenotypic analysis, seedlings were photographed by camera (Canon) or microscope (Nikon) and measured using ImageJ software. The significance of differences between two sets of data was tested using a Student's t-test. Asterisks represent different levels of significance (ns, no significance with p > 0.05, *p < 0.05, **p < 0.01 and ***p < 0.001). The significance of differences between more than two sets of data was tested by one-way analysis of variance based on Tukey's multiple comparisons test.
3 RESULTS
3.1 The IAA1/2/3/4 gene set has high sequence similarity in coding regions and AuxREs
Arabidopsis IAA1/AXR5 (AT4G14560), IAA2 (AT3G23030), IAA3/SHY2 (AT1G04240) and IAA4 (AT5G43700) are closely related homologues (Supporting Information S1: Figure 1 and 2). The distribution patterns of exons and introns in IAA1 versus IAA2 and IAA3 versus IAA4 are pretty similar (Supporting Information S1: Figure 2A). Besides their highly conserved coding regions, their cis-regulatory regions share high sequence similarity and have a unique feature (Supporting Information S1: Figure 2B; Lieberman-Lazarovich et al., 2019; Remington et al., 2004). Among the 29 Aux/IAAs, only IAA1/2/3/4 have AuxRE pairs with direct repeats of the TGTCCC motif and with a suitable space for the binding of ARF dimers (Supporting Information S1: Figure 1; Supporting Information S1: Figure 2B). Interestingly, the flanking sequences adjacent to AuxREs in the promoter regions of IAA1/2/3/4 also display deep conservation (Supporting Information S1: Figure 2B), further implying that they have arisen through a recent gene duplication. In addition, using improved calculation methods, IAA1/2/3/4 were identified as coexpressed genes (ATTED-II, http://atted.jp; ACT, https://www.michalopoulos.net/act/) (Obayashi et al., 2022; Zogopoulos et al., 2021).
3.2 Genome editing of Arabidopsis IAA1/2/3/4 genes to generate loss-of-function mutants
To generate higher-order mutants, we first knocked out IAA1/2/3/4 individually using the CRISPR/Cas9 genome editing technique (Supporting Information S1: Figure 3). The impact of each mutation on the corresponding transcript and protein product was evaluated by qRT-PCR and functional Aux/IAA domain prediction. The iaa1-2, iaa3-1 and iaa4-1 mutations had 469, 697 and 664 bp deletions, respectively, leading to frame shifts and premature stop codons. The transcription level of corresponding genes in these mutants was almost undetectable by qRT-PCR, possibly because of nonsense-mediated mRNA decay. The iaa2-1 mutation had a 43 bp deletion, eliminating the start codon ATG (Supporting Information S1: Figure 3). These mutations are loss-of-function and null. Next, we crossed these single mutants to generate the F1 hybrid, and F1 plants were self-pollinated to produce the F2 generation, which we screened to identify the double mutants. Finally, double mutants were crossed to generate triple mutants, and triple mutants were crossed to generate quadruple mutants (Table 1). Under white light control conditions and agar media containing 1% (w/v) sucrose, the quadruple mutant exhibited very weak phenotypes with slightly elongated hypocotyls and petioles compared to the WT (Supporting Information S1: Figure 4 and 5). In addition, with extended growth duration, the quadruple mutants had shorter roots (Supporting Information S1: Figure 6).
| Lines | IAA1 | IAA2 | IAA3 | IAA4 | IAA7 | IAA16 |
|---|---|---|---|---|---|---|
| iaa2/7 #1 | / | −43 | / | / | −1C | / |
| iaa2/7/16 #1 | / | −43 | / | / | −1C | +1 A |
| iaa1/2/3/4 #1 | −469 | −43 | −697 | −664 | / | / |
| iaa1/2/3/4 #2 | −305 + 1 | +2 | −697 | −664 | / | / |
| iaa1/2/3/4 #3 | −305 + 1 | −43 | −697 | −664 | / | / |
| iaa1/2/3/4 #4 | −469 | −43 | −369 + 2 | −664 | / | / |
| iaa1/2/3/4 #5 | −469 | +2 | −697 | −664 | / | / |
| iaa1/2/3/4 #6 | −469 | +2 | −369 + 2 | −664 | / | / |
| iaa1/2/3/4/7/16 #1 | −469 | −43 | −697 | −664 | −1C | +1 A |
| iaa1/2/3/4/7/16 #2 | −469 | -43 | −697 | −664 | +1 A | +1 T |
| iaa1/2/3/4/7/16 #3 | −469 | -43 | −697 | −664 | +1 T | −1G |
- Note: Using the CRISPR/Cas9 genome editing technique, single mutants of iaa1, iaa2, iaa3 and iaa4 were generated first. The iaa1/2/3/4 quadruple mutants were obtained through three rounds of crossing and screening. The iaa1/2/3/4/7/16 sextuple mutants were generated by further knocking out IAA7 and IAA16 in the iaa1/2/3/4 background, and the iaa2/7, iaa2/7/16 and iaa16 mutants were obtained by back-crossing and screening. The detail positions of genome editing-induced deletion/insertion in each gene were illustrated in Supporting Information S1: Figure 3.
3.3 The iaa1/2/3/4/7/16 sextuple mutants exhibited remarkable SAS-like phenotypes
Additional Aux/IAA proteins might perform the same function to compensate for the loss of IAA1/2/3/4 genes. Based on the above mentioned gene coexpression database, we knocked out two additional Aux/IAA genes, IAA7/AXR2 (AT3G23050) and IAA16 (AT3G04730), using the CRISPR/Cas9 technique. Three lines of sextuple mutants were successfully generated. The mutation forms of each gene in these mutants are illustrated in Supporting Information S1: Figure 3 and listed in Table 1. From here on, the mutants iaa1-2/iaa2-1/iaa3-1/iaa4-1/iaa7-3/iaa16-1, iaa1-2/iaa2-1/iaa3-1/iaa4-1/iaa7-1/iaa16-2 and iaa1-2/iaa2-1/iaa3-1/iaa4-1/iaa7-2/iaa16-4 are abbreviated as iaa1/2/3/4/7/16 #1, #2 and #3, respectively. To save space, we further simplified the iaa sextuple mutant as iaas.
Remarkably, under white light photoperiodic conditions, seedlings and adult plants of the iaas mutants exhibited a SAS-like phenotype with over-elongated hypocotyls (Figures 1a,c) and increased petiole length (Supporting Information S1: Figure 5) and leaf hyponasty (Figure 1b), indicating that IAA1/2/3/4/7/16 may participate in inhibiting the development of SAS-like responses under sufficient light. To evaluate the contribution of each gene to the SAS phenotype of the sextuple mutants, phenotypes of the iaa1, iaa2, iaa3, iaa4, iaa16, iaa2/7, iaa2/7/16 and iaa1/2/3/4 mutants were compared with the WT and the sextuple mutants. Supporting Information S1: Figure 4 indicates that iaa2 and iaa16 had slightly longer hypocotyls than the WT, while iaa2/7 and iaa2/7/16 had significantly longer ones. However, the hypocotyl lengths of iaa2/7 and iaa2/7/16 were still significantly behind that of the sextuple mutant (Supporting Information S1: Figure 4). Similar results were obtained in the ratio of petiole length to leaf length (Supporting Information S1: Figure 4). As reported, the hypocotyl length of the loss-of-function mutant iaa7-7 is similar to the WT Col-0 (Reed et al., 2018). Therefore, the IAA1/2/3/4/7/16 gene set works redundantly in regulating hypocotyl elongation, with IAA2, IAA7 and IAA16 playing the most important roles. This conclusion is consistent with their higher expression levels in hypocotyls, as estimated by the FPKM value in the RNA-seq analysis (Supporting Information S1: Table 1).
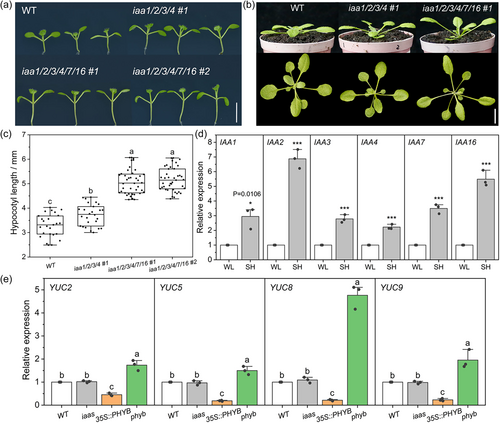
We treated WT seedlings with deep shade to check the transcription response of these genes. Shade treatment induced the expression of IAA1/2/3/4/7/16 (Figure 1d), further indicating a potential role for these Aux/IAAs in SAR.
As reported, the induced expression of YUC2/5/8/9 is required for distal auxin biosynthesis during SAR (Kohnen et al., 2016). Through qRT-PCR analysis, we found that the expression levels of these YUC genes were not changed in the leaves of the iaas mutants grown under white light control conditions (Figure 1e). By contrast, leaves of the PHYB-overexpressing line and the phyB-8 mutant displayed a reduced and enhanced expression of these YUC genes, respectively (Figure 1e). Thus, the SAS-like phenotypes in iaas mutants are not caused by the activation of auxin biosynthesis genes but, instead, by the knockout of auxin signalling repressors.
3.4 The sextuple mutants exhibited enhanced sensitivity to low light intensity and exogenous auxin
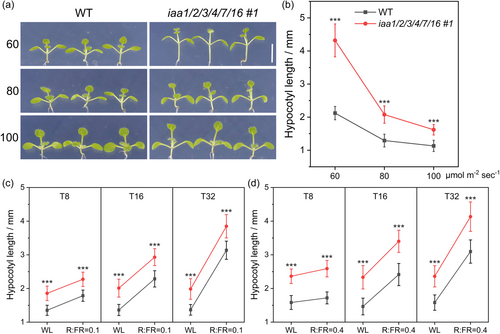
The impact of shade on Arabidopsis hypocotyl growth resulted from two separate parameters: low light intensity and low R:FR ratio (Hersch et al., 2014). We first treated the sextuple mutant with different intensities of white light from 60 to 100 μmol m−2 s−1. Results indicated that the sextuple mutant was dramatically sensitive to the reduced light intensity, developing SAS-like phenotypes including over-elongated hypocotyls (Figures 2a,b). We then treated the sextuple mutant with lower R:FR ratios, 0.1 and 0.4, at 80 μmol m−2 s−1 illuminace. Results indicated that, although the sextuple mutant had longer hypocotyls than the WT at each time node after treatment, their sensitivity to lower R:FR ratios were similar (Figures 2c,d). These results indicate that the IAA1/2/3/4/7/16 gene set is required for the optimal response of Arabidopsis seedlings to shade, especially to low light conditions.
As a basic and useful step to unravel whether auxin signalling is altered in the higher-order aux/iaa mutants, the expression levels of all canonical Aux/IAA genes, except IAA1/2/3/4 in the iaa1/2/3/4 quadruple mutants, were detected by qRT-PCR. The reason for excluding IAA1/2/3/4 is that these mRNAs in the quadruple mutants are subjected to nonsense-mediated mRNA decay because of genome editing-induced premature stop codons. Among 20 Aux/IAA genes tested, only IAA17, IAA19 and IAA29 displayed upregulated expression levels in the shoots of the quadruple mutant seedlings, indicating a robustness in Aux/IAA-mediated auxin signalling (Supporting Information S1: Figure 7). This result may also explain the weak phenotype of the quadruple mutants. Because IAA19 and IAA29 are activated by certain ARFs during auxin signalling (Krogan et al., 2014; Tatematsu et al., 2004) and by PIF4/5/7 during light/shade signalling (Nguyen et al., 2023; Pucciariello et al., 2018; Sun et al., 2013), we focused on IAA19 and IAA29. The expression levels of IAA19 and IAA29 in the hypocotyls of the sextuple mutants are significantly upregulated (Figure 3a). AUX/IAA, GH3 (GRETCHEN HAGEN 3), and SAUR (SMALL AUXIN UP RNA) are three major families of primary auxin responsive genes (Caumon & Vernoux, 2023; Leyser, 2018). Results of the RNA-seq experiment clearly showed that, two terms, ‘regulation of transcription’ and ‘response to auxin’, were highly enriched in the mutant, and multiple GH3 and SAUR genes were significantly upregulated in the hypocotyls of mutant seedlings (see below). The expression levels of certain GH3 and SAUR genes were validated by a qRT-PCR assessment in Figure 3a. Taken together, it seemed that the sextuple mutant had an enhanced nuclear auxin signalling in hypocotyls.
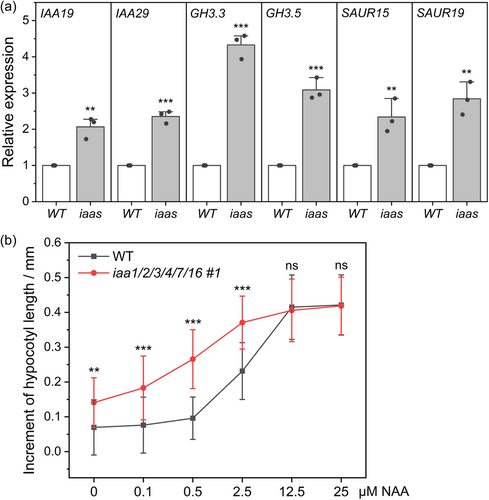
Next, we tested the auxin sensitivity of mutant hypocotyls using a synthetic auxin NAA. The molecule is membrane-permeable to trigger nuclear auxin signalling. Here, young Arabidopsis seedlings were put into liquid media containing various concentrations of NAA (0.1−25 μM). Results showed that, at 0.1 and 0.5 μM of NAA, the sextuple mutant exhibited a much larger increment of hypocotyl elongation than the WT, indicating an enhanced sensitivity to NAA treatment in the mutant. The mutant approached saturation at 2.5 μM of NAA, while the WT was not saturated until 12.5 μM of NAA (Figure 3b).
3.5 Knockouts of IAA1/2/3/4/7/16 led to abnormal expansion of hypocotyl cells
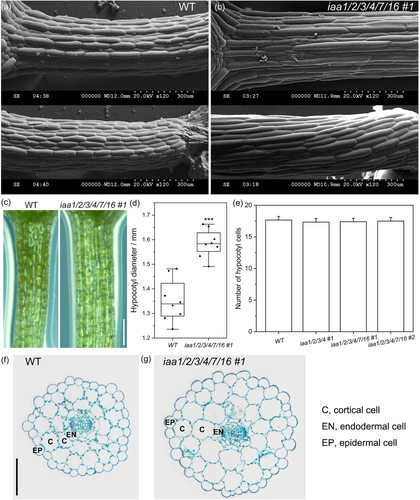
To evaluate the cytological effects of the IAA1/2/3/4/7/16 knockout, we focused on the overelongated hypocotyls of the iaas mutant. Using SEM, we observed that epidermal cells of iaas hypocotyls were dramatically longer than in the WT (from the cotyledon connection to the root connection) (Figures 4a,b). In addition to longitudinal elongation, the transverse dimension of iaas hypocotyls was also larger than in the WT (Figures 4c,d). Based on hypocotyl optical cross-sections, the volume of epidermal and cortical cells increased significantly in the sextuple mutant compared to the WT (Figures 4f,g). However, the number of epidermal cells from the top to the base of the mutant hypocotyls remained unchanged (Figure 4e). Besides, the number of epidermal cells of the periphery cell layer also remained unchanged (Figures 4f,g), indicating that epidermal cells of the mutant and WT hypocotyls do not perform postembryonic cell division. These results indicate that the hypocotyl overelongation phenotype of the sextuple mutant originated from abnormal cell expansion, longitudinally and transversely, rather than from additional cell division.
3.6 Knockouts of IAA1/2/3/4/7/16 induced the expression of cell wall-remodelling genes and shade-responsive genes
Considering that IAA1/2/3/4/7/16 encode transcription repressors, transcriptome profiling is the most appropriate approach to explore the mechanism by which these Aux/IAAs regulate cell expansion in hypocotyls. RNA-seq results revealed that 1135 genes are differentially expressed (DEGs) in the sextuple mutant's hypocotyls compared to the WT, including 1018 upregulated and 117 downregulated genes (Supporting Information S1: Figure 8A). Gene ontology (GO) analysis clearly indicated that ‘regulation of transcription’ and ‘response to auxin’ terms were highly enriched because of the knockouts of six Aux/IAAs. Interestingly, the terms ‘plant-type cell wall’, ‘extracellular region’ and ‘cell wall organisation’ were also highly enriched in the mutant (Supporting Information S1: Figure 8B). Pathway enrichment analysis highlighted ‘plant-pathogen interaction’, ‘phenylpropanoid biosynthesis’ and ‘plant hormone signal transduction’ as having the lowest p Values (Supporting Information S1: Figure 8C). Heatmap analysis based on RNA-seq data indicated that a set of cell wall-remodelling genes were upregulated in the sextuple mutant (Figure 5a). The expression levels of these genes were detected by qRT-PCR, and results indicated that these genes were significantly upregulated in the sextuple mutant compared to the WT (Figures 5b,c).
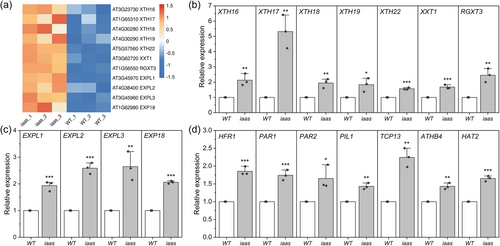
The expression levels of multiple feature transcription factor/repressor genes related to shade response were also upregulated in the hypocotyls of the sextuple mutant seedlings, although they were grown under white light control condition (Figure 5d). The expression levels of two HD-Zip genes (ATHB4 and HAT2), four PIF repressor genes (HFR1, PAR1/2 and PIL1), and one TCP gene (TCP13) were validated by qRT-PCR (Figure 5d).
3.7 IAA2 and/or IAA7 efficiently inhibit the promoter activity of certain XTH and EXPL genes
Dual-luciferase (dual-LUC) assays were conducted in tobacco (Nicotiana benthamiana) leaves to determine whether Aux/IAAs really modulate the expression of cell wall-remodelling genes. Promoters of XTH16/17 and EXPL2/L3 were individually used to drive the expression of LUC, which encodes a firefly luciferase. In each construction, the expression of REN, encoding a renilla luciferase and under a 35 S promoter, was used as an internal reference to eliminate the influence of transformation efficiency. For Aux/IAAs, we chose IAA2/7/16 for the test based on the fact that they played most important roles in regulation of hypocotyl elongation (Supporting Information S1: Figure 4). For cell wall-remodelling genes, we chose XTH16, XTH17, EXPL2 and EXPL3 for the test based on their higher upregulation in the sextuple mutant hypocotyls.
The cotransformation of the XTH16pro::LUC or XTH17pro::LUC reporter constructs with the 35 S::3 × FLAG-IAA2 effector construct led to a dramatic decrease in the LUC enzyme activity compared to the 35 S::3 × FLAG control effector (Figure 6a). Quantitatively, the LUC/REN ratio indicated that the expression of XTH16pro::LUC or XTH17pro::LUC was reduced by IAA2 to half to one-third of the control (Figure 6b). IAA7 and IAA16 could not inhibit the promoter activity of XTH16 and XTH17 (Figure 6a).
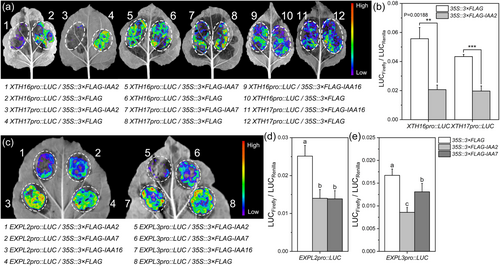
The cotransformation of the EXPL2pro::LUC or EXPL3pro::LUC reporter construct with the 35 S::3 × FLAG-IAA2 or 35 S::3 × FLAG-IAA7 effector construct weakened the LUC activity compared to the 35 S::3 × FLAG control (Figure 6c). The LUC/REN ratio indicated that the expression of EXPL2pro::LUC or EXPL3pro::LUC was reduced by IAA2 to half of the negative control. The suppression ability of IAA7 was lower than that of IAA2 (Figure 6d), while IAA16 could not inhibit the promoter activity of EXPL2 and EXPL3 (Figure 6c).
To further confirm the function of IAA2 and IAA7 in hypocotyl elongation, we performed the complementary test. As expected, both the 35 S::3 × FLAG-IAA2 construct and the 35 S::3 × FLAG-IAA7 construct can partially mitigate the over-elongation phenotype of the hypocoytls of the sextuple mutant (Supporting Information S1: Figure 9), indicating that IAA2 and IAA7 are required for the optimal regulation of hypocotyl elongation.
4 DISCUSSION
4.1 Generation of higher-order mutants to genetically dissect the functions of Aux/IAAs
In canonical nuclear auxin signalling, class A ARFs (ARF5/6/7/8/19) are transcription activators that ultimately determine which genes become upregulated by binding to the cis-regulatory element, AuxREs (Rienstra et al., 2023; Truskina et al., 2021). A previous study has confirmed that ARF6/7/8 efficiently regulate hypocotyl elongation and shade response in Arabidopsis (Reed et al., 2018). The TGTCNN motif of AuxREs has multiple variants, with CC, GG, GA and TC being the most common. Among these variants, the TGTCCC variant elicits the strongest auxin response. Evolutionarily conserved, the TGTCCC variant is enriched in a subset of Aux/IAA genes, while the TCTCGG variant is associated with cell wall biosynthesis genes (Lieberman-Lazarovich et al., 2019). The Arabidopsis IAA1/2/3/4 genes are closely related, with unique pairs of TGTCCC variants (Supporting Information S1: Figure 2; Lieberman-Lazarovich et al., 2019; Remington et al., 2004), but no higer-order mutant has been reported.
By investigating chromatin modifications and accessibility, it has been indicated that the loci of class A ARFs are constitutively open for transcription, but there is a network of transcription repressors regulating the expression levels of class A ARFs to precisely modulate the auxin signalling output. Post-transcriptionally, class A ARFs are specifically inhibited by Aux/IAAs through the interaction between the PB1 (Phox and Bem1) domains (Cancé et al., 2022; Lanctot and Nemhauser, 2020). Canonical Aux/IAAs are short-lived proteins in favour of the fine-tuning of auxin signalling (Figueiredo and Strader, 2022). However, functional redundancy among the Arabidopsis Aux/IAAs members hinders the elucidation of these fine functions.
The development of the CRISPR/Cas9 genome editing technique for Arabidopsis makes it possible to efficiently generate loss-of-function mutants for specific genes or gene sets. We first generated single mutants of Arabidopsis IAA1, IAA2, IAA3 and IAA4 genes (Figure 2). These genes being distributed on different Arabidopsis chromosomes, it is not hard to generate double, triple and quadruple mutants by crossing and screening, although the whole process is time-consuming (Supporting Information S1: Figure 3 and Table 1). However, IAA2 (AT3G23030) and IAA7 (AT3G23050) being tightly linked, it is impossible to generate double mutants by crossing and screening. Taking advantage of the CRISPR/Cas9 genome editing technique again, we knocked out IAA7 and IAA16 in the iaa1/2/3/4 background. The obvious phenotypes of the sextuple mutants (Figures 1, 2 and 5) supported the idea that higher-order mutants can contribute to the genetic dissection of Aux/IAA genes.
Since it was first developed in 2008, the Arabidopsis quadruple mutant of PIFs (pifq, pif1/3/4/5) has become one of the most used materials in light and auxin biology (Leivar et al., 2008). Combination and characterisation of all possible double, triple, quadruple, quintuple and sextuple mutants of the Arabidopsis TIR1/AFB auxin receptor genes revealed overlapping and specialised functions (Prigge et al., 2020). Recently, two research teams, one by combining the T-DNA insertion mutants and the other using the CRISPR/Cas9 genome editing technique, independently generated octuple mutant lines for the entire Arabidopsis group II GH3 genes, which encode enzymes involved in auxin conjugation. With the help of these mutants, new features of auxin homoeostasis regulation and cooperation between auxin and ABA (abscisic acid) signalling have been revealed (Casanova-Sáez et al., 2022; Guo et al., 2022).
4.2 The integration between light and auxin signalling
Under white light photoperiodic conditions, the iaas sextuple mutant remarkably exhibited SAS-like phenotypes with overelongated hypocotyls and petioles and hyponastic leaves (Figure 1 and Supporting Information S1: Figure 4 and 5). The sextuple mutants were highly sensitive to low light intensity (Figure 2) and exogenous auxin (Figures 2 and 3). Microscopic observation revealed that the hypocotyl cells of the sextuple mutant were under excessive cell expansion (Figure 4). A qRT-PCR analysis revealed that the expression level of YUC2/5/8/9 genes, which are direct targets of PIFs, remained unchanged in the sextuple mutant (Figure 1). However, IAA19/MSG2 and IAA29, two shared shade/auxin signalling targets, were upregulated (Figure 3). Besides, genes encoding cell wall-remodelling proteins and shade-responsive transcription regulators were also upregulated in the sextuple mutant (Figure 5). These results indicate that inactivation of the IAA1/2/3/4/7/16 gene set promoted shade response and auxin signalling without activating the phyB-PIFs-mediated auxin biosynthesis pathway.
In Arabidopsis, during the early stage of SAR, the role of auxin level in the cooperation between shade and auxin signalling is well-established (Franklin, 2008; Hersch et al., 2014; Iglesias et al., 2018; Korver et al., 2018; Smith & Whitelam, 1997). However, the mechanism under persistent shade, when auxin levels have declined to normal, remains unclear. Local PIFs are involved in the SAR of Arabidopsis hypocotyls to activate the expression of XTHs (Kohnen et al., 2016). Local auxin production or auxin responsiveness are crucial in SAR (de Wit et al., 2015; Michaud et al., 2017). As one of the mechanisms, PIF4 directly activates the expression of IAA19 and IAA29 to modulate auxin signalling and SAR without enhancing auxin levels (Pucciariello et al., 2018). Two new reports, PIF4/7 upregulated the expresson of IAA5/6/19 in Arabidopsis hypocotyl during SAR (Nguyen et al., 2023); and PIF4 enhaced the expression of multiple SMALL AUXIN-UP RNA genes to promote growth in response to nitrate (Pereyra et al., 2023). Previously, it has been suggested that the PIF4/5-IAA19/29 module is involved in the coordination between blue light and auxin signalling (Sun et al., 2013). Here, the SAS-like phenotypes of the sextuple mutant provide genetic evidence that the IAA1/2/3/4/7/16-mediated auxin signalling is involved in the optimal integration between light and auxin signalling pathways.
The convergence between light and auxin signalling also occurs through protein-protein interactions. As reported, photoactivated CRY1 and phyB may interact directly with IAA3/7/12/13/17 to attenuate their associations with TIR1 (Xu et al., 2018), while phyA may stabilise IAA1/3/7/17 to prevent plants from excessive SAR (Yang et al., 2018). IAA3 supposedly represses the function of PIFs to coordinate light and auxin signalling (Xi et al., 2021). With regard to ARFs—the binding target of Aux/IAA—photoexcited CRY1 and phyB may interact directly with ARF6 and ARF8 to regulate auxin-induced hypocotyl elongation (Mao et al., 2020). Alternatively, phyB may stabilise IAA14 to enhance the suppression of ARF7 and ARF19 (Li, Li, et al., 2021; Li, Zhang, et al., 2021). The brassinazole resistant 1 (BZR1)-ARF6-PIF4 module synergistically regulates many shared target genes that may ultimately trigger cell expansion during phytohormone and environmental responses (Krahmer & Fankhauser, 2024; Pereyra et al., 2023; Quint et al., 2016). The higher-order mutants generated here should help in future investigations to explore the complicated interactions among signal sensors, transcription activators and transcription repressors. Since warm temperatures also induce elongation growth of hypocotyls and petioles, and hyponasty of leaves (Casal & Fankhauser, 2023; Quint et al., 2016), it is of great interest to test these aux/iaa mutants for elevated temperature response. Such studies are important to reveal the precise role of Aux/IAA-mediated nuclear auxin signalling in the developmental control and the environmental response.
4.3 A network of cell wall-remodelling proteins
Transcriptome profiling and qRT-PCR analyses revealed that the sextuple mutant seedlings predominantly upregulated genes encoding cell wall-remodelling proteins, including XTHs and EXPLs (Figure 5 and Supporting Information S1: Figure 8). Using dual-luciferase reporter assays, we verified that IAA2 and/or IAA7 target the promoters of XTH16/17 and EXPL2/L3 to inhibit the expression of reporter genes (Figure 6). Overexpressing IAA2 or IAA7 partially rescued the overelongated hypocotyl phenotype of the sextuple mutant (Supporting Information S1: Figure 9). These results indicate that the IAA/2/3/4/7/16 set of Aux/IAAs inhibits cell expansion by negatively regulating the expression of cell wall-remodelling genes.
To control cell expansion, a set of PIFs and ARFs share the same target genes encoding cell wall-remodelling proteins, including EXPs, XTHs and EXTs (Cosgrove, 2023; Pedmale et al., 2016). The plant cell wall is mainly composed of cellulose, hemicellulose, pectin and lignin, which are connected in a complex manner (Jobert et al., 2023). Cell expansion requires water uptake and irreversible cell wall extension, including wall loosening (short time frame) and new wall material deposition (long time frame) (Perrot-Rechenmann, 2010). EXPs are activated in an acidic environment and disrupt noncovalent binding between cellulose and hemicelluloses (Cosgrove, 2023; Perrot-Rechenmann, 2010). XTHs can cut and paste xyloglucans, contributing to wall loosening by modifying wall properties or cell expansion by facilitating the integration of new wall materials. The content of xyloglucan fragments was decreased in the double mutant xth19 xth23 (Xu et al., 2020). In KEGG pathway analysis, the overrepresentation of the term ‘plant-pathogen interaction’ was most likely caused by the change in cell wall composition. As reported, changes in cell wall components may disturb plant resistance to disease (Wan et al., 2021). The enrichment of the term ‘phenylpropanoid biosynthesis’ may indicate that this pathway is related to lignin biosynthesis, which affects cell wall rigidity (Dong and Lin, 2021).
By integrating our work and previously established mechanisms, we propose a role of Arabidopsis IAA1/2/3/4/7/16 in the coordination of light and auxin signalling (Supporting Information S1: Figure 10). Auxin induces TIR1/AFBs to degrade Aux/IAAs to release ARFs, activating the transcription of downstream genes, including Aux/IAAs, forming a negative feedback loop. ARFs and PIFs physically interact to regulate a set of shared target genes, including cell wall-remodelling genes XTHs and EXPLs and the Aux/IAA genes IAA19/29. Sextuple knockouts of IAA1/2/3/4/7/16 confers SAS-like phenotype to Arabidopsis under white light control conditions because of the distribution of the optimal integration of auxin and light signalling. Considering that the Aux/IAA gene family is highly conserved, our work may also benefit agricultural and ecological studies.
ACKNOWLEDGEMENTS
We thank Dr. Holger Puchta (Karlsruhe Institute of Technology, Germany) for the CRISPR/Cas9 vectors and VIB-Ghent University for plant GATEWAYTM destination vectors. This work was supported by a China Agriculture Research System project (CARS-05-05A) to N. H., the National Natural Science Foundation of China (no. 32000363 to T. Q., no. 31971932 to H. B.), and the Natural Science Foundation of Zhejiang Province (no. LY18C020001 to J. W., no. LQ21C060006 to T. Q.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The materials that support the findings of this study are available from the corresponding author upon reasonable request.




