The SCL30a SR protein regulates ABA-dependent seed traits and germination under stress
Tom Laloum and Sofia D. Carvalho contributed equally to this work.
Abstract
SR proteins are conserved RNA-binding proteins best known as splicing regulators that have also been implicated in other steps of gene expression. Despite mounting evidence for a role in plant development and stress responses, the molecular pathways underlying SR protein regulation of these processes remain poorly understood. Here we show that the plant-specific SCL30a SR protein negatively regulates ABA signaling to control seed traits and stress responses during germination in Arabidopsis. Transcriptome-wide analyses revealed that loss of SCL30a function barely affects splicing, but largely induces ABA-responsive gene expression and genes repressed during germination. Accordingly, scl30a mutant seeds display delayed germination and hypersensitivity to ABA and high salinity, while transgenic plants overexpressing SCL30a exhibit reduced ABA and salt stress sensitivity. An ABA biosynthesis inhibitor rescues the enhanced mutant seed stress sensitivity, and epistatic analyses confirm that this hypersensitivity requires a functional ABA pathway. Finally, seed ABA levels are unchanged by altered SCL30a expression, indicating that the gene promotes seed germination under stress by reducing sensitivity to the phytohormone. Our results reveal a new player in ABA-mediated control of early development and stress response.
1 INTRODUCTION
Seed germination begins with rehydration (imbibition) and expansion of the embryo by cell elongation, which leads to rupture of the weakened seed coat and emergence of the radicle. During water uptake, a prolonged non-germinating state termed seed dormancy must be relieved before protrusion of the radicle (for reviews, see Holdsworth et al., 2008a, 2008b; Shu et al., 2016). The completion of seed germination marks a key developmental milestone in the life cycle of higher plants, being essential for the establishment of a viable plant. The germination process is highly regulated by both endogenous and environmental signals that determine the dormancy status of the seed and its aptitude to germinate.
The plant hormone abscisic acid (ABA) promotes seed maturation and dormancy while inhibiting seed germination, thus acting as a key regulator of this critical developmental step (for review, see Chen et al., 2020). In fact, mutations that affect components of ABA biosynthesis (e.g., aba2) or signaling (e.g., snrk2.2/3/6) exhibit reduced seed dormancy and precocious germination (Leon-Kloosterziel et al., 1996; Nakashima et al., 2009). ABA has also more recently been implicated in the control the seed's final size via regulation of endosperm cellularization during seed development (Cheng et al., 2014).
Apart from regulating key developmental processes such as seed germination, ABA is a known major mediator of osmotic stress responses, also in seeds where it acts as an integrator of different environmental signals to repress germination under unfavorable conditions. While numerous studies have deciphered the genetic components and transcriptional mechanisms underlying seed germination and osmotic stress responses, the involvement of posttranscriptional gene regulation, namely of alternative splicing, is beginning to unfold (Narsai et al., 2017; for reviews, see Laloum et al., 2018; Lou et al., 2020).
Serine/arginine-rich (SR) proteins are multi-domain RNA-binding factors known to regulate pre-mRNA splicing. They use one or two of their N-terminal RNA recognition motifs (RRMs) to bind specific cis-acting elements in the pre-mRNA and enhance or repress splice site recognition. SR proteins recruit core spliceosomal components to nearby splice sites through their C-terminal arginine/serine (RS) domain, which acts as a protein–protein interaction module. The RS domain is also subjected to numerous reversible phosphorylation events that control SR protein activity and subcellular localization (for reviews, see Barta et al., 2008; Morton et al., 2019; Shepard & Hertel, 2009; Zhou & Fu, 2013).
Apart from pre-mRNA splicing, noncanonical functions for SR proteins in pre-and post-splicing activities have emerged, highlighting their multifaceted roles as important coordinators of nuclear and cytoplasmic gene expression machineries. In one example, the mammalian SR protein SRSF2 was shown to mediate the activation of the paused Pol II by releasing the positive transcription elongation factor b (p-TEFb) from inhibitory 7SK ribonucleoprotein complexes, thus promoting transcriptional elongation (Ji et al., 2013). Furthermore, changes in SRSF2 levels have been shown to affect the accumulation of Pol II at gene loci (Lin et al., 2008). More generally, SR proteins influence gene transcription by directly or indirectly interacting with the C-terminal domain of RNA Pol II during their assembly as RNA processing factors. Animal SR proteins have also been shown to influence mRNA export, translation and decay by interacting with major components of the molecular complexes regulating these processes (for review, see Wagner & Frye, 2021).
Functional analyses of individual SR and SR-like proteins in plants have identified stress roles for these proteins. Knockout mutants for the Arabidopsis RS40 and RS41 display hypersensitivity to ABA during seed germination and to the inhibitory effect of salt on root elongation (Chen et al., 2013). Moreover, RSZ22 is a putative dephosphorylation target of the Clade A protein phosphatase 2C HAI1, a major component of ABA and osmotic stress signaling in Arabidopsis (Chong et al., 2019). The SR-like SR45a was recently reported to inhibit salt stress tolerance in Arabidopsis by interacting with the RNA cap-binding protein CBP20 and regulating alternative splicing of transcripts involved in the response to high salinity (Li et al., 2021). In addition to salt stress responses (Albaqami et al., 2019), the other Arabidopsis SR-like protein, SR45, regulates sugar responses by repressing both ABA signaling and glucose-induced accumulation of the hormone (Carvalho et al., 2010, 2016), with SR45-bound transcripts being markedly enriched in ABA signaling functions (Xing et al., 2015). More recently, the rice RS33 was found to modulate responses to high salinity and low temperature and to regulate alternative splicing of stress-responsive genes (Butt et al., 2022).
The Arabidopsis genome encodes 18 SR proteins, 10 of which are orthologs of the human ASF/SF2, 9G8 or SC35, while members of the RS, RS2Z and SCL subfamilies are plant-specific (Barta et al., 2010). SCL30a belongs to the latter subfamily, whose four members (SCL28, SCL30, SCL30a and SCL33) are similar to SC35 but display a distinctive short N-terminal charged extension rich in arginines, serines, glycines and tyrosines (Barta et al., 2010). SCL30a interacts with the RS2Z33 SR protein (Lopato et al., 2002) and acts redundantly with its paralog SCL33 to control alternative splicing of a specific intron in the SCL33 pre-mRNA (Thomas et al., 2012). A more recent study described pleiotropic developmental phenotypes for a quintuple mutant of the four SCL and the SC35 genes, including serrated leaves, late flowering, shorter roots and abnormal silique phyllotaxy, while the corresponding single mutants did not show obvious phenotypic alterations (Yan et al., 2017). Furthermore, all four SCL members and SC35 localize in nuclear speckles and interact with major components of the early spliceosome machinery U170K and U2AF65a (Yan et al., 2017), supporting a function as splicing regulators. Interestingly, these five Arabidopsis SR proteins were also reported recently to interact with the NRPB4 subunit of RNA Pol II, indicating a potential role in the regulation of transcription (Yan et al., 2017).
Here, we characterized the plant-specific SCL30a gene in Arabidopsis and found that the encoded protein affects ABA-related seed traits and retards germination. Loss of SCL30a function changes the splicing pattern of a surprisingly limited number of genes, but prevalently upregulates the expression of ABA-induced genes and many genes repressed during the seed germination process. In agreement, loss-of-function SCL30a mutations cause strong hypersensitivity to ABA and salt stress during seed germination, whereas overexpression of SCL30a reduces seed sensitivity to ABA and high salinity during germination. Epistatic and pharmacological analyses demonstrate that SCL30a's function in seeds and stress response depends on ABA synthesis and signaling, demonstrating a key role for this RNA-binding protein in ABA-mediated responses.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
The Arabidopsis thaliana ecotype Colombia (Col-0) was used as the wild type in all experiments. Seeds were surface-sterilized for 10 min in 50% (v/v) bleach and 0.07% (v/v) TWEEN®20, stratified for 3 days at 4°C in the dark and plated on MS media [1X Murashige and Skoog (MS) salts (Duchefa Biochemie), 2.5 mM MES (pH 5.7), 0.5 mM myo-inositol and 0.8% (w/v) agar], before transfer to a growth chamber under 16-h photoperiod (long-day conditions) or continuous light (cool white fluorescent bulbs, 18W840, 4000 K at 100 µmol m−2 s−1) at 22°C (light period) or 18°C (dark period) and 60% relative humidity. Seed imbibition (Figures 1, 2, 5, and 6c) was always performed at 4°C (equivalent to stratification). After 2–3 weeks, seedlings were transferred to soil in individual pots.
PCR-based genotyping of the SALK_041849 (scl30a-1) and SAIL_512B11 (scl30a-2) lines (obtained from NASC) with primers specific for SCL30a and the left border of the T-DNA (Table S7) followed by sequencing of the genomic DNA/T-DNA junction confirmed the insertion sites and allowed isolation of homozygous lines. The scl30a-1 line was backcrossed twice with the wild type and independently crossed with the aba2-1 (Leon-Kloosterziel et al., 1996) and abi4-101 (Finkelstein, 1994) alleles (obtained from NASC), with the corresponding double mutants being identified via PCR screening (Table S7) of F2 progeny following F1 self-fertilization.
2.2 Generation of transgenic plants
Plant transformation was achieved by the floral dip method (Clough & Bent, 1998) using Agrobacterium tumefaciens strain EHA105.
For reporter gene experiments, the 2206 bp immediately upstream of the SCL30a start codon were PCR-amplified (Table S7) from genomic DNA and subcloned into the pGEM vector (Promega), where the eGFP-GUS segment isolated from the pKGWFS7 vector (Karimi et al., 2002) using the SacII/NcoI restriction sites was fused at the 3′ end of the SCL30a promoter sequence. The entire fragment was transferred into pKGWFS7 via the SpeI/NcoI restriction sites, replacing the original CmR-ccdB-eGFP-GUS cassette, and the construct agroinfiltrated into Col-0 plants.
To generate the Pro35S:SCL30a.1 construct, an RT-PCR fragment corresponding to the SCL30a.1 transcript (Table S7) was inserted into the pBA002 backbone using the AscI/PacI restriction sites, and the construct agroinfiltrated in Col-0 plants. Two independent SCL30a-OX lines were first isolated and analyzed. After several seed-to-seed cycles, expression of the transgene in these SCL30a-OX2 and SCL30a-OX3 lines was silenced, with consequent loss of the corresponding phenotypes. A third overexpression line, SCL30a-OX1, was then generated and phenotypically characterized.
2.3 Seed measurements and composition
Wild-type (Col-0) and mutant (scl30a-1) plants were sown and grown to maturity simultaneously under identical conditions, and all assays were performed with seeds from comparable lots.
The area of dry and imbibed seeds was measured using the ImageJ software (https://rsbweb.nih.gov/ij). To determine seed weight, six groups of 1000 dry seeds were weighed using an Acculab ALC-80.4 (Sartorius) analytical balance.
For compositional analysis, dry seeds were bulk harvested by genotype and homogenized. The oil, protein and soluble carbohydrate contents were determined as described previously (Meyer et al., 2012). To analyze fatty acids, dry seeds (20 mg) were crushed and sonicated in 2 mL of heptane for 15 min at 60°C. After centrifuging for 5 min at 2000 g, 200 µL of the heptane layer were transferred to a small vial with 50 µL of trimethylsulfonium hydroxide (TMSH) in methanol and an additional 300 µL of heptane. After incubation for 30 min at room temperature, 1 µL of the upper heptane layer was used to analyze the fatty acid methyl esters, which were separated and quantified using a Hewlett-Packard 6890 gas chromatograph as described in Cahoon et al. (2001). All analyses were performed in duplicate on three independent seed batches per genotype.
2.4 Germination and dormancy assays
For germination assays, fully mature siliques from dehydrated plants were collected and stored in the dark at room temperature for at least one week before phenotypical analysis. After surface-sterilization and stratification for 3 days at 4°C in the dark, 70–100 seeds per biological replicate were sown on MS media supplemented or not with the appropriate concentrations of NaCl, ABA (mixed isomers, A1049; Sigma) or fluridone (45511, Fluka) and then transferred to long-day conditions, except for the determination of germination rates under control conditions (Figures 2c and 5c), which was conducted under continuous light to avoid the effect of long dark periods during a short time course. To assess dormancy, seeds from freshly mature siliques were collected from the tree, immediately surface-sterilized and plated on MS media before transfer to dark at 22°C, with control seeds being stratified for 3 days at 4°C in the dark before transfer to long-day conditions. Percentages of seed germination, defined as protrusion of the radicle through the seed coat, were scored over the total number of seeds. The results presented are representative of at least three independent experiments.
2.5 ABA content determination
Mature seeds harvested from Col-0, scl30a-1, SCL30a-OX2, or aba2-1 dehydrated plants and stored for 5 months were stratified for 3 days at 4°C in the dark, sown on MS media with or without 200 mM NaCl and grown for 2 days under long-day conditions. Seeds were then collected and endogenous ABA levels quantified using an immunoassay as described in (Carvalho et al., 2010).
2.6 Expression and alternative splicing analyses of individual genes
Histochemical staining of GUS activity in ProSCL30a:GUS transgenic lines was performed as described by Sundaresan et al. (1995).
For the RT-PCR analyses shown in Figures 1, S1, S2b, and S4a, total RNA was extracted from different plant tissues using TRI Reagent (T924; Sigma-Aldrich) or from dry, imbibed and up to 5-day germinated seeds using the innuPREP Plant RNA Kit (Analytik Jena). First-strand cDNA synthesis and PCR amplification were performed as described in (Carvalho et al., 2012), using the primers and number of cycles indicated in Table S7 as well as the ROC1 and UBQ10 as reference genes. Results are representative of at least three experiments.
For the RT-qPCR analyses shown in Figure 2d, seeds were stratified for 3 days at 4°C, sown on MS media, transferred to continuous light conditions, and collected after 18 h (before radicle emergence) to avoid major developmental effects. For the RT-qPCR of Figures 5a, S2c, and S5b, seedlings were grown for 1 week after stratification. Total RNA was extracted using the innuPREP Plant RNA Kit (Analytik Jena), digested with the RQ1 DNase (Promega), and first strand cDNA synthesized using 1 µg RNA, Super Script III Reverse Transcriptase (Invitrogen) and a poly-T primer. qPCR was performed using an ABI QuantStudio sequence detection system (Applied Biosystems) and Luminaris Color HiGreen qPCR Master Mix (Thermo Scientific) on 2.5 µL of cDNA (diluted 1:10) per 10-µL reaction volume, containing 300 nM of each gene-specific primer (Table S7). Reaction cycles were 95°C for 2 min (1X), 95° C for 30 s/60°C for 30 s/72°C for 30 s (40X), followed by a melting curve step to confirm the specificity of the amplified products. UBQ10 and ROC5 were used as reference genes. Each experiment was replicated at least three times.
For the analyses of alternative splicing shown in Figure S3, PCR with the NZYTaq II 2x Green Master Mix (Nzytech) was performed on cDNA from three biological replicates of germinating seeds (18 h after stratification, continuous light) using primers flanking the alternatively spliced intron (Table S7) obtained from PASTDB (pastdb.crg.edu). Reaction cycles were 95°C for 3 min (1X), 95°C for 30 s/58°C for 30 s/72°C for 5 min (35X). The PCRs products then were loaded on a 2% agarose gel and gel bands quantified using the ImageJ software (https://rsbweb.nih.gov/ij). The percent spliced-in (PSI) for each alternative splicing event was calculated after quantification of the inclusion (I) or splicing (S) for a given event as PSI = I/(I + S).
2.7 RNA-seq sample preparation and sequencing
Approximately 50 mg of Col-0 wild type and scl30a-1 mutant seeds (three biological replicates) were surface-sterilized, stratified at 4°C for 3 days and sown on MS media for 18 h under continuous light, before total RNA was extracted using the innuPREP Plant RNA Kit (Analytik Jena). The RNA-seq libraries generated from Col-0 and scl30a-1 seeds were prepared and sequenced at the Center for Genomic Regulation using the HiSeq Sequencing V4 Chemistry kit (Illumina, Inc) and the HiSeq. 2500 sequencer (Illumina, Inc), with a read length of 2 × 125 bp.
2.8 RNA-seq quantification of sequence inclusion and identification of differentially-spliced genes
We employed vast-tools v2.5.1 to quantify alternative splicing from RNA-seq for A. thaliana (Martín et al., 2021; Tapial et al., 2017). This tool quantifies exon skipping (ES), intron retention (IR), and alternative 3′ (Alt3) and 5′ (Alt5) splice sites. For all these types of events, vast-tools estimates the percent of inclusion of the alternative sequence (PSI) using only exon-exon (or exon-intron for IR) junction reads and provides information about the read coverage (see https://github.com/vastgroup/vast-tools for details). To identify alternative splicing events regulated by SCL30a we used vast-tools compare. This function compared PSI values of each alternative splicing event with sufficient read coverage in all wild-type and scl30a-1 samples being tested (three biological replicates of each genotype) and selected those with an average |∆PSI|> 15 and a |∆PSI| between the two distributions >5 (−min_dPSI 15 −min_range 5) (see https://github.com/vastgroup/vast-tools and (Tapial et al., 2017) for details). We also used the −p_IR filter to discard introns with a significant read imbalance between the two exon-intron juntions (p < 0.05, binomial test; see Braunschweig et al. (2014) for details). Moreover, to ensure that Alt3 and Alt5 are located in exons with a sufficient inclusion level, we used the option –min_ALT_use 25, which implies that the host exon has a minimum PSI of at least 25 in each analyzed sample.
2.9 RNA-seq quantification of gene expression and identification of differentially expressed genes
Quantification of Arabidopsis transcript expression from our RNA-seq experiment and public sequencing data on seed germination (GSE94459) was performed using vast-tools v2.5.1 (Tapial et al., 2017). This tool provides cRPKMs numbers for each Arabidopsis transcript as the number of mapped reads per million mapped reads divided by the number of uniquely mappable positions of the transcript (Martín et al., 2021). To identify differentially expressed genes between wild-type and scl30a-1 germinating seeds, we used vast-tools compare_expr using the option -norm (see https://github.com/vastgroup/vast-tools for details). In brief, a quantile normalization of cRPKM values with “Normalize Between Arrays” within the “limma” package of R is first performed. Next, genes that were not expressed at cRPKM > 5 are filtered out and read counts >50 across all the replicates of at least one of the genotypes compared. Graphs in Figures 3 and 4 only show expression of genes that passed these cut-offs. Finally, differentially-expressed genes were defined as those with a fold change of at least 2 between each of the individual replicates from each genotype.
2.10 Assessment of overlap between SCL30a- and ABA-regulated genes
ABA-regulated genes were obtained from the reanalysis of GSE62876 (Costa et al., 2015) using the default settings of GEO2R (https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html). Three comparisons were conducted: 0 h of ABA treatment versus 2, 12, or 24 h. Genes regulated at any of these timepoints were selected (FC > 2; adjusted p-value < 0.05). Given that ABA-regulated genes in (Costa et al., 2015) are defined based on microarray studies, which do not assess expression of all Arabidopsis genes as RNA-seq experiments do, for the comparison we discarded one SCL30a-regulated gene not represented in the microarray. We also discarded ABA-regulated genes not expressed in our RNA-seq samples (see previous section).
2.11 Gene ontology enrichment analyses
The Gene Ontology (GO) enrichment analysis shown in Figure 4, which identifies significantly enriched biological processes, molecular functions and cellular components among the genes up- and downregulated in the scl30-1 mutant, was performed using the functional annotation classification system DAVID version 6.8 (Huang et al., 2009). Only statistically significant GO categories (p < 0.05) are shown in Table S3.
3 RESULTS
3.1 SCL30a expression is markedly induced during seed germination
To initiate the characterization of the Arabidopsis SCL30a gene and investigate its expression pattern, we generated transgenic plants expressing the β-glucuronidase (GUS) reporter gene under the control of the SCL30a endogenous promoter. The SCL30a promoter was active throughout plant development (Figure 1). We observed GUS staining in vascular tissues and actively dividing cells, such as in the shoot meristem and young leaves (Figure 1a), the primary root tip (Figure 1b) and lateral root primordia (Figure 1c). At the reproductive phase, SCL30a appeared to be particularly expressed in the pistil tip, the vasculature tissue of sepals, the stamen filaments and pollen grains (Figure 1d) of developing flowers. In embryonic tissues, the SCL30a promoter was active from the early—globular and heart (Figure 1e-g)—to the late—torpedo and mature embryo (Figure 1h-j)—stages of embryo development. Finally, in imbibed mature seeds, GUS staining was detected in the whole embryo (Figure 1k) as well as strongly in the seed coat (Figure 1l), but was mainly expressed at the radicle tip during germination (Figure 1m).

In parallel, we used reverse transcription polymerase chain reaction (RT-PCR) to study the development- and tissue-specific expression pattern of SCL30a. Consistent with the established promoter:GUS expression profile, SCL30a was expressed both in young seedlings and at later developmental stages, with its mRNA being detected in different aerial tissues, such as leaves, stems, flowers and siliques, but also in roots (Figure S1). In embryonic tissues, although SCL30a transcripts were undetectable in dry seeds, gene expression was clearly observed at 3 days of seed imbibition at 4°C and increased sharply during the first hours of germination upon transfer to 22°C and light (Figure S1).
Both animal and plant pre-mRNAs encoding splicing components appear to be particularly prone to alternative splicing themselves. This has been shown to lead to a dramatic increase of the transcriptome complexity of the Arabidopsis SR protein family (Palusa & Reddy, 2010; Palusa et al., 2007), prompting us to examine the splicing pattern of the SCL30a gene. Although only one transcript has been annotated (www.arabidopsis.org), cloning and sequencing of the PCR products amplified from the SCL30a cDNA identified three alternative mRNAs (Figure S2a,b), consistent with the information available in PASTDB (https://pastdb.crg.eu), a recently developed transcriptome-wide resource of alternative splicing profiles in Arabidopsis (Martín et al., 2021). The shortest and by far most expressed SCL30a.1 transcript (Figures S1 and S2) is predicted to encode the full-length protein, while the other two splice variants encode putative severely truncated proteins (Figure S2a).
Thus, the Arabidopsis SCL30a gene, which produces only one transcript encoding a putative functional protein, displays ubiquitous expression in vegetative tissues and is induced during seed germination.
3.2 Loss of SCL30a function affects key seed traits and delays germination
To investigate the biological roles of SCL30a, we isolated a homozygous T-DNA mutant line, SALK_041849, carrying the insertion in the gene's third exon (Figure S2a). Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) analysis of SCL30a expression in this scl30a-1 mutant using primers annealing upstream of the insertion site revealed an over two-fold reduction in transcript levels when compared with the Col-0 wild type, whereas no expression was detected when primers flanking or annealing downstream of the T-DNA were used (Figure S2c). Consistent with the location of the insertion, no splice variants were detected in the mutant, which only expresses a truncated SCL30a transcript lacking the sequence corresponding to the entire RS domain as well as most of the RRM (Figure S2a,b). These results indicate that the scl30a-1 allele is a true loss-of-function mutant.
Given that we observed no notable defects in adult plants and the marked induction of the SCL30a gene during seed imbibition and germination (see Figures 1 and S1), we focused our phenotypical analysis of the scl30a-1 mutant on embryonic tissues. Notably, mature scl30a-1 seeds displayed a significant reduction in size, with dry and imbibed mutant seeds being 12% and 14% smaller, respectively, than seeds of wild-type plants (Figure 2a). Correlating with their smaller size, dry mature scl30a-1 seeds showed reduced weight when compared to the wild type, but no significant changes in their relative moisture, protein or oil content (Table S1). A more detailed compositional analysis revealed only minor changes in the relative levels of a few unsaturated fatty acids and the trisaccharide raffinose (Table S1), indicating that loss of SCL30a function does not substantially affect nutrient and water storage in embryonic tissues. Furthermore, the scl30a-1 mutant exhibited enhanced seed dormancy: after 7 days at 22°C in darkness, the germination rate of freshly-harvested, non-stratified scl30a-1 seeds was only about one-third of that of wild-type seeds (Figure 2b). The germination speed of stratified scl30a-1 mutant seeds was lower, exhibiting a significant delay when compared to wild-type seeds (Figure 2c).
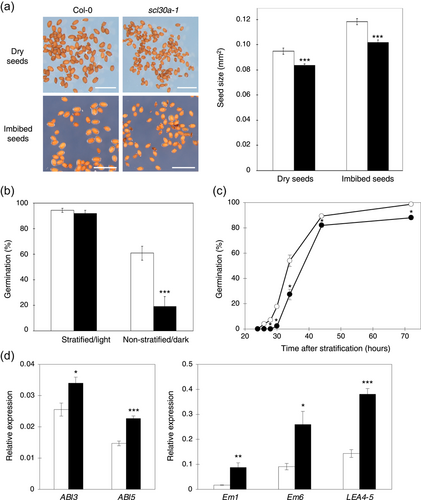
The seed phenotypes of the scl30a-1 mutant prompted us to analyze the expression of the ABI3 and ABI5 genes, two major transcriptional regulators controlling seed development, dormancy and germination (Parcy et al., 1994; Skubacz et al., 2016). RT-qPCR analyses of germinating seeds showed that the expression of ABI5, and to a lesser extent also ABI3, is significantly increased in the scl30a-1 mutant (Figure 2d). In agreement, the expression of Em1, Em6, and LEA4-5, three downstream targets of the ABI3 and ABI5 transcription factors (Bensmihen et al., 2002; Tian et al., 2020), was also upregulated in scl30a-1, even to a larger extent (Figure 2d).
These findings indicate that the SCL30a SR protein plays an in vivo role in embryonic tissues, where it affects morphological and functional seed traits, while controlling the expression of key genes that regulate seed development and germination.
3.3 The SCL30a protein affects the splicing pattern of very few genes during seed germination
To gain insight into the molecular functions of the SCL30a RNA-binding protein, we next conducted an RNA-sequencing (RNA-seq) experiment to compare the transcriptomes of wild-type and mutant germinating seeds. We used the Illumina HiSeq. 2500 system to sequence mRNA libraries prepared from Col-0 and scl30a-1 seeds 18 h after stratification and obtained a minimum of 90 million paired-end clean sequence reads per sample. Given the conserved role of SR proteins in pre-mRNA splicing, we first analyzed the alternative splicing changes caused by the scl30a-1 mutation.
At the time point sampled, we found only 22 alternative splicing events in 21 genes to be differentially regulated in scl30a-1 mutant seeds (|ΔPSI |> 15 ): seven intron retention (IR), three exon skipping (ES) and 12 alternative 3′(Alt3) or alternative 5′ (Alt5) splice site events (Table 1). Although of low magnitude (|ΔPSI| < 25 in all cases), the RNA-seq alternative splicing changes were confirmed in the four events selected for validation by RT-PCR, using wild-type and scl30a-1 RNA samples independent from those analyzed by RNA-seq (Figure S3).
| Gene ID | Gene name | AS event ID | AS event type | WT PSI | scl30a-1 PSI | ΔPSI |
|---|---|---|---|---|---|---|
| AT3G07420 | SYNC2 | AthINT0106607 | IR | 54.1 | 35.1 | 19.0 |
| AT2G19910 | RDR3 | AthINT0098183 | IR | 29.1 | 12.2 | 16.9 |
| AT3G07890 | AthINT0022974 | IR | 26.0 | 9.2 | 16.8 | |
| AT1G06500 | AthINT0001122 | IR | 53.1 | 36.6 | 16.5 | |
| AT2G46915 | AthINT0021222 | IR | 39.0 | 22.5 | 16.5 | |
| AT5G64980 | AthINT0051338 | IR | 37.1 | 20.7 | 16.4 | |
| AT5G09690 | MGT7/MRS2-7 | AthINT0086682 | IR | 27.8 | 12.2 | 15.6 |
| AT3G63445 | AthEX0008460 | ES | 97.3 | 72.4 | 24.9 | |
| AT1G45248 | AthEX0002068 | ES | 70.5 | 52.9 | 17.6 | |
| AT1G10890 | AthEX0000589 | ES | 42.4 | 25.4 | 17.0 | |
| AT1G15410 | AthALTD0000759-2/2 | Alt5 | 89.0 | 68.6 | 20.3 | |
| AT1G34340 | AthALTD0001643-2/3 | Alt5 | 78.8 | 94.9 | -16.1 | |
| AT5G24735 | SORF31 | AthALTD0009809-7/10 | Alt5 | 32.8 | 48.8 | -16.0 |
| AT5G24735 | SORF31 | AthALTD0009809-6/10 | Alt5 | 63.8 | 48.7 | 15.2 |
| AT3G05510 | AthALTA0008736-3/4 | Alt3 | 54.8 | 33.9 | 20.9 | |
| AT3G53470 | AthALTA0011304-2/2 | Alt3 | 34.5 | 55.1 | -20.5 | |
| AT3G22260 | AthALTA0010043-2/2 | Alt3 | 36.2 | 54.6 | -18.5 | |
| AT2G05210 | POT1A | AthALTA0038411-3/4 | Alt3 | 24.3 | 42.6 | -18.3 |
| AT1G06590 | APC5 | AthALTA0022122-3/4 | Alt3 | 56.6 | 72.6 | -16.0 |
| AT3G27460 | SGF29A | AthALTA0010423-2/4 | Alt3 | 25.7 | 41.2 | -15.6 |
| AT4G38330 | AthALTA0014931-4/4 | Alt3 | 74.7 | 59.2 | 15.5 | |
| AT5G47380 | AthALTA0018392-3/3 | Alt3 | 42.6 | 27.4 | 15.2 |
- Note: Genes are ordered by type of alternative splicing (AS) event—intron retention, (IR, in red), exon skipping (ES, in orange), alternative 5’ splice site (Alt5, in blue) and alternative 3’ splice site (Alt3, in green)—and decreasing absolute ΔPSI values.
Interestingly, all of the seven differentially-regulated IR events showed lower inclusion levels in the scl30a-1 mutant, suggesting negative regulation of splicing of these introns by SCL30a. On the other hand, for the three differential ES events identified, the exons were more included in the wild type, pointing to a role of SCL30a in promoting splice site recognition. Thus, IR and ES results provide contradictory clues on the role of this protein in splicing regulation, though the number of alternative splicing events retrieved is too low to draw conclusions on the mechanistic function of SCL30a.
Of the 21 genes differentially spliced in the scl30a-1 mutant, only three have been characterized previously: MRS2-7 encodes a magnesium transporter (Mao et al., 2008), POT1a a DNA-binding protein required for telomere maintenance (Surovtseva et al., 2007) known to be regulated by alternative splicing (Tani & Murata, 2005), and SGF29a is a transcriptional co-activator implicated in salt stress responses (Kaldis et al., 2011). Based on the gene annotation at TAIR (www.arabidopsis.org), another four genes appear to be also involved in transcription or different aspects of RNA metabolism, while seven play putative roles in many different processes, including lipid or nitrogen metabolism, glycolysis, cell division and protein deubiquitination. Yet, one-third of the genes whose splicing was found to be affected by the SCL30a SR protein (i.e., seven genes) are of unknown function.
3.4 The SCL30a protein regulates gene expression involved in seed germination and ABA responses
We next analyzed the gene expression changes caused by loss of function of the SCL30a gene. Our RNA-seq analysis revealed 382 genes whose expression was significantly changed by at least two-fold in the scl30a-1 mutant. Among these, 315 displayed higher transcript levels than the wild type, whereas 67 were downregulated in the scl30a-1 mutant (Table S2).
Given the seed and germination phenotypes of the scl30a-1 mutant (see Figure 2), we then asked whether the genes whose expression was affected by the SCL30a protein were transcriptionally regulated during the seed germination process. To address this question, we quantified the expression levels of the genes up- and downregulated in the scl30a-1 mutant using data from an extensive germination time-course RNA-seq experiment performed by Narsai et al. (2017) (Figure 3). Remarkably, we found that genes repressed by SCL30a (i.e., upregulated in the mutant) are in general highly expressed in dry seeds and downregulated throughout the germination process (Figure 3a). Conversely, genes whose expression is activated by SCL30a (i.e., downregulated in the mutant) show the opposite trend, being lowly expressed in dry seeds and induced during germination (Figure 3b). This finding is in line with the expression pattern of SCL30a (see Figures 1 and S1), as well as with the delay in germination exhibited by the scl30a-1 mutant (see Figure 2c), and points to this SR protein as an important positive regulator of seed germination.

Importantly, among the genes upregulated in the scl30a-1 mutant we found many involved in embryo development, seed maturation and dormancy. They include seed storage proteins (e.g., CRUCIFERIN) and genes involved in the accumulation and storage of lipidic compounds in seeds (e.g., oleosins), as well as genes involved in the acquisition of desiccation tolerance, such as many LATE EMBRYOGENESIS ABUNDANT (LEA) genes (Table S2). In agreement, Gene Ontology (GO) analysis of the scl30a-1-upregulated genes showed clear enrichment for categories related to these developmental processes, such as GO:0045735: nutrient reservoir activity, GO:0019915: lipid storage, GO:0009414: response to water deprivation or GO:0009793: embryo development ending in seed dormancy (Figure 4a and Table S3). Moreover, consistent with the key role played by the ABA hormone in the regulation of seed development, maturation, dormancy and germination, the functional category “GO:0009737: response to abscisic acid” appeared strongly enriched among the scl30a-1-upregulated genes. Indeed, the expression of genes encoding main regulators and targets of the ABA signaling pathway—including the ABI5 bZIP transcription factor (Finkelstein & Lynch, 2000), the seed-specific PP2C AHG1 (Nishimura et al., 2007) and the ABA-responsive dehydrin RAB18 (Nylander et al., 2001) — was found to be significantly enhanced in the mutant (Figure 2d, Tables S3 and S4). On the other hand, many genes found to be downregulated in the scl30a-1 mutant were related to microtubule activity and cell wall remodeling (Figure 4b and Table S3), two important processes known to be activated during germination of the seed (for reviews, see Holdsworth et al., 2008a; Yan et al., 2020).
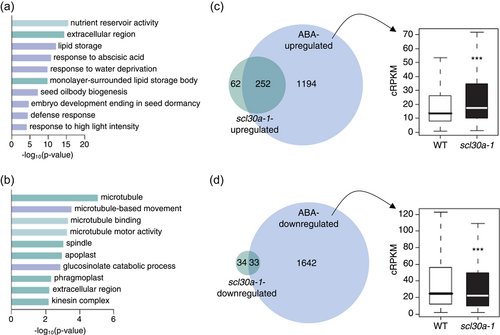
To gain further insight into the extent of SCL30a control of ABA responses during seed germination, we compared the differentially expressed genes in the scl30a-1 mutant with a list of ABA-regulated genes obtained from the reanalysis of a previous microarray experiment performed in germinating seeds submitted to a transient ABA treatment (Costa et al., 2015). Strikingly, 80% (252 genes) of the genes upregulated in the scl30a-1 mutant were also induced by ABA in wild-type germinating seeds (Figure 4c and Table S5), while 49% (33 genes) of the genes downregulated in the scl30a-1 mutant were repressed by ABA (Figure 4d and Table S6). We then analyzed the expression levels of the ABA-regulated genes defined based on [49] in our RNA-seq data. Interestingly, the 1446 genes upregulated by ABA were significantly more highly expressed in scl30a-1 than in the wild type, while the 1675 ABA-downregulated genes were downregulated in our mutant (Figure 4c,d). Together, these results suggest that an important component of SCL30a function during seed germination is to control ABA-mediated transcriptional responses.
3.5 SCL30a promotes seed germination under salt stress
To further characterize and corroborate the functional role of the SCL30a SR protein in seeds, we generated transgenic Arabidopsis lines expressing the full-length SCL30a.1 transcript under the control of the 35 S promoter in the wild-type Col-0 background. Three independent lines noticeably overexpressing the SCL30a.1 mRNA, SCL30a-OX1, SCL30a-OX2, and SCL30a-OX3 (Figures 5a and S4a), were selected for phenotypical characterization. We first assessed the impact of SCL30a overexpression on the seed traits found to be affected by the scl30a-1 mutation (see Figure 2). In contrast to what was observed for the scl30a-1 mutant, imbibed seeds from the SCL30a-overexpressing plants were significantly (10%) larger than those from wild-type plants (Figures 5b and S4b). Furthermore, stratified SCL30a-overexpressing seeds germinated slightly faster under control conditions than wild-type seeds (Figure 5c).
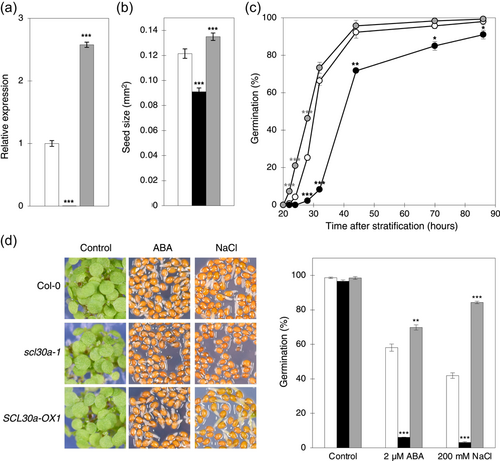
The differential expression of ABA-related genes observed in the scl30a-1 mutant prompted us to analyze ABA response of the different genotypes during germination. We found that the scl30a-1 mutant displays strong hypersensitivity to the hormone (Figures 5d and S4c), with less than 10% of the mutant seeds germinating under ABA concentrations that allowed 75% germination of the wild type (Figure 5d). In agreement, seeds from the SCL30a overexpression lines were less sensitive to the hormone during seed germination (Figures 5d and S4c). Given the established link between ABA and osmotic stress responses, we next examined the effects of loss of function and overexpression of the SCL30a gene on seed germination under salt stress. In line with the effect of exogenously applied ABA, the germination rate of mutant seeds in the presence of 200 mM of NaCl was markedly reduced when compared to those of the wild type, while the SCL30a overexpression lines were hyposensitive to high salinity, germinating twice as well as the wild type under these conditions (Figures 5d and S4c).
The phenotypes resulting from enhanced SCL30a expression reinforced the notion that this SR protein plays important roles in the seed. To further verify that the mutant phenotypes were caused by loss of SCL30a function, we isolated a second homozygous T-DNA line, SAIL_512B11, carrying the insertion at the beginning of the gene's 5′ untranslated (5′UTR) region (Figure S5a). RT-qPCR analysis of this scl30a-2 mutant using primers flanking the T-DNA insertion revealed near depletion of SCL30a expression, but only downregulation to about 50% of wild-type levels was detected when primers downstream of the insertion were used (Figure S5b). Despite the fact that this mutant allele is not a null, scl30a-2 seeds still germinated significantly later under control conditions (Figure S5c) and showed clear hypersensitivity to ABA and high salinity (Figure S5d), thus confirming that the observed germination phenotypes derive from impaired SCL30a function.
The above findings show that the full-length SCL30a SR protein plays an in vivo role in seed development and germination, substantiating the notion that it positively regulates seed germination. The opposite phenotypes under ABA and salt stress induced by loss of function and overexpression of SCL30a also demonstrate that this Arabidopsis SR protein reduces osmotic stress sensitivity during germination of the seed.
3.6 SCL30a function in seeds depends on the ABA pathway
To investigate whether the role of SCL30a in salt stress responses is mediated by ABA, we first performed stress germination assays in the presence of fluridone, an inhibitor of ABA biosynthesis (Lin et al., 2007; Moore & Smith, 1984; Ullah et al., 2002). Consistent with the well-known role of ABA as a key mediator of salt stress responses, addition of 1 μM fluridone notably relieved the inhibition imposed by NaCl on the germination of wild-type seeds (Figure 6a). Most importantly, the presence of fluridone rescued the salt stress hypersensitive phenotype of the scl30a-1 mutant, which germinated at rates similar to the wild type in NaCl (Figure 6a). This result indicates that the mutant's salt stress germination phenotype depends on endogenous ABA production.
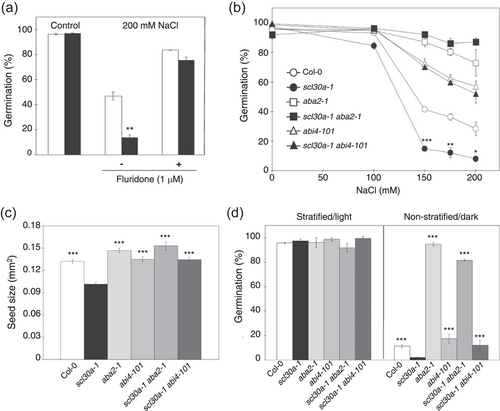
To conclusively establish the ABA dependence of SCL30a function, we next turned to epistatic analyses and assessed the genetic interaction between SCL30a and ABA2, encoding a cytosolic short-chain dehydrogenase reductase involved in the conversion of xanthoxin to ABA-aldehyde during ABA biosynthesis (Schwartz et al., 1997), or ABI4, which encodes an ERF/AP2-type transcription factor involved in ABA signal transduction (Finkelstein et al., 1998; Söderman et al., 2000). To this end, the scl30a-1 mutant was independently crossed with the ABA-deficient aba2-1 (Leon-Kloosterziel et al., 1996) and ABA-insensitive abi4-101 (Laby et al., 2000) mutant alleles to generate the corresponding homozygous double mutants. As seen in the dose–response curves depicted in Figure 6b, seeds from the scl30a-1aba2-1 and scl30a-1abi4-101 double mutants behaved as those of the corresponding single ABA mutants when germinated under high salinity, showing that SCL30a control of this stress response fully relies on functional ABA2 and ABI4 genes.
We then assessed the seed size and dormancy of the different genotypes. Both the aba2-1 and the abi4-101 mutations suppressed the reduced size displayed by scl30a-1 seeds, with the area of scl30a-1aba2-1 imbibed seeds being even significantly larger than those of the wild type, as previously reported for the aba2-1 mutant (Cheng et al., 2014) (Figure 6c). Regarding seed dormancy, the double mutants again showed strikingly similar phenotypes to those induced by single mutations in the ABA2 and ABI4 genes that, in agreement with early reports (Finkelstein, 1994; Leon-Kloosterziel et al., 1996), conferred strongly reduced and normal dormancy, respectively (Figure 6d). Therefore, both ABA2 and ABI4 are epistatic to the SCL30a gene, indicating that the seed/germination roles of the encoded SR protein are fully dependent on a functional ABA pathway.
The above findings raised the question of whether changes in SCL30a levels affect ABA biosynthesis or sensing/signaling of the stress hormone. To address this issue, we measured the endogenous ABA content of wild-type, scl30a-1 mutant and SCL30a-overexpressing seeds germinated in control conditions or under high salinity stress. Table 2 shows that Col-0, scl30a-1, and SCL30a-OX seeds responded to the presence of 200 mM NaCl by increasing their ABA content by around two-fold, with no significant differences in ABA levels being observed between the three genotypes either in the absence or presence of salt stress. As expected, the ABA content of the ABA biosynthesis aba2-1 mutant, included as a negative control, was unaltered by high salinity stress (Table 2). These results suggest that SCL30a activity does not influence endogenous ABA levels in seeds, rather affecting sensing and/or signal transduction of the hormone during seed germination.
| Genotype | Control | NaCl | NaCl/Control |
|---|---|---|---|
| Col-0 | 31.62 ± 3.07 | 59.06 ± 8.40 | *1.86 ± 0.40 |
| scl30a-1 | 36.45 ± 7.40 | 73.04 ± 16.60 | *2.00 ± 0.80 |
| SCL30a-OX2 | 36.54 ± 7.29 | 89.00 ± 19.91 | *2.44 ± 0.98 |
| aba2-1 | 27.86 ± 6.61 | 24.25 ± 4.11a | 0.87 ± 0.35 |
- Note: ABA content (means ± SE, n = 6-8), in ng/g of fresh weight, of Col-0, scl30a-1, SCL30a-OX2, and aba2-1 seeds germinated for 2 days in the absence or presence of 200 mM NaCl. Letters indicate significantly different ABA levels between genotypes under each condition and asterisks significant differences for each genotype between control and salt stress conditions (p < 0.05; Student's t-test).
4 DISCUSSION
The first indication that the Arabidopsis SCL30a SR protein was involved in regulating seed-specific traits came from our gene expression studies, showing high SCL30a induction in the embryo and testa of imbibed seeds as well as during the first stages of germination. Phenotypical characterization of an SCL30a loss-of-function mutant then revealed that this gene affects the size and dormancy of mature Arabidopsis seeds and is required for the germination process, during which it downregulates ABA signaling. In fact, germinating scl30a-1 mutant seeds display higher expression of ABA-responsive genes, and overexpression of SCL30a results in a drastic reduction of seed sensitivity to high salinity, corroborating a role for this protein as a negative regulator of ABA signaling and in promoting seed germination also under abiotic stress.
Although the SCL30a gene displays ubiquitous expression in vegetative tissues, we were unable to identify any evident phenotype at later developmental stages. This is likely due to functional redundancy between members of the SCL subfamily at the adult stage. In fact, previous phenotypic studies of adult Arabidopsis plants from single mutants in individual SCL genes did not report any visible alterations, with only a quintuple mutant of the four SCL members and the SC35 gene (scl28 scl30 scl30a scl33 sc35) exhibiting clear defects in leaf development and flowering (Yan et al., 2017). In a recent report, heterologous overexpression of the cassava (Manihot esculenta) MeSCL30a gene in Arabidopsis plants is described as increasing salt stress sensitivity during seed germination (Hu et al., 2021). However, MeSCL30a also shares high identity with the Arabidopsis SCL33 protein and was expressed at extremely high levels in Arabidopsis plants. SCL30a may hence either fulfill different functions in cassava and Arabidopsis or massive heterologous expression may have affected its biological function.
Physiological assays using an ABA biosynthesis inhibitor and epistatic analyses with the ABA-biosynthesis ABA2 (Leon-Kloosterziel et al., 1996) and the ABA-signaling ABI4 (Finkelstein, 1994) genes demonstrate that SCL30a regulation of seed traits is fully dependent on an intact ABA pathway. This is consistent with the global gene expression changes associated with the loss of SCL30a function, showing a clear enrichment of ABA-related functions among the genes upregulated in the scl30a-1 mutant. Moreover, unchanged ABA levels in mutant and overexpressing seeds, together with the enhanced and reduced sensitivity to exogenously applied ABA caused respectively by loss-of-function and overexpression of SCL30a, indicate that the encoded SR protein represses signal transduction of the phytohormone rather than its biosynthesis.
While the central roles of ABA in the induction and maintenance of seed dormancy as well as in mediating responses to salt stress are well established (for review, see Chen et al., 2020), fewer studies have addressed the involvement of this phytohormone in determining seed size. ABA has been reported to regulate final seed size via the control of endosperm cellularization during seed development, as reflected by the larger seeds of the aba2 and abi5 mutants (Cheng et al., 2014). Given the smaller and larger seeds produced respectively by the scl30a-1 mutant and the overexpressor lines, and our discovery that SCL30a is a major modulator of ABA-related gene expression, the SR protein could regulate endosperm development, and thereby seed size, by controlling the expression of key ABA components such as the ABI5 gene, which is upregulated in the scl30a-1 mutant.
Seeds challenged with osmotic stress undergo an arrest in germination that is triggered by a rise in their ABA content (Lopez-Molina et al., 2001, 2002). Our results indicate that by decreasing sensitivity to this phytohormone, the SCL30a SR protein promotes seed germination under salt stress. The derepression of a subset of ABA-responsive genes and the seed germination delay associated with the loss of SCL30a function in the absence of stress suggest that the SR protein is already able to repress ABA signaling under optimal growth conditions. Therefore, the stress hypersensitivity displayed by the scl30a mutants could result from an already active ABA signaling state, with the stress stimulus inducing an overaccumulation of ABA-responsive transcripts in the mutants. Alternatively, the stronger mutant phenotype under stress when compared to control conditions could indicate stress regulation of SCL30a activity. The fact that the SCL30a expression and splicing pattern is unaffected by ABA or salt in seeds (data not shown and Costa et al., 2015) points to posttranslational regulation of this RNA-binding protein. In support of this notion, SR proteins are known to undergo extensive phosphorylation at their RS domain, and stress cues affect both the phosphorylation status and activity of Arabidopsis SR and SR-related proteins (Albuquerque-Martins et al., 2023; Ali et al., 2003; Chong et al., 2019; Rausin et al., 2010; Tillemans et al., 2006; Umezawa et al., 2013; Wang et al., 2013).
Quite surprisingly, our large-scale transcriptome analysis revealed only 22 alternative splicing events in 21 genes affected in the scl30a-1 mutant (|ΔPSI| > 15 ), thus precluding deep mechanistic insight into the splicing function of this SR protein. Our results contrast with a main expected role for SCL30a as a splicing regulator and raise the question of whether this protein is involved in regulating other steps of gene expression. Beyond splicing, animal SR proteins have been shown to play important roles in coordinating several steps of gene expression, including transcriptional activation, nonsense-mediated decay, mRNA export and translation (for reviews, see Howard & Sanford, 2015; Jeong, 2017; Wagner & Frye, 2021). In Arabidopsis, SCL proteins can interact with the NRPB4 subunit of the RNA Polymerase II, pointing to a potential role in the regulation of gene transcription, and simultaneous disruption of the four SCL subfamily genes and SC35 causes drastic transcriptional changes (Yan et al., 2017). Therefore, and although it is entirely possible that the transcriptional changes are mediated by the few splicing targets identified, an important component of SCL30a function during seed germination could lie in the regulation of gene transcription. Nonetheless, the RNA-seq experiment performed reflects the transcriptome of scl30a-1 germinating seeds at a specific time point (18 h after stratification), and the possibility that the observed gene expression changes are a consequence of earlier alternative splicing defects cannot be ruled out. Future identification of the direct targets of SCL30a using RNA immunoprecipitation methods should elucidate the molecular functions of this protein during seed germination and stress responses.
We have disclosed a role for the Arabidopsis SCL30a RNA-binding protein in modulating seed-specific traits and stress sensitivity during germination via targeting of the ABA pathway. Whether SCL30a control of ABA- and germination-related gene expression is mediated by its few splicing targets or occurs directly via a noncanonical role in transcriptional regulation remains unknown. Nevertheless, the function of SCL30a in governing seed sensitivity to salt stress, which dramatically reduces plant productivity worldwide, underscores the high potential of this SR protein for biotechnological applications.
ACKNOWLEDGMENTS
We thank V. Nunes (IGC Plant Facility) for technical assistance and excellent plant care. This work was funded by Fundação para a Ciência e a Tecnologia (FCT) through Grants PTDC/AGR-PRO/119058/2010 and PTDC/ASP-PLA/2550/2021, as well as PhD Fellowship SFRH/BD/28519/2006 awarded to S.D.C. Funding from the research unit GREEN-it “Bioresources for Sustainability” (UIDB/04551/2020) is also acknowledged. T.L was supported by Marie Skłodowska-Curie Individual Fellowship MSCA-IF-2015 (project 706274), and G.M. was supported by MSCA-IF-2016 (project 750469) and an EMBO Long-Term Fellowship (ALTF 1576- 2016).
Open Research
DATA AVAILABILITY STATEMENT
Raw sequencing data and transcript expression results were submitted to the Sequence Read Archive (accession number GSE181122). The data that support the findings of this study are available from the corresponding author upon reasonable request.




