AAAAI–EAACI PRACTALL: Standardizing oral food challenges—2024 Update
Abstract
This common statement of the American Academy of Allergy, Asthma and Immunology (AAAAI) and The European Academy of Allergy and Clinical Immunology (EAACI) provides an update of the 2012 published guidelines on food challenges. The guidelines equally address food challenges in the research and the clinical settings. They first address the diagnostic tests which can guide the decision to conduct a challenge. Safety of food challenges is prime, and the various procedures and safety issues as well as medications potentially involved in challenges are extensively discussed. Challenges are suggested to be conducted with semi-logarithmic incremental doses based on the protein content, typically for IgE-mediated food allergy with intervals of 20–30 min between doses. Specific protocols for other types of reactions such atopic dermatitis or gastrointestinal food allergy are detailed separately. Proper stopping criteria are essential in order to reduce the risk of false-positive diagnoses, but also severe reactions. The guidelines recommend criteria based on “go on,” “stop,” or “observation.” These revised guidelines will clearly provide much needed guidance for food challenges in the research and clinical settings. They will continue to evolve with new diagnostic tests or new needs in the field of food allergy.
1 INTRODUCTION
In 2012, the PRACTALL Consensus Report on standardizing the double-blind placebo-controlled oral food challenge (DBPCFC) was published by a working group from the American Academy of Allergy, Asthma and Immunology (AAAAI) and the European Academy of Allergy and Clinical Immunology (EAACI).1 The intent of this report was to develop an international standard for conducting and interpreting DBPCFCs that would enable the comparison of studies involving the diagnosis, natural history, and therapeutic outcomes of trials in food allergy throughout the world. Over the past decade, the field of food allergy has seen tremendous advances and the 2012 DBPCFC Consensus Report has served as the “gold standard” in the evaluation of novel diagnostic approaches and therapeutic trials. Importantly, both the European Medical Agency (EMA) and the US Food and Drug Administration (FDA) have accepted the DBPCFC for evaluating therapeutic efficacy. This methodology was used in a therapeutic trial showing the efficacy of oral desensitization induction for peanut, which led to the approval of the first therapy for food allergy in 2020,2 and more recently to the FDA approval of omalizumab for IgE-mediated food allergies in patients 1 year of age and older.3 With the experience gained using the DBPCFC in thousands of patients over this period, investigators have suggested various modifications to the initial protocol. Consequently, the two Academies agreed to form a new consensus-working group to review and update the 2012 DBPCFC Consensus Report. This Update is meant to complement the 2012 PRACTALL DBPCFC Guidelines, adding new information gleaned over the past 12 years and expanding to include protocols for non-IgE-mediated food allergy challenges and office-based practice challenges. Besides these guidelines specifically referring to oral food challenges (OFC), the reader is referred to the recently published EAACI guidelines on the diagnosis of IgE-mediated food allergy, as well as to the AAAAI practice parameters for food allergy diagnosis.4, 5 The aim of this consensus report is to provide recommendations specifically for OFCs, based on a consensus discussion after expert analysis of the available literature. The procedure leading to an OFC, and the methodology used for the various clinical and research situations are multi-faceted. This is reflected in this consensus report which does not represent a classical guideline, but an expert-driven, literature-based discussion on the good practice of OFCs. For a recent systematic review of the various diagnostic procedures, the reader might refer to the EAACI guidelines on the diagnosis of IgE-mediated food allergy.4 Statistical analyses and appropriate reporting of results are referred to in the 2012 Guidelines1 and expanded here.
2 PRE-CHALLENGE ASSESSMENT
2.1 Clinical factors
- Diseases that may interfere with assessment. The challenge should be postponed if a patient is experiencing unstable or exacerbated disease(s), (e.g., asthma, atopic dermatitis, urticaria, acute infection or allergic rhinitis), based on the physician's judgment of the severity and the condition of the patient. Chronic atopic diseases (e.g., rhinitis, atopic dermatitis, asthma) should be stable and optimally controlled before the challenge. If the challenge is performed for clinical reasons, then the clinician should discuss the limitations of attempting to interpret a food challenge with underlying unstable symptoms, for example, erythema and pruritus in patients with eczema, through shared decision making with the patient or their parent/guardian. If the challenge is being performed for research purposes, then unstable allergic disease (e.g., severe asthma or severe atopic dermatitis) is likely to be at least a temporary exclusion criterion.
- Diseases and conditions that may affect safety. A challenge should not be performed if the patient has a known chronic medical condition that would pose a significant health threat in the event of anaphylaxis or treatment of a reaction. Chronic medical problems that may interfere with treatment of an allergic reaction include unstable coronary or other cardiac disease, dysrhythmias, severe chronic lung disease, and pregnancy. If the patient is acutely ill (e.g., febrile illness, upper respiratory infection), then the challenge should be deferred.
-
Medications that may interfere with assessments or affect safety. Medications that may interfere with symptoms of an allergic reaction include antihistamines, medications with antihistamine-like properties (e.g., tricyclic antidepressants, benzodiazepines, and atypical antidepressants), bronchodilators, anti-IgE monoclonal antibodies and mast-cell stabilizers. If a patient is taking an anti-IgE monoclonal antibody, for example, omalizumab, it is possible they could have a decrease in skin test reactivity (although additional prospective studies are needed to confirm this finding), modified IgE levels,8, 9 and an increased eliciting dose threshold.3, 10 The effect of other biologics that might interfere (e.g., dupilumab, tezepelumab) have not been systematically studied. The impact of biologics should be taken into consideration when interpreting the outcome and when making recommendations regarding avoidance or repeating the challenge if the anti-IgE monoclonal antibody is discontinued.
Epidemiological data suggest that concurrent use of beta blockers and/or ACE-inhibitors may increase reaction severity, although this effect may be confounded by the underlying reasons for the prescription (e.g., cardiovascular disease, age), which have also been reported to impact severity.11 Systemic glucocorticoids should be avoided for 7–14 days prior to the challenge because disease rebound could affect interpretation. Preliminary data in a small trial indicates dupilumab may not have a significant effect on OFC outcomes,12 although more studies are needed. NSAIDs, alcohol, proton pump inhibitors and H2 blockers have been reported to affect reaction severity; however, the evidence is limited and inconsistent.11, 13 As a general rule, medications with a potential of suppressing symptoms should be removed for a time-period equivalent to 5 half-lives prior to the challenge. Table 1 provides a list of medications that may interfere with OFC interpretation. An extensive list of medications can be found in Table S1.
| Medication | Recommended last dose based on suppression of skin prick test wheal diameter | T½ (h) | Five half-lives (h) |
|---|---|---|---|
| Antihistamines (oral) | |||
| First generation H1-blocking | |||
| Brompheniramine | >2 to 4 days | 24.9 ± 9.3 | 78–171 (3–7 days) |
| Chlorpheniramine | 3–6 days | 27.9 ± 8.7 | 96–183 (4–7 days) |
| Clemastine | 5–10 days | 21.3 ± 11.6 | 48.5–164.5 (2–7 days) |
| Cyproheptadine | 9–11 days | 16 | 80 (3 days) |
| Diphenhydramine | 2–5 days | 9.2 ± 2.5 | 33.5–58.5 (1–2 days) |
| Hydroxyzine | 5–8 days | 20 ± 4.1 | 79.5–120.5 (3–5 days) |
| Promethazine | 3–5 days | 9–16 | 45–80 (2–3 days) |
| Tripolidine | 3–7 days | 3.2 | 16 (<1 days) |
| Second generation H1-blocking | |||
| Acrivastine | 3 days | 1.4–3.1 | 7–15.5 (<1 days) |
| Cetirizine | 3 days/3–5 days | 7–11 | 35–55 (1–2 days) |
| Desloratadine | 7 days | 7.8 ± 4.2 | 18–60 (1–3 days) |
| Fexofenadine | 2 days/3–5 days | 14.4 | 72 (3 days) |
| Levocetirizine | Unknown | 7 ± 1.5 | 27.5–42.5 (1–2 days) |
| Loratadine | 7 days/3–5 days | 7.8 ± 4.2 | 18–60 (1–3 days) |
| Recommended last dose based on theoretical ability to interfere with OFC interpretation (recommendation based on 5 half-lives [T½ in hours of product] unless otherwise stated) | |||
| T½ (h) | |||
| Short-acting bronchodilator§ | |||
| Albuterol | 8 h | ||
| Isoproterenol | 8 h | ||
| Metaproterenol | 8 h | ||
| Terbutaline | 8 h | ||
| Medium-acting bronchodilator | |||
| Ipratropium | 24 h | ||
| Long-acting bronchodilator | |||
| Salmeterol | Continue at lowest dose possible and on fixed schedule because withdrawal could result in exacerbationDiscontinuation at least 8 h prior to the OFC has been recommended. | ||
| Formoterol | |||
2.2 Surrogate markers
Food challenges are time-consuming, resource intensive and carry a risk of anaphylaxis, although there may be benefits to the experience of a food challenge including reported improvement in health-related quality of life14, 15 – something noted to be greater in those who self-treated with epinephrine following anaphylaxis at challenge compared to those who experienced more mild reactions.11
The most commonly used and reliable adjunctive tests for diagnosing food allergy are skin prick tests and serum specific IgE levels.16, 17 However, these tests measure IgE-sensitization which does not necessarily imply clinical reactivity. Thus, interpretation of all surrogate biomarkers is reliant on a clinical history supporting the ongoing presence of IgE-mediated food allergy. Accordingly, clinical interpretation of “positive” surrogate biomarkers relies on either a suggestive clinical history of IgE-mediated specific food allergy, or a positive OFC when the ingestion history is unclear.
2.3 Case history
In the clinical setting, the case history is perhaps the most important component and the cornerstone for diagnosis, usually in conjunction with positive IgE tests. The case history should support characteristic symptoms of IgE-mediated disease, that is, symptoms consistent with mast cell degranulation and inflammatory mediator release occurring within minutes to 1–2 h of ingestion, except in cases of galactose-alpha-1, 3-galactose (alpha-gal) allergy that generally occurs within 3–8 h of mammalian meat ingestion.18 Patients with a history of severe anaphylaxis (i.e., required intubation or intravenous inotropes, or with neurologic compromise) have typically been excluded from interventional research therapies or food challenges because of the theoretical risk of a further severe reaction. Surrogate biomarkers are poorly predictive of reaction severity, so case history should be considered when undertaking an assessment of risks pertaining to food challenges for individual patients. It is largely known that self-reported food allergy exceeds the challenge-proven incidence suggesting that OFCs should always be considered in doubtful cases.19
2.4 Skin prick testing
Skin prick testing (SPT) may be performed using commercially available extracts or fresh food. Protein content within commercial extracts is not standardized, extracts may lack relevant allergens (Bet v 1 homologues, LTP, oleosins, etc.) and variability may exist from batch-to-batch and between manufacturers.11 The type of SPT device used may also contribute to variability in results.20, 21 Fruit and vegetable extracts are likely to lose potency over time and prick/puncture testing with fresh fruits and vegetables, including use of different cultivar strains (e.g., apples) is more reliable when indicated based on the patient history.20
Efforts have been made to correlate challenge outcomes with SPT results, particularly in the pediatric population; however, because of the inherent variability in SPT results, reported standards must be interpreted with caution. A positive skin test result is generally considered a SPT wheal ≥3 mm greater than the negative control; however, as mentioned above, this indicates sensitization only and does not equate to food allergy.22 In general, an SPT wheal ≥3 mm has low specificity and can have a poor positive predictive value, which may lead to over diagnosis. At least in children, a larger SPT wheal diameter correlates with a higher likelihood of clinical reactivity23-26 while a negative SPT typically implies the absence of IgE-mediated food allergy. However, since positive and negative results are not 100% reliable for any food, the clinical history must be taken into context when considering an observed challenge versus home introduction. Given a diagnosis of peanut allergy, SPT wheal size does not reliably predict the severity of symptoms.11
Efforts have been undertaken to improve the diagnostic accuracy of SPTs by use of single allergenic proteins to predict the OFC outcome and have been reported for several foods.4 SPTs are not able to predict tolerance or reactivity to baked-milk or baked-egg in children with allergy to raw/fresh milk and egg, respectively.13, 27 Thus, the OFC remain the diagnostic standard for establishing tolerance to baked-milk and baked-egg.
2.5 Serum allergen-specific IgE
Allergen-specific IgE testing is readily available; like SPT, its presence represents sensitization, and not clinical reactivity, that is, allergy to a food. Therefore, allergen-specific IgE testing is not a reliable surrogate for a food challenge to confirm diagnosis. Decision-points for some foods (e.g., milk, egg, peanut, hazelnut) have been proposed using the ImmunoCAP or UniCAP (Thermo Fisher Scientific, Uppsala, Sweden) system as well as by Skin Prick Tests. Table 2 is modified from the recent meta-analysis by Riggioni et al. on the accuracy of diagnostics for food allergy, which included 149 studies evaluating heterogeneous populations. Such meta-analyses enable the synthesis of findings from various sources, which then enhances the reliability and generalizability of the overall results.25 Predictive levels established with the ImmunoCAP system do not necessarily translate to other diagnostic platforms.28 Decision points established for milk and egg may vary by age, and even within the same clinical center.29-32 Even combined with the clinical history, they provide only limited accuracy regarding the potential outcome of an OFC.
| Diagnostic test | Sensitivity (95% CI) | Specificity (95% CI) | Cut-off median | Interquartile [IQ] range |
|---|---|---|---|---|
| Peanut | ||||
| SPT to peanut | 0.84 (0.69; 0.92) | 0.86 (0.79; 0.91) | 4 | 3–8 |
| sIgE to peanut | 0.81 (0.71; 0.88) | 0.83 (0.71; 0.90) | 4.3 | 0.35–10 |
| Ara h 2-sIgE | 0.82 (0.77; 0.86) | 0.92 (0.87; 0.95) | 0.44 | 0.3–1.3 |
| Ara h 6-sIgE | 0.87 (0.47; 0.98) | 0.94 (0.76; 0.99) | 0.4 | 0.1–0.9 |
| BAT to peanut | 0.84 (0.76; 0.90) | 0.90 (0.83; 0.94) | 5.0 | 4.7–7.1 |
| Egg (cooked) | ||||
| SPT to egg white | 0.68 (0.37; 0.88) | 0.77 (0.64; 0.86) | 5 | 3–8 |
| sIgE to egg white | 0.85 (0.77; 0.90) | 0.73 (0.63; 0.80) | 3.5 | 1.7–5.5 |
| Ovomucoid-sIgE | 0.74 (0.54; 0.87) | 0.91 (0.87; 0.93) | 0.8 | 0.35–3.7 |
| Ovalbumin-sIgE | 0.65 (0.43; 0.82) | 0.92 (0.83; 0.97) | 1.2 | 0.2–2.8 |
| Cow's milk | ||||
| SPT to fresh cow's milk | 0.90 (0.53; 0.94) | 0.80 (0.25; 1.00) | 4 | 3–9 |
| sIgE to cow's milk | 0.82 (0.59; 0.94) | 0.92 (0.80; 0.97) | 3.5 | 0.9–10.5 |
| Casein-sIgE | 0.67 (0.53; 0.78) | 0.93 (0.85; 0.97) | 2.6 | 1.0–5.3 |
| β-Lactoglobulin-sIgE | 0.68 (0.53; 0.80) | 0.89 (0.73; 0.96) | 1.7 | 1.6–1.8 |
| Hazelnut | ||||
| SPT to hazelnut | 0.82 (0.68; 0.91) | 0.78 (0.44; 0.94) | 5 | 3–7 |
| sIgE to hazelnut | 0.79 (0.71; 0.85) | 0.62 (0.38; 0.81) | 2.34 | 0.6–6.3 |
| Cor a 9-sIgE | 0.69 (0.46; 0.85) | 0.81 (0.73; 0.88) | 0.83 | 0.35–1.4 |
| Cor a 14-sIgE | 0.73 (0.53; 0.87) | 0.95 (0.90; 0.98) | 0.64 | 0.35–3.5 |
| Cashew | ||||
| SPT to cashew | 0.93 (0.89; 0.96) | 0.92 (0.82; 0.96) | 5 | 4–6 |
| sIgE cashew | 0.94 (0.89; 0.97) | 0.64 (0.54; 0.74) | 1.1 | 0.6–3.1 |
| Ana o 3-sIgE | 0.96 (0.91; 0.98) | 0.94 (0.88; 0.97) | 0.4 | 0.2–0.6 |
| Walnut | ||||
| sIgE to walnut | 0.87 (0.60; 0.97) | 0.82 (0.60; 0.93) | 2.8 | 0.2–11.4 |
| Jug r 1-sIgE | 0.77 (0.58; 0.89) | 0.90 (0.78; 0.96) | 0.2 | 0.1–0.3 |
| Almond | ||||
| sIgE to almond | 0.72 (0.62; 0.80) | 0.95 (0.43; 1.00) | 3.4 | 1.2–10.5 |
| Sesame | ||||
| SPT to sesame | 0.70 (0.55; 0.82) | 0.89 (0.76; 0.95) | 8 | 4–10 |
| sIgE to sesame | 0.70 (0.23; 0.95) | 0.83 (0.26; 0.99) | 7.5 | 0.9–50 |
| Ses i 1-sIgE | 0.77 (0.64; 0.86) | 0.87 (0.77; 0.92) | 2.0 | 0.3–4.0 |
| BAT to sesame | 0.89 (0.80; 0.94) | 0.93 (0.76; 0.98) | 10.9 | 8.2–11.6 |
| Soy | ||||
| SPT to soy | 0.47 (0.11; 0.87) | 0.79 (0.63; 0.89) | 3 | 2–6 |
| sIgE to Soy | 0.73 (0.62; 0.82) | 0.75 (0.44; 0.92) | 3.0 | 0.1–8.7 |
| Gly m 4-sIgE | 0.61 (0.36; 0.81) | 0.69 (0.30; 0.92) | 0.2 | 0.1–17.6 |
| Wheat | ||||
| SPT to wheat | 0.53 (0.23; 0.81) | 0.72 (0.57; 0.84) | 3 | 3–5 |
| sIgE twheat | 0.72 (0.54; 0.84) | 0.79 (0.68; 0.86) | 0.6 | 0.35–5.6 |
| ω-5 gliadin-sIgE | 0.79 (0.68; 0.88) | 0.78 (0.66; 0.86) | 0.3 | 0.1–0.6 |
| Shrimp | ||||
| SPT tshrimp | 0.62 (0.44; 0.77) | 0.90 (0.31; 0.99) | 3 | 3–5 |
| sIgE to shrimp | 0.96 (0.42; 1.00) | 0.63 (0.46; 0.78) | 1.2 | 0.5–3.1 |
| Pen a 1-sIgE | 0.62 (0.45; 0.76) | 0.89 (0.75; 0.95) | 1.1 | 0.6–4.4 |
- Note: Median cut-off values: SPT mean wheal diameter in mm; specific IgE (sIgE) in kUA/L; and basophil activation (BAT) in %CD63+ basophils.
Component resolved diagnostics (CRD) measure specific IgE levels to individual proteins derived from a food, and aim to better identify clinically relevant sensitization compared with irrelevant cross reactivity; however, CRDs do not consistently predict allergic reactivity.11, 22, 32 For example, IgE to Ara h 2 has been found to predict peanut allergy due to primary sensitization, whereas sensitization to Ara h 8 is more consistent with pollen-food allergy syndrome due to primary sensitization to Bet v 1. However, diagnostic cutoffs for Ara h 2 vary greatly between studies, and do not reliably predict reaction severity.22 CRDs are available for tree nuts such as hazelnut (Cor a 1, Cor a 8, Cor a 9, Cor a 14), walnut (Jug r 1, Jug r 3), cashew (Ana o 2, Ana o 3), Brazil nut (Ber e 1), and also for other foods such as sesame (Ses o 1), kiwi fruit (Act d 1, Act d 2, Act d 5, Act d 8), soy (Gly m 4, Gly m 5, Gly m 6) or shrimp (Pen m 1, Pen m 2, Pen m 4). Limited studies suggest better accuracy using CRDs for the respective tree nuts over whole extract; however, additional studies are needed.22 Some studies have suggested potential utility with predicting baked-milk and baked-egg outcomes using CRDs for milk allergens (Bos d 4, Bos d 5, Bos d 6, Bos d 8) and egg allergens (Gal d 1, Gal d 2, Gal d 3, Gal d 4, Gal d 5); however, data across studies are not consistent, nor do they demonstrate superior predictive ability over SPT and/or whole allergen specific IgE.13 Omega-5-gliadin (Tri a 19) is a wheat allergen associated with wheat dependent exercise induced anaphylaxis.33, 34 It has been correlated with IgE-mediated wheat allergy in Japanese and Swedish populations, but the usefulness in other populations is unclear.11, 22, 32 As with SPT, specific IgE testing (including CRD) has been shown not to predict severity with high accuracy and precision.11
Specific IgE testing to allergen epitopes is an emerging diagnostic procedure that has been shown to identify patients with persistent cow's milk allergy35 and has been shown to more accurately predict peanut allergy36 and potentially eliciting dose thresholds.37 Broad application to other allergens is currently limited, due to the need to validate the assays in large cohorts of well-characterized food-allergic patients.38
2.6 Other tests
Basophil activation testing (BAT) is an in vitro assessment of allergen-induced basophil activation. Recent studies of BAT have reported some advantages of BAT over SPTs and specific IgE testing in peanut, milk and hazelnut allergy due to its higher specificity.39-43 Limitations to broad implementation in clinical practice are the requirement for fresh blood (optimally, BAT should be performed within 4 h); impact of transportation on basophil reactivity (vibration and temperature changes may cause degranulation); patient-specific factors including non-responders (around 10% of individuals), concomitant inflammatory diseases, infection and others; and the need to identify large cohorts of well-characterized food-allergic patients to validate the assays.44
Mast cell activation testing (MAT) is being investigated as a potential diagnostic tool for peanut allergy, although data are limited at the present time.45, 46 MAT uses plasma or serum to sensitize mast cells derived from either a cell line or primary cells from peripheral blood, tissue or engineered cell lines.38, 46 The MAT has demonstrated similar specificity in diagnosing peanut allergy as the BAT, but may be less sensitive, at least when using cell lines, for example, LAD2.32 MAT using primary, blood-derived mast cells showed improved sensitivity and specificity compared to BAT in one study.47 Recently a Hoxb8 mast cell line, an immortalized progenitor line from the bone marrow of mice that are transgenic for the human high-affinity IgE receptor (FcεRIa) was established46; preliminary studies suggest that this may also provide a more sensitive and reproducible assay for diagnosing allergy.46
Among other tests, atopy patch testing has been investigated as a surrogate marker for determining clinical allergy in patients with atopic dermatitis, eosinophilic esophagitis and FPIES,48 but its utility for diagnosis in food allergy is highly controversial and has been largely abandoned. The lack of standardized food extracts is a major limiting factor, and additional studies are needed to validate its use in diagnosis of food allergy.20, 49 Intradermal skin testing for foods is potentially dangerous, overly sensitive, increases the chance of a false-positive result and is not recommended.20 Other modalities lacking sufficient evidence to recommend their use include allergen-specific IgG or IgG4 testing, pulse testing, electrodermal testing, lymphocyte activation testing, cytotoxic testing and applied kinesiology testing.49
2.7 Conclusion
Oral food challenges remain the standard for diagnosing food allergy. The clinical history is the most important component to direct testing, and then a step-wise approach to confirmation typically includes skin prick testing and/or serum IgE testing with consideration for the use of CRD and/or epitope-specific IgE testing to support the diagnosis. The BAT is not currently widely available; however, there are efforts to bring BAT to clinical practice. MAT is an emerging diagnostic tool that may further assist with diagnosis in the future. Multivariate regression models have been developed and validated to predict peanut allergy and reaction severity outcomes from the DBPCFC with good diagnostic accuracy40, 50; however, at this time, surrogate biomarkers have not replaced the need for a DBPCFC in the research setting.
3 CHALLENGE SETTINGS AND SAFETY ISSUES
Food challenges, whether DBPCFC, single-blind or open, pose certain safety issues that have to be addressed prior to starting.1 Factors that must be considered include the purpose of the food challenge, the type of expected allergic symptoms and the patient's previous clinical history of a reaction, among others are important factors to consider.1, 13, 51-55
3.1 Purpose of challenge
The indication for the challenge is important: whether it is being done for clinical reasons to prove or disprove a patient is allergic to a certain food, or for research purposes to confirm both clinical reactivity and establish the reaction threshold (eliciting dose) of food protein needed to cause a clinical reaction.56, 57 In addition, an OFC may be indicated for follow-up of food allergy when there is a significant chance that the patient has lost his allergy. The likelihood of a “reactive” challenge will depend upon the patient's history of previous reactions and clinical evidence suggesting possible resolution. The rate of reaction at OFC may vary according to the indication for the challenge58, 59; of note, only around 50% or less of patients thought to have a food allergy will react, depending on the clinical setting.52, 60, 61 Other relevant factors include whether the suspected allergic reaction is likely IgE or non–IgE-mediated, the age of the patient and the food to be challenged.
3.2 Safety/challenge setting
There are several options for conducting OFCs: the intensive care unit, a regular hospital room, a hospital clinic room, an outpatient clinic room, an emergency room, a private practice office, or the patient's own home.62, 63 There is an inherent risk of severe reactions, including to very low doses. Around 10% of patients react with objective symptoms to doses under 10 mg food protein, and around 5% of these reactions will be anaphylaxis.64 This holds true even in patients whose allergy may be perceived to be less significant. In one series of food challenges to raw milk/egg conducted in children tolerant to the allergen when present in baked foods, 18/87 (21%) developed dyspnea and/or mild hypotension,58 some of them at milligram-level doses. Thus, the starting dose and schedule of dose escalation is part of the overall safety equation.
Certain foods seem to cause more severe reactions during OFC than others in different geographical areas, even when correcting for prevalence.11 Peanut/tree nuts are the most common triggers in most regions; however, cow's milk (and other mammalian milks) and seafood are increasingly common causes of fatal and non-fatal anaphylaxis, and are even responsible for a higher rate of severe anaphylaxis than peanut in some regions.65 In the European Anaphylaxis Registry, wheat has been reported to be responsible for more severe reactions compared to other foods in adults, possibly due to a greater influence of cofactors.66, 67 Surrogate tests, including allergen-specific IgE, CRD and BAT, do not correlate with the severity of symptoms provoked during OFC.11 Some studies show a better association between sensitization to specific IgE components and likely severity, for instance sensitization to the 2S albumins in tree nut allergy.11 The likelihood of a late-phase reaction or delayed symptoms should be considered prior to initiating an OFC, in order to determine the appropriate place and period of observation.52-54 Other factors to consider include the age of the subject, the presence of other concomitant diseases such as atopic dermatitis, asthma, cardiovascular disease, or whether they have reacted to milligram amounts of the food by clinical history, the criteria used for determining a reactive challenge (objective and subjective symptoms), and whether the patient was on an elimination diet prior to challenge.1, 13, 53, 68-70
An experienced physician will always have to make a safety assessment prior to any OFC, based on the various factors developed above. The setting will be chosen based on the pre-test risk assessment, keeping in mind that all OFC have an inherent risk of reaction.
3.3 Personnel
The personnel involved in a challenge procedure must be specifically trained in the management of acute allergic reactions, and anaphylaxis practice drills should be conducted periodically with the challenge team. Dedicated centers for doing food challenges are desirable, especially for challenges conducted for research purposes, so that personnel have adequate experience in conducting food challenges.71
3.4 Medications
All necessary medication should be readily available if needed during the food challenge. Epinephrine, oxygen, antihistamines, inhaled beta-agonists and intravenous fluids may all be needed to treat symptoms.1, 13, 71-73 Patients with a history of a prior severe anaphylaxis should have intravenous access established prior to initiating the challenge. The need for intravenous access should otherwise be guided by the ease by which access could be achieved in an emergency. The vast majority of individuals undergoing food challenge do not require intravenous access as a matter of routine. Patients with severe asthma, even without a history of prior anaphylaxis to food, and older children and adults with difficult intravenous access also should be considered for prior line insertion.53
Patients can generally be discharged after an observation period of at least 1–2 h, provided no reaction occurred.13 If the clinical history indicates that allergic symptoms on the initial reaction occurred later, then a longer period of observation might be necessary. If the patient experienced significant clinical symptoms, then a period of up to 4 h of observation may be needed before discharge. If a patient experiences a severe systemic allergic reaction needing extensive treatment, then he/she should be kept under observation at the hospital overnight with appropriate treatment.52, 74
3.5 Conclusions
Safety is an important consideration when deciding where and when a challenge should be performed. A food challenge may be done for various reasons, generally to identify a food that the patient should be avoiding or a food that the patient can add into their diet. It is important that OFCs be conducted in a location that is fully equipped to deal with anaphylaxis, by staff who are trained in dealing with such reactions. Appropriate settings also may include allergists' offices, since many allergists administering immunotherapy are fully equipped to manage anaphylaxis and are well trained in the management of OFCs.
4 CHALLENGE SCHEDULES
4.1 General considerations
Prior to publication of the previous PRACTALL consensus document in 2012, numerous dosage schedules had been used for research DBPCFCs.29, 52-54, 57, 61, 75, 76 For example, Figure 1 shows several schedules for DBPCFCs with cow's milk. While schedules for other foods are similar, calculation from protein content to weight of food in either dried or native form may vary, especially when protein content of such foods are high (such as in fish) or low (such as in celery).52 As can be seen from Figure 1, starting doses vary widely from the low microgram level for studies aimed at determining the lowest observable adverse effect level (LOAEL) and no observable adverse effect level (NOAEL), to doses from the low milligram level to several hundred milligrams in schedules used for other purposes. Incremental scales vary from 10-fold increases through 5-fold,57, 77 semi-logarithmic (i.e., 1 – 3 – 10 – 30 – 100 – 300 – 1000 – 3000 mg etc.),29, 52 doubling doses78 or even smaller increases,54, 61, 75 the latter being associated with schedules evaluating cumulative doses. Dosing intervals for various foods in relation to the aim of the challenge will be discussed in more detail later. In one study with peanut-allergic children undergoing OFCs in which the challenge was not discontinued on the emergence of symptoms except for anaphylaxis, 21 (78%) eventually developed anaphylaxis, but only three as the initial presenting symptoms. Thirteen (48%) children experienced initial non-anaphylactic symptoms, but then developed anaphylaxis with further peanut ingestion.79 This implies that the incremental dosing used in OFCs can reduce the risk of anaphylaxis. However, in an analysis of 734 DBPCFCs in The Netherlands, dose predicted only 4.4% of the variance in reaction severity, that is, the relationship between dose and severity is complex and unclear.80 This may be due to different reaction patterns in patients, with some demonstrating a dose–response but others not.81 Most datasets show that severe reactions can occur at all levels of allergen exposure, including milligram doses of allergenic food.11
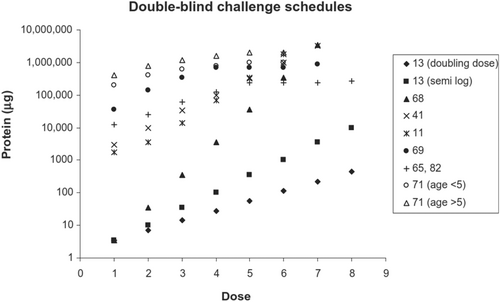
Due to differences in the reporting of safety and validity outcomes resulting from studies using different schedules, robust evidence demonstrating the relative superiority of any one schedule is limited. Comparing schedules is further hampered by the inability to assess the contribution of a given schedule parameter to a purported difference in outcome. Many parameters have been established on the basis of practical considerations, such as the length of time required to complete the challenge procedure.
Schedules developed for NOAEL/LOAEL determination employ starting doses at the low microgram level. However, it is more difficult to achieve meaningful top doses with acceptable increments in an acceptable period of time. A further consideration is the potential for partial desensitization (analogous to “rush desensitization”) and false negative results.82 A combination of logarithmic and semi-logarithmic increases, as done in CoFAR studies and EuroPrevall, can be helpful.83
A distinction must be made between dosing schedules designed for different purposes: diagnosing a food allergy, performing an OFC before starting oral immunotherapy, determining reaction thresholds (for example, to counsel individuals on the degree of allergen-avoidance required), and conducting clinical trials. Several non-PRACTALL dosing regimens have been proposed in clinical settings. The dosing schedule typically includes between 3 and 6 steps, and a maximum dose (equivalent to an age-appropriate serving size) has been described for a selection of common foods (Table 3 and Table S2).13 There are scant recommendations in the literature concerning the number of challenge doses for open OFCs in a patient with a high likelihood of passing an OFC and without factors interfering with the development of an allergic reaction. In clinical practice, a similar total serving portion may be divided into fewer doses for a low-risk patient. Individuals who are sensitive to very low (milligram) levels of exposure can be assessed using a one dose challenge.56 High starting doses may be associated with more severe reactions if utilized in an OFC.84 Starting doses at the low milligram level are generally safe and seem to result in fewer severe reactions.85, 86
| Food protein | Pasteurized cow's milk (3.3% protein) | Pasteurized whisked hen's egg (12.8% protein) | Peanut butter (24% protein) | Gluten powder (80% protein) | Soy drink (3.3% protein) |
|---|---|---|---|---|---|
| 3 mg | 91 mg ≈ 0.1 mL | 23.4 mg | 12.5 mg | 3.8 mg | 91 mg ≈ 0.1 mL |
| 10 mg | 303 mg ≈ 0.3 mL | 78.1 mg | 41.7 mg | 12.5 mg | 303 mg ≈ 0.3 mL |
| 30 mg | 909 mg ≈ 0.9 mL | 234.4 mg | 125 mg | 37.5 mg | 909 mg ≈ 0.9 mL |
| 100 mg | 3.03 g ≈ 3 mL | 781.3 mg | 416.7 mg | 125 mg | 3.03 g ≈ 3 mL |
| 300 mg | 9.09 g ≈ 9.1 mL | 2.344 g | 1.250 g | 375 mg | 9.09 g ≈ 9.1 mL |
| 1000 mg | 30.3 g ≈ 30.3 mL | 7.813 g | 4.166 g | 1.250 g | 30.3 g ≈ 30.3 mL |
| 3000 mg | 90.9 g ≈ 90.9 mL | 23.438 g | 12.500 g | 3.750 g | 90.9 g ≈ 90.9 mL |
The top dose required to avoid false non-reactive DBPCFCs is variable according to the protein content of the food being tested, but seems to be at least 2–3 g of food protein for most allergens. Sicherer et al.61 reported approximately 5% false negative test results with a schedule utilizing a maximum dose of 875 mg (cumulative dose 3500 mg) protein. Schedules with maximum doses of approximately 1750 mg (2190 mg cumulative dose) report a false negative rate of approximately 9% and 12.7%,87 as gauged by recurrence of symptoms during subsequent introduction of the food. With the publication of population-based, dose-distributions for the majority of “priority” food allergens, there is now much more certainty as to the dose required to ensure a false negative test rate of under 5%. Suggested top-doses depend on both the proportion of protein in a particular allergenic food, but also need to acknowledge that some foods are less “potent” than others; for example, the amounts of protein to elicit an objective allergic reaction in 5% of people allergic to that food (ED05) are higher for white fish (12 mg, 95% CI 4.5, 43.9 mg) and even more so for prawn/shrimp (280 mg, 95% CI 69.3, 880 mg) compared to other priority allergens56 as can be seen in Table 4. Top doses for these foods also need to be higher: published data on a safe schedule for fish and shrimp OFC have used a top dose of 12 g fish protein88 and 9–16 g shrimp protein.88, 89 However, the top dose also needs to be adapted depending on the age of the patient, to account for age-related differences in serving size. For example, because of difficulties in getting infants to consume a 3 g protein amount, some studies have used smaller quantities (e.g., single top dose of approx. 2 g peanut protein90 instead of 3 g).
| Allergen | Food | Food protein per serving | 4–11 months | 1–3 years | 4–8 years | >9 years |
|---|---|---|---|---|---|---|
| Egg | French toast (1 egg per 1 slice of bread)* | 6 g if made with 1 large egg | ½–1 slice | ½–1 slice | 1 slice | 1–2 slices |
| Hard-boiled or scrambled egg | 6 g/1 large egg | ½–1 egg | ½–1 egg | 1 egg | 1–2 eggs | |
| Milk | Infant formula | 2–3 g/140 mL (5 oz) | 4–8 oz | |||
| Milk | 8 g/220 mL (8 oz) | 110–220 mL (4–8 oz) | 110–220 mL (4–8 oz) | 220 mL (8 oz) | ||
| Cottage cheese | 10–14 g/4 oz | ¼–½ cup | ¼–½ cup | ½–1 cup | ½–1 cup | |
| Hard cheese | 6–8 g/28 g (1 oz) | 7–14 g (¼–½ oz) | 14 g (½ oz) | 28 g (1 oz) | 28 g (1 oz) | |
| Yogurt (NOT Greek style) | 8 g/226 g (8 oz) | 60–120 g (¼–½ cup) | 60–120 g (¼–½ cup) | 120–240 g (½–1 cup) | 120–240 g (½–1 cup) | |
| Peanut | Peanut (whole) | 2 g/w8 peanuts | 16 pieces | 16 pieces | ||
| Peanut butter | 3 g/1 tbsp | 1 rounded tbsp | 1–2 tbsp | 1–2 tbsp | 2 tbsp | |
| Peanut flour or peanut butter powder | 3 g/1 tbsp original or 2.25 g/1 tbsp chocolate flavor | 1 rounded tbsp | 1–2 tbsp | 1–2 tbsp | 2 tbsp | |
| Tree nut | Almond | 3 g/11 whole nuts | 11 pieces | 11 pieces | ||
| Cashew | 3 g/10 whole nuts | 10 pieces | 10 pieces | |||
| Hazelnut | 3 g/3 tbsp hazelnuts or hazelnut meal | 3 tbsp | 3 tbsp | |||
| Pecan (halves) | 3 g/25 halves | 10–25 halves | 25 halves | |||
| Pine nuts | 3.5 g/3 tbsp pine nuts | 3 tbsp | 3–4 tbsp | |||
| Pistachio | 3 g/20 whole nuts | 20 pieces | 20 pieces | |||
| Walnut (halves) | 3 g/10 halves | 10 halves | 10 halves |
Data suggest that up to 13% of OFCs may be false negative due to the challenge methodology using incremental doses that might induce transient desensitization in some patients, which seems to be independent of the allergen tested.82 At the same time, in other patients, the incremental exposure might “prime” the immune system resulting in less being tolerated during the OFC compared to a single exposure.91 Irrespective of the outcome, individuals who are non-reactive during an OFC should receive an age-appropriate serving size of the allergen in question following the challenge to confirm absence of reactivity. Some centers may choose to undertake this more formally, as a single-dose open food challenge performed on the next day or 2 h after the incremental challenge.
Data on the utility of a labial food challenge (LFC) as a starting dose are scarce. In one report, nine of 108 patients reacting to a LFC also reacted to subsequent OFC, while out of the 99 non-reactive LFCs, 27 OFCs turned out to be reactive.92 Another trial found that LFCs had poor sensitivity, and recommended against LFC as an alternative or initial step to formal OFC.93 On this basis, including a labial dose in a challenge protocol is not recommended.
Japanese guidelines for food allergy include a stepwise OFC (on various days according to the reactions) for diagnosis and assessment of a food allergy with total cumulative loading dose, including low, medium, and full doses.94 Stepwise OFCs can clarify the dosage of food that can be safely consumed95-97 and allows to stratify individuals' long term prognosis.98
Schedules will be different if the purpose is to observe for more delayed reactions, such as those seen in atopic dermatitis (AD), alpha-gal hypersensitivity, food protein-induced enterocolitis syndrome (FPIES), eosinophilic esophagitis (EoE) and food protein-induced allergic (eosinophilic) proctocolitis (FPIAP), as detailed below.
4.2 Other protocols
4.2.1 Galactose-α-1,3-galactose syndrome (alpha-gal)
Although most cases of Alpha-gal Syndrome can be diagnosed based on history and serum IgE to alpha-gal, there are a number of situations that require an oral food challenge for clarification. There are no standardized alpha-gal oral challenge protocols. One group recommends challenging patients with 2–3 pork sausage patties (~70 g each) openly (or the equivalent in a blinded fashion).99 The dose is generally given as a single dose. Larger doses are generally given when the history of reactivity is inconsistent. Given the length of time until symptoms typically begin (3–6 h), the challenge may be initiated at home, depending upon the patient's history of possible reactivity. Patients should be observed for 4–6 h depending on their history of reactivity. The severity of reactions is quite variable and may be severe; 15%–20% of food challenge reaction reportedly require one or more doses of epinephrine and/or emergency medical transport.
4.2.2 Atopic dermatitis (AD)
Patients with AD and evidence of food-IgE sensitization usually undergo OFCs per standard IgE protocols to evaluate for immediate reactions, following two or more weeks of food avoidance.13 If delayed, cell-mediated reactions are suspected, a longer observation period prior to discharge is advisable.100 If, based on the history, eczematous rash appears or worsens following several feedings, either a multi-day hospitalization or multi-day challenge at home may be needed, accompanied by the physician or family documenting rash severity. In addition, a placebo arm is required since AD can flare independently of food over a 2-week period.101 Blinding of a home challenge is difficult and requires provision of masked challenge material for the duration of home feeding. SCORAD, EASI and IGA are validated scoring systems that can be used in medical settings, whereas POEM can be used by a caregiver.102, 103
4.2.3 Eosinophilic esophagitis (EoE)
Eosinophilic esophagitis (EoE) is a food-responsive disease, with or without systemic IgE sensitization to a specific food.104 If dietary elimination is preferred for the management of EoE, foods for elimination are chosen empirically, with cow's milk being the most frequently implicated food allergen, followed by wheat, egg, soy and peanut.105, 106 Reintroduction of the suspected food allergen is typically performed after a minimum 4 weeks of avoidance.107 Both symptoms and histological remission following elimination or reoccurrence following reintroduction are required. Histology is measured by esophageal endoscopy with biopsies in at least four locations in the esophagus. The biopsy should be evaluated for eosinophils per high power field. The normal esophageal eosinophil count is zero and greater than 15 eosinophils per high power field is consistent with active EoE. Symptoms can be monitored with one of several validated symptom scores including the Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS), Eosinophilic Esophagitis Activity Index (EEsAI) or the dysphagia symptom score.108-110 With concerns about IgE-sensitization and reactivity, patients usually first undergo an OFC per standard IgE protocol to evaluate for immediate-type reactions. If the supervised OFC excludes immediate IgE-mediated reactions, long-term feeding continues at home. In the absence of detectable systemic food-sIgE, food reintroduction is usually performed at home, in an open manner.
4.2.4 Food protein-induced enterocolitis syndrome (FPIES)
Food protein-induced enterocolitis syndrome (FPIES) is classically defined as a non–IgE-mediated food allergic disorder, however in a subset, referred to as “atypical FPIES,” food-sIgE is detectable. FPIES symptoms can be dramatic and lead to hypotension and shock.111
There is no universal agreement on the optimal FPIES OFC protocol, and several different approaches have been proposed.91 Generally, FPIES OFCs are done openly, in a supervised setting.112 Securing intravenous access is recommended in patients with prior severe FPIES reactions, that is, with a prior reaction with hypotension and requiring fluid administration. Criteria for a reactive challenge have been proposed; see Table 5.111 Treatment of FPIES reactions entails rehydration (oral or intravenous), ondansetron (oral or parenteral), and for more severe symptoms, parenteral methylprednisolone, although the efficacy of methylprednisolone has not been established. Baseline and post-challenge (at 4–6 h time mark) complete blood counts with differential are useful to document a ≥1500 cells/mL rise in absolute neutrophil count, which confirms a reactive FPIES reaction.113 A marked rise in neutrophil count is especially helpful when symptoms are more subjective (e.g., nausea, abdominal pain) because it supports interpretation of the challenges as reactive.114
| Major criterion | And ≥2 minor criteria |
|---|---|
| Vomiting in the 1- to 4-h period after ingestion of the suspect food and the absence of classic IgE-mediated allergic skin or respiratory symptoms |
(1) Lethargy (2) Pallor (3) Diarrhea 5–10 h after food ingestion (4) Hypotension (5) Hypothermia (6) Increased neutrophil count of ≥1500 neutrophils above the baseline count |
| The OFC will be considered diagnostic of FPIES (i.e., positive) if the major criterion is met with ≥2 minor criteria. | |
| Important caveats | |
|
(1) With the rapid use of ondansetron, many of the minor criteria, such as repetitive vomiting, pallor, and lethargy can be averted; (2) Not all facilities performing challenges have the ability to perform neutrophil counts in a timely manner; (3) In adults, severe abdominal pain is more common than vomiting. Therefore the treating physician might decide that a challenge be considered diagnostic in some instances, even if only the major criterion was met. |
|
In one approach, the total challenge dose is calculated as 0.06–0.3 g of food protein per kilogram body weight (maximum 3 g protein), administered as a single serving or as 2–3 servings every 15–30 min, followed by 4 h observation. Patients are usually discharged after an asymptomatic initial feeding and advised to incorporate the food into their usual diet with gradual progression to a full serving over the course of several days. A modification of this approach has been reported by which FPIES OFCs with 1/3 of the food portion for age is done under physician observation followed by a home titration to a full dose.115 Using this protocol, there was a very low rate of mild (mostly diarrhea) delayed reactions later during the day of the OFC or within the first few days of home dosing. Given that symptoms were mild and resolved without treatment in all cases, it was suggested that low-dose OFCs with home titration to a full serving may be safe and offer considerable benefits over the traditional OFC protocol that frequently induces more severe symptoms during a single feeding with a higher dose of food. Of importance, recent reports highlight the possibility that even following a successful supervised OFC, FPIES symptoms might recur at home.114, 116
4.2.5 Atypical FPIES
Presence of food-sIgE in patients with atypical FPIES requires incremental administration of the initial dose of challenge food to account for potential immediate symptoms, followed by same observation as in classic FPIES. In atypical FPIES, treatment for IgE-mediated symptoms, including anaphylaxis, must be immediately available.
4.2.6 Other non–IgE-mediated gastrointestinal disorders (food protein-induced allergic proctocolitis (FPIAP), food protein-induced enteropathy (FPE), non-specified gastrointestinal food allergy)
Oral food challenges for other non–IgE-mediated gastrointestinal disorders are usually done openly, over several days, and at home.117 Non-specific gastrointestinal symptoms may emerge over several days (FPIAP) or weeks/months (FPE). Symptoms may include diarrhea, abdominal discomfort, visible or occult fecal blood, fecal mucus, fatty stools, and/or poor weight gain. Validated gastrointestinal symptom scoring algorithms may be utilized to evaluate the outcome of the challenge.108, 109
4.3 Food challenge schedules in the research setting
- cereals containing gluten (e.g., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains)
- crustacea
- eggs
- fish
- milk
- peanuts
- sesame
- specific tree nuts (almond, cashew, hazelnut, pecan, pistachio and walnut).
The semi-logarithmic dosing protocol results in a similar proportion of individuals allergic to the index allergen reacting to each dosing level (see Figure 2) and can be used to inform challenge doses for both research and non-research OFCs. Equivalent doses of priority food allergens are shown in Table S3, using a 7-dose regimen. For non-research challenges where there is less of a concern as to “first-dose” reactors (i.e., left-censored data), doses 1–3 can be combined resulting in a 5-dose regimen. For some foods, higher challenge doses are required, either due to low protein content or a lower potency of the allergen in provoking reactions (such that higher doses are needed); the latter is a particular concern with respect to shrimp and fish.88 In addition, for some foods, the dose that would be expected to cause a reaction in the vast majority of individuals allergic to that food is significantly less than a typical serving portion (e.g., for cow's milk, soy milk, wheat-based products). Therefore, for these foods, an additional final “portion-size” dose is recommended (see Table S3).
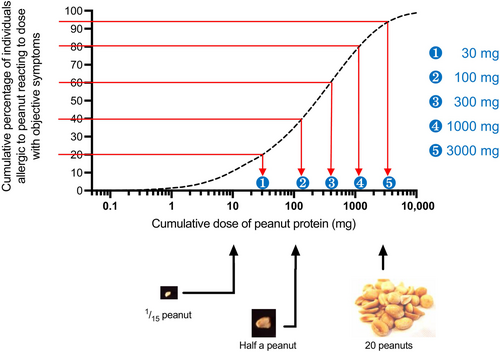
4.4 Double-blind, placebo-controlled food challenges (DBPCFC) and open food challenges
The preferred method for DBPCFCs is the administration of active and placebo challenges on separate days. If the active and placebo test challenges are administered on the same day, the active and placebo challenge should be separated by at least 3 h. However, such schedules are not useful for diagnosing reactions that occur more than 3 h following the challenge. The working group does not recommend administration of active and placebo doses in an interspersed fashion, that is, administering both placebo and active doses in the same challenge, since reactions cannot then be attributed to a specific dose. If subjective symptoms occur and the observer is unsure whether a true reaction is developing, it is preferable to delay the next dose to see if symptoms progress.
Starting doses at the low milligram level are generally safe and seem to result in fewer severe reactions than higher doses.85, 86 For example, Hourihane et al.110 demonstrated that a dose of 1.5 mg peanut protein (equivalent to an ED05 exposure, that is, the amount of allergen that would be expected to trigger an objective reaction in at least 5% of the population allergic to that allergen) resulted in only mild symptoms in peanut-allergic children using a single dose-challenge. A starting dose of 3 mg peanut protein seems also to be safe as children with high levels of IgE-sensitization undergoing an OFC experienced only mild to moderate symptoms at this dose.118, 119
Based on the above recommendations, a typical dosing schedule for a DBPCFC should include the following doses: 3, 10, 30, 100, 300, 1000 and 3000 mg of food protein (Table 3 and Table S3). Such a semi-logarithmic schedule is likely to be appropriate for most foods, patients, clinical situations and settings. In the clinical setting where open OFCs are undertaken in individuals who are not suspected to be low-dose reactors, the first three doses could be combined into a 43 mg protein dose, resulting in a 5-dose schedule. Sample schedules are provided for the priority food allergens in Table S3.
4.5 Dosing intervals
The previous PRACTALL document1 recommended an interval of at least 15–20 min between doses. However, time from ingestion to reaction is longer than 15 min for most allergens and median time from ingestion to objective allergic reaction varies depending on the allergen: from 20 to 30 min for milk,120, 121 55 min (range: 5–210 min) for peanut122 and 50–75 min for some forms of cooked egg.120, 121 These data support the hypothesis that shorter dose intervals may lead to an overlap effect and result in patients receiving higher doses, which could cause more severe reactions.100 This may be a particular issue with food challenges where the allergen is in a non-native form, such as “baked-egg” and “baked-milk” challenges.123 Ke et al.124 studied variable dosing intervals of up to 30 min in milk-allergic children undergoing OFCs prior to immunotherapy and reported that longer dosing intervals were associated with less need for rescue treatment with multiple doses of epinephrine. Studies have reported less severe reactions using dosing intervals of 60 versus 30–40 min for milk,125 egg,125, 126 boiled egg125 and wheat.125, 127 On this basis, a dosing interval of at least 30 min is recommended for food challenges conducted in the research setting, and 20–30 min for clinical OFCs. Longer dosing intervals (e.g., 60 min) may be justified in some patients with a history of more severe reaction, or alternatively, clinicians might wish to extend the dosing interval where a patient is suspected to meet challenge stop criteria if the next dose is delayed (e.g., if 30 min after the dose, symptoms are evolving rather than resolving).
5 OFC MATERIALS
Materials used in DBPCFC and open OFC should be carefully selected, free from cross-contamination with other food allergens, microbiologically safe, and validated to ensure accuracy and reproducibility.128 Standardized recipes and vehicles are mandatory for DBPCFCs to minimize variability and ensure comparability between different studies.83 Materials for research DBPCFC are provided by the investigators, whereas for open OFCs in clinical scenarios, the patient or caregivers can provide the challenge food per the specific instructions. Commercial materials for OFC may also be available in some countries.129, 130
The preparation of food allergen depends on the purpose of the challenge. To confirm complete tolerance, usually the most raw form of food is used, e.g., fresh cow's milk, pasteurized raw egg white powder or peanut flour. When evaluating tolerance to processed foods such as baked or cooked, such foods are prepared according to specific recipes. The effect of heating on the allergenicity of proteins is greater in eggs than in milk and is influenced by the duration, temperature and presence of other foods, such as wheat.131
The food matrix may be chosen to minimize or maximize allergenicity, as certain matrices, such as high starch and gluten muffins, have been shown to alter the bioavailability of allergens in a way that is more similar to baked goods, such as bread, than to low water activity matrices such as cookies.132 A protein-rich matrix has a protective effect on absorption.130
Baked milk and eggs have been found to be tolerated by 70%–85% of children with IgE-mediated allergies. Tolerance to baked milk and egg has been shown to be a marker of milder and transient allergy.133, 134 However, it is important to note that reactions to baked milk and egg challenges may be severe and more delayed with symptoms beginning at ≥60 min after OFC completion. Therefore, modified dosing and prolonged observation should be considered.123
For open OFCs, the food used should contain only the causative antigen and a vehicle with a low frequency of other allergies.98, 135, 136 The specific food and dosage used can be tailored to an individual patient's age and history.96, 97
6 SCORING AND STOPPING ORAL FOOD CHALLENGES
6.1 General considerations
To facilitate comparisons of DBPCFC outcomes, standards must be followed with respect to reporting results, including which symptoms are classified as subjective or objective, and what outcomes constitute a reactive challenge. It is not possible to have universally agreed upon published parameters, likely because clinical judgment is needed, and circumstances may vary by patient or study characteristics. Decisions to discontinue dosing may be made for reasons that vary according to requirements of specific study protocols, the symptoms observed, safety issues and patient characteristics. In response to safety concerns, dosing may be discontinued prior to eliciting clear objective reactions, but this in turn may reduce the diagnostic accuracy of the procedure.
Food challenges are typically considered reactive (positive), and dosing stopped, when objective symptoms are present. In some situations, however, mild objective symptoms may be considered insufficient to discontinue dosing or to consider a challenge reactive (e.g., one or two transient perioral urticarial lesions from contact with the food or one episode of vomiting in a child with anxiety and a distaste for the challenge substance).51 Subjective symptoms may in some circumstances indicate a reactive response to challenge and present a good reason to discontinue dosing, for example, by having repetitive symptoms or multiple subjective symptoms in several organ systems, Table 6. However, stopping a challenge with only subjective symptoms increases the risk of a false-positive test compared to discontinuing the challenge only for objective symptoms. Subjective or initially mild objective symptoms may be subtle in a young child, who may become suddenly quiet or have behaviors such as food refusal or scratching in the ears or at the tongue and neck.
| General nonspecific pruritus |
| Scratching |
| Nasal pruritus |
| Ocular pruritus |
| Dyspnea (without objective signs) |
| Throat “tightness” |
| Nausea |
| Abdominal pain |
| Oral/throat pruritus |
| Complaints of weakness, dizziness, not feeling well, etc. |
At a minimum, the parameters for stopping and declaring a challenge reactive (positive) or negative should be pre-specified in challenge protocols and the details reported in publications. Symptoms typically considered subjective are discussed below. Regarding the options for discontinuing dosing and considering a challenge reactive, options include “worsening” subjective symptoms, repeated elicitation (e.g., on three doses) or persistence of symptoms (e.g., 2 dosing intervals, or 40–60 min).51-53 These parameters for stopping a challenge and declaring a challenge reactive have not been evaluated with regard to impact on the sensitivity and specificity of the test. When mild objective or subjective symptoms occur, decisions include stopping the challenge, waiting longer for the next dose or repeating a dose.51, 70 Judgments about proceeding must balance safety against the certainty of the challenge outcome. Although more time consuming, undertaking additional challenges (e.g., 5 oral challenges per food with three challenges containing placebo and 2 challenges containing the food allergen or vice versa, randomly) can greatly increase the accuracy of conclusions when symptoms are subjective.52
6.2 Stopping criteria for OFCs
Recent evidence supports the concept that there are significant differences in reaction thresholds when less objective symptoms are used to define the stopping point in food challenges, even with the same dosing schedule.137 Less stringent criteria (i.e., modified CoFAR 3 versus prior PRACTALL guidelines) appear to underestimate the challenge reaction threshold.138 This may be problematic in research studies where a precise eliciting dose/cumulative reactive dose (greatest tolerated dose/cumulative tolerated dose) define the primary endpoint, but not in clinical settings where the aim is to determine simply whether a patient is reactive (allergic) or tolerant to a specific food.
Typically, when food-allergic individuals react to their trigger allergen, there are two patterns of symptom evolution: the majority of individuals initially experience subjective symptoms (such as oral and/or pharyngeal pruritus or mild gastrointestinal discomfort) – although this can be difficult to communicate in very young children – prior to progressing to the onset of objective symptoms with or without further challenge doses being given. However, some individuals have a different pattern, where objective symptoms (which may include anaphylaxis) are the presenting symptoms (Figure 3).81 Ideally, OFCs (whether blinded or unblinded) should be stopped on the basis of reproducible and objective symptoms. Subjective symptoms (Table 6) are common during OFCs and often precede the emergence of objective symptoms by a number of dosing intervals.
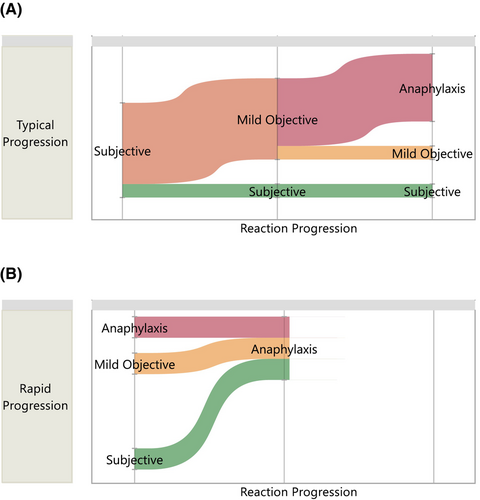
Subjective symptoms, which can be substantial and persistent, are also reported during placebo OFCs,77 which complicates the decision of when to discontinue an OFC and confirm the presence of food allergy. For example, if symptoms such as nausea or dyspnea (without objective signs) are a result of anxiety rather than true food allergy, this could lead to a false diagnosis of food allergy. Furthermore, there is considerable variability in how clinicians interpret subjective symptoms that occur during food challenge, and whether they indicate a reactive reaction.139, 140
Mild nasal symptoms are common during food challenges in both food-allergic and non-allergic subjects141 and are therefore not usually considered indicative of true reactivity. Subjective oral symptoms, such as tongue and throat tingling or pruritus, present a particular challenge as they are difficult to distinguish from local oral symptoms. A retrospective chart review of 652 OFCs found that subjective oral symptoms occurred in over one third of food challenges.142 Subjective oral symptoms occurred with reactive OFCs, but the false-positive rate for subjective symptoms in predicting a reactive challenge was 30% – that is, among those who initially experienced subjective oral symptoms, 30% were able to complete the challenge without developing any objective symptoms. There was no difference in the occurrence rate of anaphylaxis during reactive challenges among those with and without subjective oral symptoms. In a separate study, which combined individual participant data from four interventional studies (n = 592), the use of less objective symptoms to define challenge stopping criteria underestimated both the challenge threshold and increased the rate of placebo reactions.137 Together, these studies demonstrate the impact that subjective symptoms can have on the reliability of food challenge results, which is important in both research and clinical settings.
A clear approach is therefore needed to determine when to stop a diagnostic challenge and yet avoid the risk of false-positive tests. Furthermore, using subjective symptoms in isolation can significantly impact the dosing level reported to cause a reaction, which is typically used to inform primary outcomes in trials of therapeutic interventions in food allergy.137 Therefore, an approach is needed to achieve a balance between safety (which may be impaired if excess, unnecessary doses are given during a challenge) and confirmation of true clinical reactivity.
A variety of approaches for handling subjective symptoms have been proposed, including waiting for the symptoms to resolve before continuing the challenge,143 discontinuing the challenge if the subjective symptoms persist for a sufficient period of time, for example, 45–60 min,144, 145 or discontinuing the challenge if subjective symptoms occur over three consecutive doses.7 The previous PRACTALL guideline suggested that subjective symptoms that recur over three doses, or persist (e.g., 40 min) are more likely indicative of a reaction than when such symptoms are “transient and not reproducible”. However, this must be weighed against the observation that some subjective symptoms are less indicative of an allergic reaction than others (e.g., oral pruritus), and that a recent analysis of 592 reactive challenges from four separate studies found no significant adverse impact on rates of anaphylaxis during food challenges when excluding subjective symptoms from defining challenge stoppage.137
- Green symptoms: Not generally sufficient to alter dosing or consider a challenge reactive.
- Orange symptoms: These are more likely to indicate a reaction, particularly if they recur or persist (for more than a single dosing interval, i.e., 20–30 min). Clinicians may wish to consider delaying the next dose on the occurrence of one orange symptom, particularly if this involves an intermittent cough/throat tightness/persisting abdominal pain. Two “orange” symptoms from different organ systems and occurring at the same dose are more likely to represent a true response and thus considered sufficient to stop the OFC.
- Red symptoms: A single red symptom is likely to indicate a true response, and if present, the challenge should be halted.
| Suggested challenge stopping criteria: one red or two orange symptoms from 2 distinct organ categories | ||
|---|---|---|
| I. Skin | ||
| Rash: erythema |
Few areas of faint erythema <50% of body surface area Generalized (>50% body surface area) |
|
| Rash: urticaria |
Limited to perioral region or due to contact 1–2 lesions (not perioral or due to contact) ≥3 lesions (not perioral or due to contact) |
Local skin reactions due to contact (including lip contact with challenge dose) excluded |
| Angioedema |
Prominent lip or ear edema Facial edema (and new-onset uvula edema) Generalized edema |
Facial (including periocular) swelling should be prominent and not due to local rubbing or crying. If crying/rubbing causes local swelling, consider delaying the next FC dose to see if other symptoms develop |
| Pruritus | Scratching (any) | Not considered a stopping criterion |
| II. Eyes/Upper respiratory | ||
| Eyes |
Minimal reddening, rubbing of eyes Conjunctival hyperemia (without prior rubbing) |
Periocular rubbing or crying is a common cause of conjunctival reddening |
| Nasal |
Mild, infrequent rhinitis Persistenta and significant rhinorrhea/sneezing/rhinitis |
Note mild nasal symptoms are common during FC and therefore a poor indicator of objective reaction |
| III. Respiratory | ||
| Coughb |
Intermittent cough associated with throat clearing Frequent cough without respiratory compromise Cough with respiratory compromise* *Manage as anaphylaxis |
If cough is present without evidence of respiratory compromise (e.g., significant tachypnoea, fall in oxygen saturations, use of accessory muscles, wheezing, PEFR decrease >20% with good technique), consider whether to terminate the FC (which could lead to an equivocal result if no other symptoms develop) or adopt “watchful waiting” (and delay the next FC dose) |
| Wheezing |
Any wheeze* *Manage as anaphylaxis |
Reduced air entry or “added sounds” on auscultation may precede wheeze |
| Chest tightness |
Isolated chest tightness Chest tightness with fall in peak flow of ≥20%* (with good technique) *Manage as anaphylaxis |
Chest tightness is subjective and should not trigger challenge-stop in isolation (but may prompt extending the dosing interval). If peak flow is being assessed, then a decrease of ≥20% from baseline (assuming satisfactory technique) can be considered a stopping criteria |
| IV. Oropharyngeal | ||
| Oral cavity | Itchy mouth | |
| Throat/Laryngeal |
Itchy throat, intermittent throat clearing Persistenta throat tightness or pain Non-transient hoarseness/stridor |
Subtle vocal changes are presumably due to mild laryngeal edema and should therefore trigger the FC to be stopped if non-transient in nature |
| V. Gastrointestinal | ||
| Abdominal discomfort |
Nausea (any severity/frequency) Mild abdominal pain Persistenta non-distractable abdominal pain (usually with a decrease in activity level in children) Persistenta severe abdominal pain |
Abdominal pain is a subjective symptom and should not trigger challenge-stop in isolation. Persistent severe abdominal pain would normally be accompanied by other symptoms. Where this is present, further FC doses should be deferred to allow additional time for other symptoms to evolve |
| Vomiting |
Vomit due to gag or taste aversion 1+ episode (where investigator considers this is due to allergic reaction) |
If vomiting occurs during or shortly after the FC dose, then this is more likely to be due to gag or taste aversion. If other symptoms subsequently develop, clinicians should reconsider whether the episode was non-allergic in origin |
| Diarrhea |
1 episode 2+ episodes |
|
| VI. Cardiovascular | ||
| Cardiovascular |
Mild tachycardia Clinically significant hypotension Cardiovascular shock/collapse |
Hypotension defined as a decrease in systolic BP greater than 30% from that person's baseline, OR
|
| VII. Neurological | ||
| Neurological |
Feeling weak, tired, upset/agitated Significant change in cognition or GCS* Loss of consciousness* *Manage as anaphylaxis |
Allergic mediators such as histamine are also neurotransmitters; neurological impairment can occur independently of cardiovascular compromise during allergic reactions |
- Note: Objective symptoms are shown in bold italics.
- Abbreviations: FC, food challenge; GCS, Glasgow Coma Score.
- a Persisting = ongoing symptom (without evidence of resolution) for at least one dosing interval (20–30 min).
- b Cough may be upper respiratory, laryngeal or lower respiratory in origin, and it can be difficult to determine the source.
Under the revised system, OFCs should be stopped if a single red symptom or two orange symptoms from different target systems are present during the same dosing interval. These criteria can be applied to both “open” (i.e., unblinded) and DBPCFCs. Staff performing challenges need to be suitably trained and supported to have confidence in implementing this guidance and not discontinuing challenges prematurely.
Peak flow can be assessed in most children over 6 years of age using a portable peak flow meter and can be a useful measure of bronchoconstriction (so long as technique is satisfactory). Where feasible, peak flow can be conducted at baseline and then as indicated during the challenge, to help assess whether subjective dyspnea is associated with objective change in respiratory function. Some groups have proposed using peak expiratory flow to help inform challenge stop criteria, on the basis that a fall in PEF of ≥20% from baseline is likely to indicate an objective impairment in ventilation.55, 146, 147 An assessment will need to be made as to whether any change in PEFR is due to poor technique/lack of effort or reflects bronchoconstriction. Modest reductions in peak flow can occur with abdominal pain by adversely impacting technique, but this is usually apparent to the observer.
When food challenges are used to evaluate reported delayed symptoms (e.g., isolated gastrointestinal reactions and atopic dermatitis) or more controversial atypical symptoms (migraine, behavior, and arthritis), different approaches, such as multiple DBPCFCs (with more than 1 active and 1 placebo challenge to assess reproducibility of symptoms), symptom diaries and SCORAD scores, might be needed. Similarly, evaluation of isolated oral symptoms from pollen-food allergy syndrome requires modification of outcome measures. Challenge protocols might require modifications to accommodate specific ages (e.g., smaller portion sizes/doses) or disease outcomes; nevertheless, it is essential to explicitly detail how symptoms are assessed with regard to stopping dosing and determination of reactive, negative, or inconclusive challenge results to allow comparison of outcomes. The 2012 iteration of PRACTALL guidelines contains a full discussion of right censoring statistical design and analysis issues for the DBPCFC when individuals exhibit subjective symptoms,1 which can be found in Appendix S1.
7 ORAL FOOD CHALLENGE—REPORTING RESULTS
7.1 OFC for research versus clinical settings, and reporting results
The DBPCFC is the “gold standard” for diagnosis used in research settings, and until a more reliable surrogate biomarker is developed, it is very likely that placebo-controlled challenges will be a standard part of research studies for the foreseeable future. In both research and clinical settings, the DBCPFC may be used to provide a definitive diagnosis of food allergy, to determine the dose-triggering threshold (eliciting dose) of an allergen, and to validate the acquisition of desensitization or tolerance to a full serving of the allergenic food.1, 94 The use of a placebo control allows for minimization of bias from both the participant and clinician administering the challenge; however, in clinical practice, placebo-controlled challenges are rarely used, especially in a double-blind manner.4 Single-blind challenges may be employed on occasion when performing a food challenge in a patient with a significant amount of anxiety that may prohibit their ability to reliably ingest a serving of the food without interference from subjective symptoms often provoked by anxiety. Open OFCs are the most common method of assessing tolerance development or validating the diagnosis of IgE-mediated food allergy in clinical practice. Open food challenges may be sufficiently reliable in infants who are unable to communicate subjective symptoms, or if stopping criteria requires objective evidence of an allergic reaction.
Reporting of protocols used and outcomes of DBPCFCs are key aspects to support reproducibility, assess for potential biases and to enable comparisons between studies. Where different stopping criteria are applied, this can preclude comparison of results based on food challenges. For example, the results of a trial that required ingestion of 300 mg of peanut protein without reaction to define desensitization compared to a trial that required ingestion of 1000 mg, are not directly comparable without acknowledging this key difference. The reasons for stopping a challenge should be reported, along with the number of participants in each category, in terms of objective symptoms only and then how subjective symptoms were handled (e.g., repeated subjective, worsening subjective, or persistent subjective symptoms). The number of patients with severe systemic reactions and their characteristics should also be reported. Caution should be applied in the use of epinephrine to treat a reaction as a measure of reaction severity, since multiple patient factors, site practices (e.g., the threshold to administer epinephrine might be lower in an outpatient setting), and physician judgment are often subjective, variable and will affect the treatment of a reaction. Nuanced aspects of the DBPCFC should be included in publications, irrespective of the study design (e.g., RCTs, observational studies etc.) including the type of matrix used for mixing the allergenic food, components of the placebo, including blinding ingredients, and the volume of the product required to ingest at each step of the food challenge. Table 8 includes a summary of data to report in studies using an OFC. Figure 4 illustrates the definitions of terms used in trial reporting. The PRACTALL working group recommends that trials report both the eliciting dose and cumulative tolerated dose in milligrams of protein prior to eliciting an objective, dose-limiting reaction. Investigators are encouraged to make individual data available when possible in on-line repositories.
| 1. Demographics including age, sex, ethnicity, age at diagnosis, SPT results, in vitro specific IgE levels (and methodology used), other atopic disorders |
| 2. Oral food challenge inclusion/exclusion criteria |
| 3. Food matrix used and ingredients, including fat content (percent). If matrix was validated, how was this accomplished? |
| 4. Description of challenge food's physical state (e.g., raw, cooked, dehydrated or defatted) |
| 5. Method to validate blinding between placebo and verum |
| 6. Dose escalation schedule (expressed in mg of protein, amount of time between doses, and whether repeat doses were allowed) |
| 7. Challenge setting (e.g., hospital, research unit, etc.) |
| 8. Scoring system with predetermined stopping/delaying/repeating/progressing decision points and how subjective symptoms are used in the scoring system |
| 9. Percentage of patients stopped for objective and subjective responses. Transparent reporting of subjective responses should include whether those with subjective responses only were deemed allergic, inconclusive, or offered a repeat food challenge |
| 10. Eliciting dose in mg of protein (i.e., single administered dose eliciting objective reaction leading to dose-limiting symptoms) |
| 11. Cumulative tolerated dose in mg of protein (i.e., sum of all doses ingested prior to administered the eliciting dose) |
| 12. Cumulative reactive dose in mg of protein (i.e., sum of all doses ingested including the eliciting dose) |
| 13. Percentage of positive, negative or inconclusive results with criteria defined in advance |
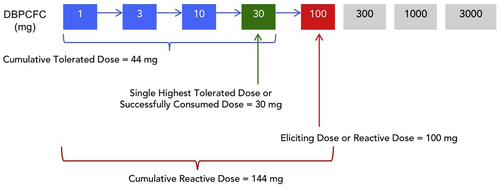
Placebo responses should also be reported, including subjective and objective symptoms, and data should be reported in a similar fashion to the data from allergen challenges, including severity grading. The rate of placebo reactions (false positive rate) during OFC should be reported and accounted for in the design and analysis of the study.148 Descriptions of symptoms elicited by an OFC may provide data for grading the severity of a reaction and should be reported. While severity grading has been extensively studied and discussed, it is not within the scope of the present guidelines.149, 150
8 CONCLUSIONS
As noted in the Introduction, the 2024 PRACTALL Food Challenge Guidelines is meant to update and complement the 2012 Guidelines. Sections on the food challenge setting and safety parameters are highlighted, but remain largely unchanged, and the section on reporting clinical trial results has been expanded, while the reader is referred to the 2012 Guidelines for a discussion of statistical analyses. Studies over the past 12 years have improved precision to the standard diagnostic techniques, for example, skin prick tests and serum IgE quantitation, and the use of IgE to food component proteins and epitopes have provided improvements in diagnostic accuracy. Other diagnostic tests, such as the basophil activation assay and the mast cell activation assay may soon become clinically available and add another diagnostic tool to the allergist's armamentarium. Use of a standardized DBPCFC protocol has provided a great deal of new information on the effects of challenge dose, timing and material utilized for the food challenge, and provided guidance on the use of different protocols for routine clinical diagnosis versus research trials in IgE-mediated food allergy. In addition, new protocols for use in IgE-mediated alpha-gal allergy and non-IgE-mediated food allergy such as eosinophilic esophagitis and gastroenteropathies, food protein-induced enterocolitis syndrome, food protein-induced colitis syndrome and others. Systematic reviews of the many studies employing the 2012 Guidelines' challenge protocols have provided a wealth of information on challenge-induced signs and symptoms that prompted the need to revise the scoring and stopping rules for oral food challenges, which are extensively discussed in these Guidelines. It is hoped that one day laboratory-based biomarkers will replace the need for oral food challenges, but until that time, the DBPCFC will remain the “gold standard” for diagnosing food allergy and will serve as the standard benchmark for new diagnostic tests and novel therapeutics to treat food allergies.
8.1 Key recommendations for conducting oral food challenges
- In the clinical setting, food challenges are conducted as a diagnostic or monitoring procedure and should be carried-out in conjunction with other diagnostic tests when possible and following joint decision-making with the patient.
- In the research setting, food challenges often determine the primary outcome of the study and should be designed to have the most definitive and robust results.
- Safety and reliability are key when deciding on the indication, design and setting of an oral food challenge.
- Food challenge doses should be given in incremental amounts separated by at least 30 min intervals in the research setting, and at 20–30 min intervals in the clinical setting.
- Food challenge protocols should be adapted to the clinical type of food allergy under investigation (immediate type IgE-mediated food allergy, FPIES, EoE, AD).
- DBPCFCs are preferred in research settings and in clinical settings when symptoms expected are likely to be subjective or the patient being challenged is highly anxious.
- Observed signs and symptoms can be grouped into three categories to aid in the decision of when to discontinue or proceed to the next challenge dose: no significant reaction (green = proceed), possible reaction (orange = pause and reassess) and definite reaction (red = stop).
- A food challenge is deemed non-reactive when the amount of food protein ingested is equivalent to a normal serving of the food prepared in the usual manner has been consumed and tolerated with no adverse symptoms.
AUTHOR CONTRIBUTIONS
Hugh A. Sampson: Conceptualization; methodology; writing – original draft; writing – review and editing; project administration; supervision. Stefania Arasi: Conceptualization; methodology; writing – original draft; writing – review and editing. Henry T. Bahnson: Conceptualization; methodology; writing – original draft; writing – review and editing. Barbara Ballmer-Weber: Conceptualization; methodology; writing – original draft; writing – review and editing. Kirsten Beyer: Conceptualization; methodology; writing – original draft; writing – review and editing. Carsten Bindslev-Jensen: Conceptualization; methodology; writing – original draft; writing – review and editing. J. Andrew Bird: Conceptualization; methodology; writing – original draft; writing – review and editing. Katarina Blumchen: Conceptualization; methodology; writing – original draft; writing – review and editing. Carla Davis: Conceptualization; methodology; writing – original draft; writing – review and editing; project administration; supervision; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; conceptualization; methodology; writing – original draft; writing – review and editing; project administration; supervision. Motohiro Ebisawa: Conceptualization; methodology; writing – original draft; writing – review and editing. Anna Nowak-Wegrzyn: Conceptualization; methodology; writing – original draft; writing – review and editing. Nandinee Patel: Conceptualization; methodology; writing – original draft; writing – review and editing. Rachel L. Peters: Conceptualization; methodology; writing – original draft; writing – review and editing. Scott Sicherer: Conceptualization; methodology; writing – original draft; writing – review and editing. Jonathan Spergel: Conceptualization; methodology; writing – original draft; writing – review and editing. Paul J. Turner: Conceptualization; methodology; writing – original draft; writing – review and editing. Noriyuki Yanagida: Conceptualization; methodology; writing – original draft; writing – review and editing. Philippe A. Eigenmann: Conceptualization; methodology; writing – original draft; writing – review and editing; project administration; supervision.
CONFLICT OF INTEREST STATEMENT
Hugh A. Sampson, MD; Funding to his institution for grants from NIH/NIAID and having received consulting fees from DBV Technologies, S.A., N-Fold Therapeutics, LLC, and Siolta, Inc., Alpina Biotech, royalties from Elsevier, and stock options from DBV Technologies and N-Fold Therapeutics outside the scope of this work. Stefania Arasi, MD, PhD, MSc; Advisory board member for Aimmune and Novartis, as speaker/discussant for Ulrich and DBV, Stallergenes Greer outside the submitted work. Henry T. Bahnson, MPH; Consulting fees from King's College London and stock options from DBV Technologies, where he is currently employed. Barbara Ballmer-Weber, MD; Advisory Board honoraria and honoraria for talks outside of the submitted work from Novartis, Sanofi, ALK, Stallergenes, ThermoFisher, Menarini, Aimmune. Kirsten Beyer, MD; Funding to her institution for grants from the German research foundation, the German Federal Ministry of Education and Research (BMBF), the German Federal Ministry of Food and Agriculture (BMEL), Aimmune, Danone, DBV, Hipp, Infectopharm, Hycor, Novartis; and having received speaking honoraria from Aimmune, ALK, Danone, Infectopharm, Nestle, Hycor, ThermoFisher, Kantar Health, Labor 28, Stallergenes, consulting fees from Hipp, Novartis and advisory board honoraria from Aimmune, ALK, Danone, Viatris, Nestle, Allergy Therapeutics, Novartis, Kantar Health, Primus Consulting, Stallergenes outside the scope of this work. Carsten Bindslev-Jensen, MD, PhD DMSci; Advisory Board honoraria from ALK-Abello, novartis ans Sanofi outside this work. J. Andrew Bird, MD; Institutional funding from Aimmune, Astellas, DBV Technologies, FARE, Genentech, NIH NIAID, Novartis, Regeneron and Siolta; consulting from Allakos, AllerGenis, Allergy Therapeutics, DBV Technologies, FARE, Genentech, HAL Allergy, Novartis, Nutricia and Parexel; speaking honoraria from DBV Technologies; and DSMB participation for Infinant Health. Katarina Blumchen, MD; Institutional grants by Novartis Pharma GmbH, Aimmune Therapeutics, DBV Technologies, and Hipp GmbH; Consulting fees by Novartis Pharma GmbH, Nestle, Bencard Allergie, Aimmune Therapeutics, ALK, and DBV Technologies; Honoraria by Aimmune Therapeutics, DBV Technologies, Novartis Pharma GmbH, Thermo Fisher Scientific, Viatris Healthcare GmbH, Allergopharma, and Danone; Meeting/travel support by DBV Technologies, Novartis Pharma GmbH, and Aimmune Therapeutics; and Participation Data Safety Monitoring Board/Advisory Board by Novartis Pharma GmbH. Carla Davis, MD; Contract funding from DBV Technologies, Regeneron, and Aimmune Therapeutics, grant funding from the National Institute of Allergy and Infectious Disease (grant no. R34AI157948) (CoFAR and Consortium of Eosinophilic Gastrointestinal Disease Researchers), and the Food Allergy Research and Education. Motohiro Ebisawa, MD; Speaker and advisory board honoraria: Viatris, Sanofi, Novartis, and ARS-Pharmacuticals. Anna Nowak-Wegrzyn, MD, PhD; Research grants from DBV Technologies and Siolta Therapeutics, Speaking and consulting: ThermoFisher Scientific, Nestlé Health Sciences, Danone. Nandinee Patel, MD; No conflict of interest to declare. Rachel L. Peters, PhD; Grants from National Health and Medical Research Council of Australia, Allergy and Immunolgoy Foundation of Australasia and the Thoracic Society of Australia & New Zealand, paid to their institution; research prize from Stallergens Greer Foundation, paid to their institution; and personal fees from Stallergenes Greer; all outside the submitted work. Scott Sicherer; Grants to his institution from the National Institute of Allergy and Infectious Diseases (NIAID), from Pfizer, and from Food Allergy Research and Education (FARE), outside the submitted work; and Royalties from Johns Hopkins University Press and UpToDate; and Honoraria from the American Academy of Allergy, Asthma and Immunology for the role of deputy editor of J Allergy CIin Immunol: In Practice. Jonathan Spergel, MD; Grant support from Regeneron, Sanofi, Novartis, Consultant-Novartis, Allakos, Regeneron, Sanofi, Readysetfood, Bristol Myers Squib. Paul J. Turner, MD; Grants from UK Medical Research Council, the UK Food Standards Agency, The Jon Moulton Charity Trust, NIHR/Imperial Biomedical Research Centre, outside the submitted work; personal fees from UK Food Standards Agency, UpToDate, Elsevier, ALK, and Allergenis outside the submitted work. Noriyuki Yanagida, MD; No conflict of interest to declare. Philippe A. Eigenmann, MD; Speaker and advisory board honoraria: DBV technologies, Novartis, ThermoFisher Scientific, Nestlé Health Sciences, Synlab, GSK; Stocks and Stock options: DBV technologies.




