Region-specific effects of the cysteine protease papain on gastric motility
Abstract
Background
Papaya is a traditional remedy for gastrointestinal complaints in the folk medicine. On this basis, papain, a cysteine protease of the fruit, is sold as a nutritional supplement, although scientific data on its effects in the gastrointestinal tract are lacking. We aimed to explore the effect of papain on gastric motility in vitro.
Methods
Guinea pig antrum and corpus strips were mounted in organ bath.
Key results
Papain reversibly increased the amplitude of ongoing phasic contractions in both circular and longitudinal antrum strips without having an effect on the frequency or on the muscle tone. All three tested doses of papain (end cc.: 12.5 mg L−1, 50 mg L−1, 100 mg L−1) were similarly effective. Contrarily, in the corpus circular and longitudinal muscle strips, papain caused a dose-dependent relaxation, which was preceded by a transient contraction in most tissues. The effect was resistant to tetrodotoxin (1 µM), but diminished by the cysteine protease inhibitor E64 (4.5 µM) in both regions. In the corpus, L-NAME (100 µM) and the protease-activated receptor (PAR)-1 antagonist SCH79797 (5 µM) or the PAR-2 antagonist GB 83 (3 µM) did not change the effect of papain significantly. This demonstrates that the effects of papain are not neurally mediated and nitrergic pathways are not involved in the mechanism. The effects are linked to the enzymatic activity, but not executed via PAR-1 or 2.
Conclusions and inferences
Papain alters gastric motility in a region-specific manner, which could at least partly explain its claimed beneficial effects in functional gastrointestinal disorders.
Key Points
- Papain, a cysteine protease of papaya, has region-specific effects on gastric motility.
- This may at least partly explain its observed beneficial effects in functional gastrointestinal disorders.
- The aim of our study was to test the effects of papain on stomach motility in vitro.
- Corpus and antrum strips from guinea pigs were mounted in organ bath.
- Papain increased the amplitude of ongoing phasic contractions of the antrum, but did not change the muscle tone and contraction frequency.
- In the corpus, it caused a transient contraction in most cases, followed by a sustained relaxation.
1 INTRODUCTION
Papain is a cysteine protease found in the papaya plant (Carica papaya), dominantly in its latex and unripe fruit. Papaya is known as a traditional remedy for gastrointestinal complaints in countries where it grows.1 In Ayurvedic medicine, the latex is used to relieve dyspepsia, while the fruit is applied as a stomachic and digestive.2 Papain in combination with other enzymes is applied as a treatment for functional dyspepsia in India.3 Furthermore, papaya extracts or purified papain are commercially available as a nutritional supplement in many countries. As an oral preparation, purified papain has been applied in various situations such as a digestive supplement for children with autism,4 a protease supplement to reduce inflammation in eccentric exercise5 or to treat esophageal meat impaction.6
Despite its easy access over internet and various applications, scientific data on the effects of papain in the gastrointestinal tract are extremely scarce. In an early study, oral papaya extract and crystallized papain both protected from experimentally induced gastric ulcer in rats, while the number of gastric parietal cells remained unchanged in the gastric mucosa.7 Papaya is used as a traditional remedy also to improve bloating and stool irregularities, but little is known about the effect of papain on gastrointestinal motility. In a rodent model, papaya extracts significantly reduced small intestinal propulsion.8 In a placebo-controlled, randomized, double-blind study, Caricol, a preparation of organically cultivated papaya, was tested on 139 volunteers with functional gastrointestinal complaints.1 Caricol was significantly more effective in ameliorating constipation; painful, strenuous bowel movements, and flatulence than placebo. An oral enzyme supplement from India containing papain was tested on 100 non-ulcer dyspepsia patients and showed a significant reduction in frequency and severity of all recorded symptoms of indigestion (fullness, belching, bloating, flatulence, and postprandial distress) after 14 days of treatment.9 Nevertheless, none of these studies were performed with pure papain. It is to consider that in case of consuming papaya fruit, the fibers of the plant and many other components may also play a role in its effect on gastrointestinal motility. Kiwifruit is known to relieve constipation and the symptoms of IBS with constipation (IBS-C).10 Besides fibers, its effects are partially assigned to its actinidin content, which is a cysteine protease with a structural homology to papain.11 In a rat model, actinidin has been shown to increase gastric emptying in case of specific diets.11 However, this effect was attributed to the proteolysis of some dietary components by actinidin rather than its direct influence on gastric motility. In humans, an effect of kiwifruit rich in actinidin on gastric emptying could not be detected, but it reduced bloating and other measures of gastric discomfort, while kiwifruit without actinidin did not show the same beneficial effect.12 Interestingly, in gravid and non-gravid rat uterine in vitro preparations, papaya latex extract induced dose-dependent sustained contractions, while pure papain evoked transient increase in frequency and amplitude of contractions.13 These results corroborated the common belief in countries where papaya grows that consumption of the fruit during pregnancy may provoke a spontaneous abortion.
Based on these observations, we aimed to expand knowledge on the gastrointestinal actions of papain by exploring its effects on gastric smooth muscle motility.
2 MATERIALS AND METHODS
2.1 Animals
Male guinea pigs weighing 280–400 g (Dunkin Hartley, Charles River, Sulzfeld, Deutschland) were kept in isolated airflow units at a temperature of 20–24°C and a 14:10 h light/dark cycle. Standard laboratory food pellets and drinking water were provided ad libitum. Animals were killed by a percussive blow to the head followed by exsanguination. All animal work was conducted according to the German guidelines for animal care and welfare (Deutsches Tierschutzgesetz) and approved by the Bavarian state ethics committee (Regierung Oberbayern, which serves as the Institutional Care and Use Committee for the Technische Universität München) according to §4 and §11 Deutsches Tierschutzgesetz under reference number 32-568-2.
2.2 Drugs
Papain from papaya latex (enzyme activity: min. 10 U mg−1 protein, Merck) was dissolved first in distilled water and then diluted in Krebs solution (300 µL in total). Tetrodotoxin (TTX, Tocris), the cysteine protease inhibitor E64 (Merck), and N(ω)-nitro-L-arginine-methylester (L-NAME, Merck) were all dissolved first in distilled water to obtain a stock solution and then further diluted with Krebs solution to a final concentration of 1 µM, 4.5 µM, and 100 µM, respectively. The PAR-1 antagonist SCH79797 (Abcam) was diluted in DMSO to a 10 mM stock solution and then added to Krebs solution to reach a final concentration of 5 µM. The PAR-2 antagonist GB 83 (Axon Medchem) was diluted also in DMSO to a stock solution of 12.2 mM and then further diluted with Krebs to 3 µM in the organ bath.
2.3 Gastric motility experiments
The entire stomach was removed and immediately immersed in ice-cold, carbogen-aerated (95% O2, 5% CO2) Krebs solution (pH 7.4, composition in mmol L−1: 117 NaCl, 4.7 KCl, 2.5 CaCl2 (2H2O), 1.2 MgCl2 (6H2O), 20 NaHCO3, 1.2 NaH2PO4 and 11.0 Glucose). The stomach was cut along the greater curvature, rinsed in Krebs solution, and fixed mucosal side up with metal pins in Sylgard-coated Petri dishes. The mucosa was carefully removed under an Olympus SZ51 stereomicroscope (Olympus, Hamburg, Germany). Muscle strips (1 cm2) were cut along both the circular and longitudinal muscle axis of gastric corpus and antrum and mounted in a four-chamber, 25 mL automatic organ bath (Panlab, Barcelona, Spain). The strips were maintained constantly in carbogen-bubbled Krebs solution at 37°C and pH between 7.3 and 7.4. The muscle strips were attached to an isometric tension transducer connected with a Quad Bridge and a MacLab/4S analog/digital converter (MacLab, AD Instruments, Spechbach, Germany). Motility was recorded and analyzed employing LabChart 7 software (MacLab, AD Instruments) on a computer. After setting a preload of 15 mN, tissue preparations were equilibrated for 60 min. Electric field stimulation (EFS) was performed with a Grass SD9 stimulator (100 V, 10 Hz, pulse width of 0.5 ms, 10 s) to judge tissue viability. Tissues not responding to EFS with a change in tension were excluded from further testing. Viable tissues responded with a biphasic response, with an initial contraction followed by a relaxation. In case of antral muscle strips, the contractile response was more dominant, while corpus muscle strips typically displayed a smaller contractile response and a pronounced relaxation. Tissues were thoroughly rinsed after each electrical stimulation.
2.4 Drug applications
After application of papain to the organ bath chamber, motility was recorded for 15 min and then an EFS was performed and papain was thoroughly washed out. Motility was further recorded after washout to observe if the effects are reversible after washout. Three different doses, with a final cc. of 12.5 mg L−1, 50 mg L−1, and 100 mg L−1 papain in organ bath chamber were used. To test the mechanism of action, different drugs were combined with papain in cc. of 100 mg L−1. Tetrodotoxin (TTX, 1 µM) was administered before papain on both corpus and antrum preparations and compared in paired experiments with papain alone. In another set of experiments, papain was applied with or without the cysteine protease inhibitor, E64 (4.5 µM) on corpus and antrum, and their effect was compared. In other experiments, N(ω)-nitro-L-arginine-methylester (L-NAME, 100 µM) was added 25 min before papain to corpus strips and compared to the effect of papain alone. In the last set of experiments, the PAR-1 antagonist SCH79797 (5 µM) or the PAR-2 antagonist GB 83 (3 µM) was added 20 min before papain to corpus strips and compared to the effect of their vehicle, DMSO + papain alone.
2.5 Data analysis
The number of animals used to obtain the tissues for each experiment is shown after the number of tissues in parenthesis. The changes in muscle tension evoked by papain were compared to the baseline tension before adding papain and expressed as ∆mN. In case of antrum muscle strips, the effect of papain on the amplitude and frequency of spontaneous contractions was also analyzed. In case of paired experiments, paired Student's t test was used. At multiple comparisons, one-way analysis of variance or in case of data with a non-Gaussian distribution, Kruskal-Wallis one-way analysis of variance on ranks was used. Statistical significance was determined as p < 0.05. In antrum, all results were normally distributed and data are presented as mean ± SEM. In corpus, not all results were normally distributed, thus for better comparability all results, also those with a normal distribution, were presented as median [25%/75%].
3 RESULTS
3.1 Antrum
3.1.1 The effect of papain on antrum circular and longitudinal muscle strips
There was no difference either in the basal tone (n tissues [animals] = 7 (7); 10.1 ± 1.0 vs. 13.6 ± 1.8 mN, p = 0.11) and in the basal amplitude of contractions (2.6 ± 0.4 vs. 3.9 ± 1.0 mN, p = 0.23) between circular and longitudinal antrum strips. Papain (100 mg L−1) evoked a similar, significant increase in the amplitude of spontaneous contractions of both circular and longitudinal antrum muscle strips, with no difference in the magnitude of effect (1.7 ± 0.4 vs. 2.6 ± 0.7 ΔmN, p = 0.29); therefore, the results have been pooled for further analysis. The effect started 3 min after application (199 ± 24 s), reached its maximum after 8 min (495 ± 54 s) and remained till a washout was performed (Figure 1A). Washout reversed the effect of papain on the amplitude of spontaneous contractions (p = 0.29).
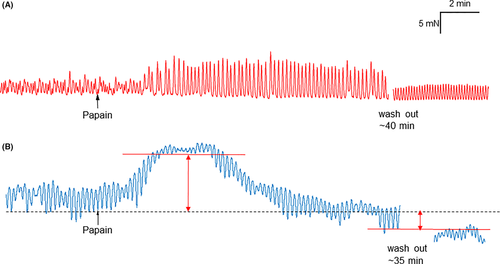
Papain did not change the frequency of spontaneous contractions (5.6 ± 0.3 vs. 5.4 ± 0.3 contractions per minute, p = 0.67) or the muscle tone (11.8 ± 1.1 vs. 12.3 ± 1.1 mN; p = 0.24) in antrum.
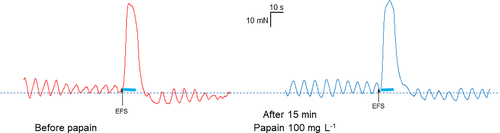
Papain did not change either the contractile (before papain: 35 ± 8.8 mN vs. with papain: 37.1 ± 8.7 mN; p = 0.6) or the relaxatory response (before papain: −1.8 ± 0.4 vs. with papain: −2.6 ± 0.9, p = 0.44) evoked by electric field stimulation (Figure 2).
3.1.2 Effects of different doses of papain on antral contractility
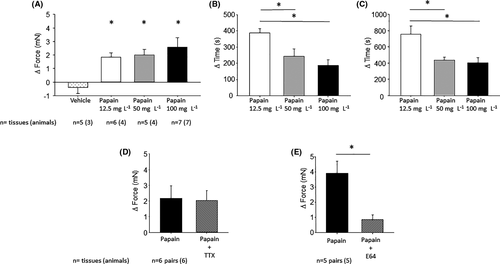
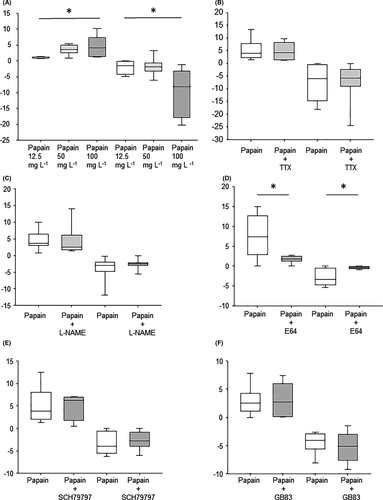
All three tested doses of papain (12.5 mg L−1, n = 6 (4); 50 mg L−1, n = 5 (4); 100 mg L−1, n = 7, (7)) increased the amplitude of antral contractions significantly, contrary to the vehicle group (n = 5 (3); −0.4 ± 0.4 mN, p = 0.38; Figure 3A). The differences in the magnitude of increase between the three doses were present but marginal (1.9 ± 0.3 vs. 2.0 ± 0.4 vs. 2.6 ± 0.7; p = 0.50). However, at the lowest dose, the effect started significantly later than at the higher doses (388 ± 25 vs. 243 ± 44 vs. 186 ± 35 s p = 0.001; Figure 3B), and the maximal effect was also reached significantly later (755 ± 104 vs. 436 ± 36 vs. 401 ± 65 s, p = 0.01; Figure 3C).
3.1.3 Pharmacology
The neurotoxin tetrodotoxin did not change the effect of papain on the amplitude of spontaneous contractions (n = 6 pairs (6); Δ ampl. papain: 2.2 ± 0.8 vs. papain + TTX: 2.0 ± 0.6 mN, p = 0.89; Figure 3D). The beginning of the effect (331 ± 88 s vs. 272 ± 46 s; p = 0.53) and the time till the maximal effect (553 ± 102. s vs. 538 ± 128 s; p = 0.95) were also unchanged. The cysteine protease inhibitor E64 significantly reduced the effect of papain (n = 5 pairs (5); 3.7 ± 0.96 vs. 0.8 ± 0.3 mN, p = 0.036; Figure 3E), but did not change the time to the onset and maximal effect of papain (189 ± 38 s vs. 259 ± 106 s; p = 0.63, 560 ± 132 s vs. 365 ± 89 s, p = 0.4).
3.2 Corpus
3.2.1 The effect of papain on corpus circular and longitudinal muscle strips
Papain consistently evoked a relaxation on both circular and longitudinal corpus muscle strips. The relaxation was preceded by a transient contraction within the first 3 min after application in 8/10 (80%) of corpus circular and 5/7 (71.4%) of corpus longitudinal muscle preparations (Figure 1B). There was no difference either in the contractile (4.8 [1.9/8.0] vs. 4.61 [2.4/9.7] mN, p = 0.74) or in the relaxatory response (−5.8 [−8.7/-2.4] vs. −3.6 [−6.0/-3.1] mN; p = 0.45) to papain between the circular and longitudinal muscle strips; therefore, the results have been pooled for further analysis. The reduction in the muscle tone did not completely return to baseline after washout (30.7 [27.01/37.36] vs. 25.9 [19.9/34.1] vs. 27.0 [17.5/27.7] mN; p = 0.015).
Papain significantly increased the contractile response after EFS (6.6 [4.7/18.0] vs. 16.6 [6.9/19.7] p = 0.002; Figure 4). Nevertheless, the maximal absolute force reached by the contraction remained unchanged (39.7 [31.5/43.0] vs. 37.3 [35.7/46.2] mN; p = 0.105). Papain significantly decreased the relaxatory response to EFS (−9.6 [−11.8/-6.5] vs. −5.8 [−8.1/-2.4] mN; p = 0.02). Similarly to the contractile response, the minimal absolute force at the relaxatory response reached comparable values (15.6 [12.5/21.9] vs. 16.1 [11.1/20.6] p = 0.847).
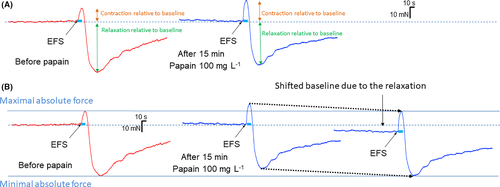
As most corpus samples did not show a regular spontaneous phasic activity, these parameters were not analyzed.
3.2.2 Effects of different doses of papain on corpus
In case of 12.5 mg L−1 papain, 5 of 8 samples (62.5%) showed a contraction within the first 3 min after application. This value was 7 out of 8 (87.5%) in case of 50 mg L−1 and 9/10 (90%) in case of 100 mg L−1. The highest dose of papain caused a significantly greater contraction than the lowest (12.5 mg L−1: 1.13 [0.8/1.3] mN vs. 50 mg L−1: 3.53 [2.57/5.06] mN vs. 100 mg mL−1: 4.03 [1.37/7.45] mN, p = 0.016; Figure 4A). In case of vehicle, only one sample out of 8 (12.5%) showed a contraction within 3 min after application. All three tested doses of papain (12.5 mg L−1, 50 mg L−1, and 100 mg L−1) caused a significant relaxation; the effect of the highest dose was significantly stronger than that of the lowest (−1.5 [−4.3/−0.1] vs. −1.9 [−3.3/−0.6] vs. −8.1 [−17.9/−3.1], respectively, p = 0.013; Figure 5A).
The contraction started shortly after 1 min in all three groups (71 ± 18 s vs. 74 ± 13 s vs. 65 ± 11 s, p = 0.88), and the time to maximum was comparable (248 ± 23 s vs. 209 ± 48 s vs. 167 ± 20 s, p = 0.22). There was also no difference in the onset (512 ± 82 s vs. 534 ± 71 s vs. 343 ± 51 s; p = 0.097) and maximum of relaxation (703 ± 60 s vs. 695 ± 62 s vs. 692 ± 52 s; p = 0.99) between the groups.
3.2.3 Pharmacology
Tetrodotoxin did not change either the contraction (papain: n = 7/10 pairs (6); 3.870 [2.21/7.74] mN vs. papain + TTX: 4.12 [1.36/8.21] mN; p = 0.59) or the relaxation (papain: −6.0 [−15.0/−0.5] vs. papain + TTX: −5.9 [−9.1/−2.3]; p = 1) evoked by papain on the motility (Figure 5B).
L-NAME also did not change either the contraction (papain: 7/9 (6); 3.6 [3/6.5] vs. papain + L-NAME: 2.6 [1.7/6.2]; p = 0.597) or the relaxation (papain: −3 [−4.8/−1.9] vs. papain + L-NAME: −2.5 [−3.0/−2.1] evoked by papain (Figure 5C).
The cysteine protease inhibitor E64 per se had no effect on the motility. E64 dramatically reduced the effect of papain on the muscle strips (n = 8 pairs (6), contr.: papain: 7/8 strips, 7.4 [2.8/12.7] mN vs. papain + E64: 1.9 [1.3/2.5] mN, p = 0.018; relax. papain: −3.3 [−4.7/−0.5] mN vs. papain + E64: −0.4 [−0.6/0] mN, p = 0.0073; Figure 5D).
The PAR-1 antagonist did not change the effect of papain significantly (n = 6 pairs (6), contr.: 6/6, papain + vehicle: 3.8 [2.0/8.1] mN vs. papain + SCH79797: 6.2 [1.8/7.0] mN, p = 0.87; relax. papain: −3.9 [−5.5/−0.6] mN vs. papain + SCH79797: −2.8 [−4/−0.8] mN, p = 0.611; Figure 5E). Similarly, the PAR-2 antagonist did not alter the effect of papain (n = 6 pairs (5), contr.: papain + vehicle: 5/6, 2.5 [1.2/4.3] mN vs. papain + GB 83: 2.8 [0.1/56.0] mN, p = 0.93; relax. Papain + vehicle: −4.0 [−5.6/−2.9] mN vs. papain + GB 83: −5.1 [−7.6/−3.0] mN, p = 0.54; Figure 5F).
4 DISCUSSION
Here, we report for the first time that papain exerts a region-specific effect on the motility of the guinea pig stomach. While it increases the amplitude of ongoing spontaneous contractions in the antrum longitudinal and circular muscle strips, it does not change the muscle tone and the contraction frequency. This effect is sustained, but reversible upon washout. To the contrary, in the corpus longitudinal and circular muscle preparations, papain causes a sustained relaxation, which is preceded by a transient contraction in the majority of cases. Similar region-specific effect has been described in our laboratory with the same methodology using STW-5 (Iberogast®),14 an herbal preparation known to ameliorate the symptoms of functional dyspepsia.15 As a comparison, the magnitude of effect of the lowest tested doses of STW-5 was similar to the highest tested doses of papain, while higher doses of STW-5 exerted a much stronger effect. The three different concentrations of papain tested in the current study were chosen to model the following situations. 12.5 mg L−1 in its enzymatic activity corresponds approximately to the cysteine protease activity measured in the stool of some constipation-predominant irritable bowel syndrome (IBS-C) patients.16 50 mg L−1, meaning cca. 0.5 U mL−1 (in BAEE units) may develop in the stomach in case of 1 L stomach volume after eating one green papaya for example in a salad, which is usual in many tropical countries (calculation based on16), while 100 mg L−1, cca. 1 U mL−1 (in BAEE units) equals to the enzymatic activity which is reached in the stomach in case of 1 L stomach volume after taking the recommended dose of commercially available papain preparations. We intentionally did not test doses of papain over 100 mg L−1, to avoid its proteolytic effect on the tissue. Papain is widely used for the isolation of smooth muscle cells, as it is considered more delicate than trypsin. The highest dose in our experiments was 15 times lower than which is used for digestion of stomach smooth muscle.17 In the antrum, the effect of papain was completely reversible, making a permanent damage to the tissue unlikely. In the corpus, the effect of papain did not fully recover after washout. To test if papain damaged the tissue, we performed an electric field stimulation (EFS) before and 15 min after the application of papain. The response to the EFS was unchanged in antrum. In corpus, the contractile response was increased and the relaxatory response decreased, but the absolute values remained the same, indicating that the changes are related to the relaxation-evoked change in muscle tone by papain and not to any damage of the tissue (Figure 4). In some experiments, multiple washouts were performed during 2–3 h to see if the baseline returns to normal, but this was not the case, while the tissues still responded to the EFS. In a study on rats, feeding with crystallized papain reduced gastric acid secretion induced by methacholine, histamine and tetragastrin already after 2 h of administration, lasting up to 48 h, but the effect disappeared in 96 h.18
In rat uterine preparations, papain showed an effect already at a concentration of 2.5 µg mL−1, but the maximal effect was seen at a final concentration of 10–12.5 µg mL−1 in the organ bath.13 In our experiments, the lowest tested concentration was 12.5 µg mL−1. In the antrum, this concentration seemed to have a maximal effect, similarly to the rat uterine preparations. Nevertheless, the highest dose reached a similar magnitude of effect faster. In the corpus, the effect was subtle, but significantly different from zero. By increasing the dose, the effect significantly increased, while the response to the electric field stimulation was not diminished. Based on data on the absorption of bromelain, it can be speculated that the local concentration of papain in the gastrointestinal tract is higher than in other organs, which justifies the use of different papain concentrations in case of uterine and stomach preparations.19 In another study, the effect of papaya crude latex on pregnant rat uterus was comparable to oxytocin or prostaglandin F2α, while ripe papaya juice (which does not contain much active papain) had no effect.20 The authors concluded that it is probably the papain content which induces contractions, as they have also observed the potent uterine-stimulating effect of papain and chymopapain on isolated guinea pig uterus (unpublished results). In this publication, the dosage of papain was not indicated. The effect of papain also depends on other conditions than the dosage. Papain is often activated by L-cysteine, but it is also active in the absence of added activators.21 We intentionally did not use L-cysteine or other activator substances in our experiments to avoid its possible influence on the muscle strips per se and to mimic conditions after taking a nutritional supplement or eating papaya. Oral consumption of a protease mixture consisting of fungal proteases, bromelain, papain, and calcium citrate showed a systemic effect by significantly reducing muscle strength losses after eccentric exercise, accompanied by a decrease in COX-2, interleukin 6, and interleukin 12 in the blood serum and an increase in the number of circulating eosinophils and basophils.5 Citrate is also a known activator of papain, while the enzymatic activity of papain was not mentioned in the manuscript, making a direct comparison with our findings difficult. Our experiments were performed on a pH of 7.4. Papain functions in a very broad pH spectrum (pH 2.5–9), and its pH optimum varies according to the actual protein substrate, being around pH 5–7 for many proteins.22 Under pH 2.5, papain is rapidly deactivated but not destroyed, as its activity is reversible after resetting the pH.22 As a consequence, the low pH in the stomach does not destroy the enzymatic activity. In our study, the experiments have been performed with a muscle preparation lacking the mucosa, while in case of an oral administration, the mucosal penetration also has an influence on the dose reaching the smooth muscle. The gastrointestinal tract, particularly the stomach, is known for their selective absorption capacity and papain has a high molecular weight (23.4 kDa). However, evidence shows that there are numerous other factors determining the absorption, which are more important than the molecular weight.23 Experiments on Caco-2 cell monolayers demonstrated that serine and cysteine proteases are able to increase the permeability of the epithelium reversibly.24 Out of the tested four proteases, papain caused the highest increase in permeability to the fluorescein marker. Strikingly, fluorescent dyes with a molecular weight as high as 600 kDa were also absorbed. It has been shown that the effect of papain on permeability is reversible and does not destroy the overall intestinal epithelium.25 Papain is able to degrade tight junction components such as occludin, which can contribute to the observed elevation in epithelial permeability.26, 27 This mechanism can likely facilitate not only the absorption of other larger molecules but also that of papain itself.23 Papain has been recently increasingly used as a permeation promoter of various active compounds,28 such as furosemide29 or vancomycin,30 as it significantly enhances oral bioavailability of these drugs without being cytotoxic. There is also stable evidence that cysteine proteases of plant origin are readily absorbed from the gastrointestinal tract in their active form after oral administration.23 Extensive studies with the cysteine protease of pineapple, bromelain, are available. They have shown that bromelain was recovered from human blood samples in undegraded form31 and increased the ability of human blood serum to digest casein after oral administration, demonstrating that the absorbed bromelain was functional.32 In rats, a 50% absorption rate of orally applied bromelain has been demonstrated after 6 h.19 In case of papain, data are more scarce, but measurements in rats showed a 26% total absorption rate after oral administration, based on calculations from blood serum and lymph.33 No data are available on the concentration reached in different organs with papain. However, based on studies with bromelain the concentration is likely the highest in the gastrointestinal tract compared to other organs.19
We also performed experiments with pharmacological tools to explore the mechanism of action of papain. Tetrodotoxin, a fast voltage-gated sodium channel blocker, did not influence the effect of papain either in antrum or in corpus. Furthermore, the nitric oxide synthase inhibitor L-NAME did not reduce the relaxation caused by papain in corpus. These results suggest that papain directly affects the smooth muscle, without the involvement of nitrergic pathways. In our experiments, the cysteine protease inhibitor abolished the effects of papain in both the antrum and the corpus, which shows that its effect on the motility is linked to its enzymatic activity. Many proteases act by cleaving protease-activated receptors (PARs) via their enzymatic activity. In some cell types, papain has activated one or more of the four known PARs (PAR-1, PAR-2, PAR-3, and PAR-4). In HeLa cells, transfected with individual PARs, papain induced a calcium mobilization in cells expressing PAR-2 and PAR-4, but not in nontransfected cells or those expressing PAR-1 and PAR-3.34 Papain has been shown to activate PAR-2 also in airway epithelial cells.35 In rodent gastric smooth muscle preparations, the activation of PAR-1 and PAR-2 evoked both contractions and relaxations depending on the experimental settings.36, 37 Therefore, we tested specific antagonists of PAR-1 and PAR-2 in the corpus, where papain caused changes in the tone, but we could not detect the involvement of these receptors.
We have demonstrated a genuine, region-specific effect of papain on the stomach motility in vitro, which could support its observed beneficial effects in functional gastrointestinal disorders such as functional dyspepsia. Nevertheless, this effect is very subtle compared to, for example, the previously tested herbal preparation, STW-5.
Papain is used in research as a “model cysteine protease” as it is readily available and has a strong structural homology not only with actinidin from the kiwifruit, but also with animal cysteine proteases such as cathepsin B and H from the rat or cathepsin L from the chicken.38 The main differences can be observed in the middle region, far removed from the active site of the enzymes, while the structures in the N-terminal and C-terminal regions are highly conserved.38 This highlights our results from an interesting additional point. In a subgroup of IBS-C patients, an increased cysteine protease activity has been measured in the stool, which correlated with their abdominal pain.39 Repeated intracolonic infusions of fecal supernatants from IBS-C patients with high cysteine protease activity caused a hypersensitivity to rectal distension in mice, which could be reproduced by papain.39 Furthermore, in mucosal biopsy supernatants from IBS patients, cysteine proteases such as cathepsin C and L1 were significantly more abundant and cathepsin Z less abundant than in supernatants from healthy controls.40 Therefore, it can be speculated that papain-like cysteine proteases may also have direct effects on the gastrointestinal muscles which could contribute to the symptoms in IBS.
ACKNOWLEDGMENTS
The authors thank Marlene Redl for her technical assistance.
DISCLOSURE
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the author.