A randomized double-blind placebo-controlled crossover pilot study: Acute effects of the enzyme α-galactosidase on gastrointestinal symptoms in irritable bowel syndrome patients
Magnus Simrén and Stine Störsrud shared senior authorship.
ABSTRACT
Background
Postprandial symptoms presumably related to intestinal gas production are common in patients with irritable bowel syndrome (IBS). The aim of the study was to assess if oral α-galactosidase is superior to placebo in reducing gastrointestinal (GI) symptoms and intestinal gas production after ingestion of carbohydrate-rich meals in adult patients with IBS.
Methods
We studied the effect of 1200 GaIU/meal α-galactosidase (Nogasin®) or placebo capsules on GI symptoms in patients with IBS after three standardized, meals high in oligosaccharides, in a randomized, double-blind, crossover study. The intensity of eight GI symptoms was rated, and breath hydrogen and methane were measured every 30 min during 7.5 h. The severity of GI symptoms the following morning was assessed and compared with baseline.S
Key Results
Twenty adult patients with IBS (19 females), mean age 49 years (range 22–75 years), were included. All test meals were well tolerated but induced a gradual increase in GI symptom severity. Neither GI symptom ratings over time, nor hydrogen and methane concentrations differed between the days with α-galactosidase or placebo. The severity of abdominal pain and bloating was lower the following morning, but with no differences between α-galactosidase and placebo.
Conclusions & Inferences
The use of α-galactosidase together with meals high in oligosaccharides was in this pilot study not superior to placebo in reducing postprandial GI symptoms or the concentration of hydrogen and methane in expired air in IBS.
Key Points
- The enzyme α-galactosidase could theoretically reduce gastrointestinal symptoms due to fermentation of oligosaccharides in sensitive individuals.
- The enzyme α-galactosidase was not superior to placebo in reducing postprandial gastrointestinal symptoms after ingestion of provoking meals in IBS patients.
- The effect of digestion enzymes in the treatment of functional gastrointestinal diseases is important to study and might replace restrictions in the diet.
1 INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits.1 The prevalence of IBS is estimated to 5%–20% of the global population.2 The majority of IBS subjects experience that food is important triggers or an aggravating factor for GI symptoms.3 Many of the symptoms are potentially related to effects of intestinal gas.4-6 In order to relieve gas-related GI symptoms in IBS, several drug-based approaches have been used with limited or no success, such as prokinetics to accelerate GI transit7 and antifoaming agents to ease the passage of and increase the tolerance for gas.8, 9 Based on the assumption that gas-related symptoms are due to an aberrant gut microbiota, the bactericidal effects of specific antibiotics have also been reported as useful in this condition.10 However, the effects are transient and there are concerns that this strategy may cause adverse effects in the long run.11 On the other hand, to use probiotics12-16 or increase food items with potential prebiotic effects, stimulating growth and activity of beneficial colonic bacteria17 is more attractive from a safety point of view. However, a potential drawback with prebiotics such as fructo-oligosaccharides (fructans) and galacto-oligosaccharides (GOS), and other fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs), is that they are not absorbed in the small intestine and thus pass unaltered to the colon.18 These short-chain carbohydrates are osmotically active, which increases the water volume in the intestinal lumen,19 and are rapidly fermented, leading to production of gas. The resulting increase in luminal water volume and gas production leads to bowel distension,20 potentially aggravating symptoms in IBS.21
The enzyme α-galactosidase with amylase-like activity is derived from the mold Aspergillus niger and can break down fructans and GOS in the small intestine before they are metabolized by colonic bacteria. Through this mechanism, α-galactosidase has the potential to facilitate the intestinal absorption and to minimize the bacterial gas production, which has been reported to be effective in reducing gas production and relieving gas-related symptoms in healthy subjects and in children with IBS.22-25 However, it is not yet established if α-galactosidase is efficient in alleviating GI symptoms in adult patients suffering from IBS, as the results are inconclusive.26, 27
The aim of the present study was to test the hypothesis that the enzyme α-galactosidase is superior to placebo in reducing GI symptoms after ingestion of meals high in FODMAPs, in adult IBS patients.
2 MATERIAL AND METHODS
2.1 Subjects
Patients ≥18 years of age that previously had taken part in studies at our unit were invited to participate in the current study during 2011–2012. They were diagnosed with IBS according to the Rome III criteria1 and had specific complaints of frequent bloating, abdominal distension, and/or flatulence. Written and verbal informed consent was obtained before any study-related procedures, and the study was performed as outlined below and as approved by The Regional Ethical Review Board at the University of Gothenburg. The study was registered at ClinicalTrials.gov with identifier: NCT01483287.
2.2 Study design
This was a randomized, double-blind, placebo-controlled, crossover study where the patients were assigned to receive either 3 capsules containing the digestive enzyme α-galactosidase (Nogasin®, Oy Verman Ab, Kerava, Finland) or a corresponding placebo with identical capsule design taken together with the first bites of the three study-specific meals. Each capsule with the active enzyme had a total content of 400 galactosidic units (GaUI) where 1 GaUI equals the amount of enzyme that releases 1 µmol of galactose from the substrate in 1 min. The total dose of the enzyme given at each meal was 1200 GaUI, according to what has been shown to reduce gas production in another study.23 The treatment order was decided from a computer-generated randomization list provided by Oy Verman Ab, who otherwise was not involved in the conduct or planning of the study. The randomization code was not broken until after the completion of the study and after entering of all data into a database.
2.3 Study visit 1 and 2
The patients arrived at the hospital after an overnight fast. They had been instructed to avoid consumption of food containing large amounts of dietary fibers and FODMAPs the day before. Before intake of the first meal, each patient completed baseline symptom questionnaires (see below). Breakfast was served at 8 a.m. after which the first breath test for hydrogen, and methane content was performed and a specific symptom questionnaire assessing GI symptoms and digestive comfort was administered. Consecutive breath tests with the accompanying symptom questionnaires were thereafter collected every 30 min with lunch served at 11.30 a.m., shortly after the 8th breath test. Both the breakfast and the lunch were high in fructans and GOS. The patient left the hospital after the 16th breath test and was provided with the instruction to eat a prefabricated dinner meal, with low content of FODMAPs, within the time span of 3 h after leaving the hospital. There was a washout period of at least 2 weeks between the two study visits.
2.4 The test meals
The breakfast (618 kcal, 17.7 g total FODMAPs; 9.9 g oligosaccharides) and lunch (587 kcal, 13.6 total FODMAPs; 2.7 g oligosaccharides) meals were composed in order to provoke tolerable, but clear GI symptoms (Table 1) and were supposed to represent a high, but not unrealistic amount of oligosaccharides, and able to provoke symptoms in IBS subjects. We performed a pilot study preceding this study where six patients with IBS and complaints of frequent bloating, abdominal distension, and/or flatulence tested the meals in order to establish if the requested effects on gas production occurred and also to establish the acceptance of the meals to avoid dropouts. The food was regarded as palatable and all six patients experienced the expected GI symptoms and a significant increase in gas production but stated that the meals did not produce intolerable symptoms.
| Time | Amount | Food item | Calculated energy content |
|---|---|---|---|
|
Breakfast 8.00 |
220 g | Rye porridge | 618 kcal |
| 40 g | Jam (lingonberry jam) | 12.3 g protein | |
| 2 dl | Lactose free-milk (3% fat) | 18.1 g fat | |
| 40 g | Wholemeal bread | 83.3 g carbohydrates | |
| 6 g | Margarine (70% fat) | 9.7 g dietary fibers | |
| 20 g | Cheese (28% fat) | 7.3 g fructose in excess of glucose | |
| 30 g | Bell pepper | 0.9 g GOS | |
| 2 dl | Apple juice | 9.0 g fructans | |
| 0.5 g polyols | |||
| 0.0 g lactose | |||
|
Lunch 11.30 |
455 g | Chicken casserole | 587 kcal |
| (contents; beans, chickpeas, onion, garlic, mushrooms, tomato, chili, rape oil, black pepper, salt, stock cube, thyme) | 45.0 g protein | ||
| 16.3 g fat | |||
| 59.6 g carbohydrates | |||
| 8.5 g dietary fibers | |||
| 9.8 g fructose in excess of glucose | |||
| 30 g | White bread (wheat) | 0.4 g GOS | |
| 6 g | Margarine (70% fat) | 2.3 g fructans | |
| 20 g | Cheese (28%fat) | 1.1 g polyols | |
| 2 dl | Apple juice | 0.0 g lactose | |
| Supper | 600 g | Fish au gratin (prefabricated) | 690 kcal |
| 2,5 dl | Apple juice | 52.8 g protein | |
| 18.2 g fat | |||
| 75.0 g carbohydrates | |||
| 4.8 g dietary fibers | |||
| 7.4 g fructose in excess of glucose | |||
| 0.0 g GOS | |||
| 0.0 g fructans | |||
| 0 polyols | |||
| 0.0 g lactose |
During the whole study, breakfast and lunch were prepared by one of the authors (L.B.), but dinner consisted of a prefabricated meal. All patients received the same meals at both visits. No other food or beverages except for water were allowed until the next morning.
2.5 Hydrogen and methane breath test
Samples of expired air were collected at 16 time points as described above to assess the concentrations of hydrogen and methane as a measure of fermentation. All breath samples were end expiratory and collected in a system used for the sampling and storing of alveolar air (GaSampler System, QuinTron Instrument Company, Milwaukee, WI, USA). Analysis was done by use of a gas chromatograph (QuinTron Breath Tracker, QuinTron Instrument Company, Milwaukee, WI, USA). The concentration of hydrogen and methane was given in parts per million (ppm).
2.6 Symptom assessment
2.6.1 IBS symptoms
The IBS-SSS was used to assess the severity of IBS symptoms.28 The questionnaire was completed at the beginning of each of the two visits. In order to specifically follow the effects of the study medication the day after each test meal, we used a modified questionnaire version, which only included the current severity of three of the VAS ratings (severity of abdominal pain, abdominal distension, and dissatisfaction with bowel habits). The IBS-SSS has a total score range of 0, meaning no symptoms, to 500, indicating maximal symptom severity. It consists of five questions, addressing abdominal pain severity, abdominal pain frequency, bloating severity, bowel habit dissatisfaction, and daily life interference, each ranging from 0 to 100.
2.6.2 Meal-related GI symptoms questionnaire
The severity of eight GI symptoms: gas, bloating, abdominal discomfort, abdominal distension, nausea, stomach rumbling, urgency to have a bowel movement, and abdominal pain, was rated on a scale ranging from 0 to 20. Zero corresponds to no symptoms and 20 to the worst conceivable symptoms. In addition, the degree of digestive comfort was rated and bowel movements were reported. This scale has previously been validated and used to assess meal-related GI symptoms in IBS patients and healthy controls.29
2.7 Data analysis and statistics
Descriptive data are presented as proportions (%) for categorical variables and as mean values and standard deviation or standard error for continuous variables. Data were analyzed using linear mixed models implemented using the mixed procedure in SAS 9.4 software (SAS Institute, Cary, NC, USA). To test the response to the first two meals, the first 16 time points (up to 450 min) were used. For the analysis, the first time point (0 min) served as the pre-meal baseline time point (reference category). Different models were estimated for each symptom and hydrogen and methane as the dependent variables. Drug and time were entered as categorical within-subject independent variables. Main effects and the drug*time interaction effect were included in the model. The Kronecker product of an unstructured (drug) and first-order autoregressive (time) variance-covariance matrix was used to model the data as this provided the best fit based on the minimization of Akaike's information criterion (AIC). To test our hypothesis of a differential symptom or breath test hydrogen response to the meal for active drug versus placebo, the drug*time interaction effect was followed by a planned contrast comparing the difference between the average of all post-meal time points and the pre-meal baseline time point between active drug and placebo.
Since this study was testing a hypothesis where the potential treatment effect was uncertain, no formal power calculation was done. Instead, we used the same type of crossover design as in previous studies evaluating the effects of α-galactosidase in adults aiming at sample size that was larger22 or at least of similar size23 to maximize power.
3 RESULTS
3.1 Subject characteristics
Sixty-two persons were initially asked to participate in the study. Thirteen persons did not reply; 24 declined and 25 gave their consent to participate. However, four changed their mind due to lack of time, and one became ill before study start, which means that 20 persons (19 females, 95%) started and completed the study. The mean age was 49 years (range 22–75 years) and the mean BMI 27.2 ± 6.0 (SD) kg/m2. The mean (±SD) overall IBS symptom severity (IBS-SSS total score) was at baseline 265.5 (±57.7)) and the IBS-SSS bloating severity 66.6 (±26.6). According to IBS-SSS, severe IBS symptoms were reported by 5 subjects (25%), moderate symptoms by 13 (65%), and mild symptoms by 2 subjects (10%).
3.2 Effects of the test meals
The three test meals were generally well tolerated by all participants, and no deviations from the protocol were recorded. All subjects completed and returned the symptom questionnaires during and after the meal intake. No adverse events apart from induction of GI symptoms after the meals were reported.
3.2.1 GI symptoms induced by the test meals
A significant effect of time was found for the symptom ratings of gas (F = 3.35, p < 0.0001), bloating (F = 3.01, p = 0.0002), discomfort (F = 2.88, p = 0.003), distension (F = 3.09, p = 0.0001), urgency to have a bowel movement (F = 2.28, p = 0.005), and abdominal pain (F = 2.08, p = 0.014), but not for nausea (F = 1.45, p = 0.13) or stomach rumbling (F = 1.24, p = 0.24), from baseline to 450 min, when the subjects left for home. There were no significant main effects of drug (α-galactosidase versus placebo) for any of the rated GI symptoms. A time*drug interaction effect was found for bloating (F = 2.8, p = 0.0004), discomfort (F = 2.15, p = 0.008), distension (F = 1.73, p = 0.045), and abdominal pain (F = 1.8, p = 0.034), but not for gas (F = 1.51, p = 0.10), nausea (F 0.90, p = 0.57), or stomach rumbling (F = 1.24, p = 0.24). However, in the planned contrast comparing the difference between the average of all post-meal time points and the pre-meal baseline time point between active drug and placebo, no significant differences could be found for any of the symptoms. Separating the time period into two sections (first meal 0–180 min, second meal 210–450 min) and repeating the same analyses described for the whole time period above did not show a significant main effect of drug nor drug*time interaction effect for any of the symptoms (data not shown). In conclusion, the changes in GI symptom ratings over time analyzed after the two meals were not different from each other during the two test days regardless if α-galactosidase or placebo was ingested before meals. The meal response for each GI symptom over time is outlined in Figure 1A–H.

3.2.2 Hydrogen and methane concentration in breath tests after the test meals
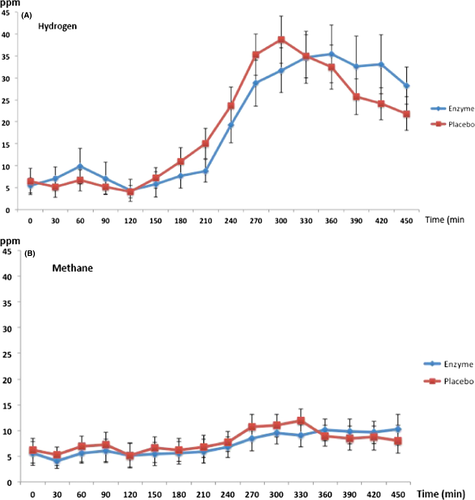
A significant effect of time was found for both hydrogen (F = 6.2, p < 0.0001) and methane (F = 1.7, p = 0.044) concentrations in breath tests from baseline to 450 min when the subjects left for home. There were no significant main effects of drug (α-galactosidase versus placebo) for hydrogen (F = 0, p = 0.95) or for methane (F = 0.05, p = 0.82) concentrations in the breath tests. No time*drug interaction effect in hydrogen (F = 0.90, p = 0.57) or methane (F = 0.97, p = 0.49) concentrations in breath tests was seen either. This means that the changes occurring in hydrogen and methane concentrations in the breath tests analyzed after the two meals were not different from each other in any significant way during the two test days regardless if α-galactosidase or placebo was ingested before meals. The meal effect on hydrogen and methane concentrations in expired air is outlined in Figure 2A,B.
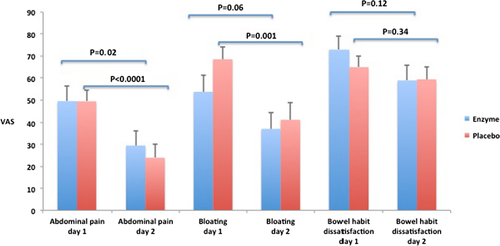
3.2.3 GI symptoms the day after the test meal
When comparing the three items of IBS-SSS in the morning after each test meal day with the baseline score, the scores for abdominal pain were significantly lower on day 2, both after intake of α-galactosidase (p = 0.02) and placebo (p < 0.0001). The intensity of bloating was lower on day 2 after placebo intake (p = 0.001), but only reaching a trend for a decrease after enzyme intake (p = 0.06). There were no significant differences for the ratings of the dissatisfaction of bowel habits comparing the 2 days of meal intake and the following days after meal intake, regardless of enzyme or placebo intake. No differences between the groups were detected in the change of individual IBS-SSS items day 1 vs. day 2. Data are summarized in Figure 3. Regarding the meal-related GI symptoms questionnaire that also was repeated in the morning the day after each test meal day, no significant differences between α-galactosidase and placebo were found for any of the rated symptoms (data not shown).
4 DISCUSSION
We have evaluated the acute effects of the enzyme α-galactosidase on GI symptoms and gas production after symptom-provoking meals high in FODMAPs in adult patients with IBS. No significant effects on GI symptom intensity or the concentrations of hydrogen and methane gas in breath tests were observed in this pilot study. Our results are in contrast with some published studies.22-24, 26
Complaints of “too much gas” causing abdominal pain, bloating, abdominal distension, and flatulence are common in IBS. This phenomenon is probably of multifactorial origin where intestinal gas production itself is only one component of the sensation. It may also include factors like effects on intestinal motility, GI sensitivity, or response to intestinal gas unrelated to its total volume.30, 31 There is no clear evidence suggesting that IBS patients have abnormal volume or composition of intestinal gas, even though abnormal colonic fermentation has been suggested as an alternative explanation.32 Complex carbohydrates escape small bowel absorption, resulting in gas production within the colon33 with the potential to provoke symptoms in IBS patients. This knowledge forms the theoretical basis for the use of enzymes that can digest these complex carbohydrates and thereby improve their absorption and limit the amount entering the colon. Supplementation with an enzyme would be an attractive approach to relieve symptoms from partial or complete maldigestion in the same manner as with lactase supplementation.34-36 Currently, a dietary regime that focuses on reducing the total load of FODMAPs, among them fructans and GOS,18 has gained most of the interest among patients with IBS. If use of α-galactosidase could overcome the need to adhere to this rather strict diet, most IBS patients would probably find it to be useful.
There are only a few studies, with differences in subjects included and study design, that have investigated the efficacy of α-galactosidase in alleviating GI symptoms.22-25 Supplementation with α-galactosidase has shown beneficial effects for controlling excessive gas production and some gas-related symptoms in healthy volunteers22, 23 and in IBS subjects.26 However, one study of healthy subjects included subjects with troublesome complaints of intestinal gas, where one of the subjects possibly had IBS, which makes the study population a bit heterogeneous.23 Another study included only 8 patients, which makes the results weaker even if significant positive effects on both symptoms and intestinal gas production were found.22 In both of these studies a crossover design, similar to our study was used. Di Nardo et al.24 investigated the efficacy of α-galactosidase in 52 IBS children, ingesting enzyme or placebo for 2 weeks in a double-blind, randomized, parallel-group design. The enzyme group reported reduced global distress, bloating, and flatulence compared to the placebo group. The study design was different from ours, and the positive results may potentially be explained by an accumulating effect from using the enzyme during a longer period of time, as compared with our study. In the study from Tuck et al., the overall symptoms were reduced only in 21 (68%) of the patients defined as GOS-sensitive. Breath hydrogen production was minimal in all treatment groups with no statistically significant differences between groups.26 The randomized, double-blind, placebo-controlled study, where α-galactosidase or placebo was given daily for 12 weeks in 125 adult IBS subjects, did not show any statistical significant differences between the two treatment groups.27 Hence, heterogeneity among published studies regarding subjects included, study design, and results clearly exists.
IBS patients often relate aggravation of symptoms to certain food items.3, 37-40 The idea of our test meal composition was to evaluate the potential of α-galactosidase supplementation to reduce both gas production and symptoms. Food items rich in fructans and GOS were included to test the hypothesis that a more complete digestion of these complex carbohydrates would prevent the aggravation of GI symptoms.18 The dose of oligosaccharides given for breakfast and lunch can be questioned as not being sufficiently provocative. However, it was more than double the intake observed in adult IBS subjects in general41 and still within a reasonable range of an intake that can be expected in IBS subjects. Nevertheless, the composition of the meals, including the high intake of fructose in excess of glucose, may have accounted for the lack of enzyme effect, and this is a limitation of the study.
The subjects included in the present study had not in a structured way shown a positive response to a low FODMAP diet, even though they verbally reported sensitivity to certain FODMAP-rich foods, nor been tested for GOS or fructan sensitivity, fructose, or lactose intolerance prior to inclusion of the study. We can therefore not be completely sure that the symptoms in these subjects are related to the intake of FODMAPs, fructans, or GOS, as 25%–50% of IBS patients do not respond to a low FODMAP diet and are assumed not to be sensitive to FODMAPs. The GI symptoms and the lack of effect of the enzyme can thus partly be explained by other factors in the diet. All the meals served in the study were lactose free, so lactose intolerance should not be a factor of importance for our results. The rather high amount of fructose in excess of glucose in the meals, but within the range observed in IBS subjects,41 could theoretically be responsible for part of the GI symptoms reported and also overshadow the effect of the enzyme on symptoms.
The additive effects of other components in the study meals may have overshadowed the effects of fructans and GOS and the potential benefit of their breakdown induced by α-galactosidase. The content of fructose should have been limited in order to help us to more clearly detect the effect of the enzyme. Moreover, an overall effect of dietary fibers on GI symptoms is possible, as the content of dietary fibers was fairly high, 23.0 g/day, but still in accordance with nutrition recommendations.42 Taken together, our negative results may potentially be explained by complex effects of different ingredients in the study meals over-shadowing the effects of fructans and GOS.
The rather high dose of enzymes (1200 GaIU) per meal provided in our study can also be viewed as a potential limitation, as one study27 showed a potential negative effect of high doses of α-galactosidase (1200 GaIU), with a high proportion of subjects terminating the study prematurely because of more severe abdominal pain and diarrhea. This study was not available when the present study was planned and initiated. However, no patients withdrew from our study after inclusion because of potential side effects and we have therefore no reason to believe that the enzyme had adverse effects, but the optimal dose of the enzyme in order to break down non-absorbable oligosaccharides without producing adverse effects should of course be further assessed in future studies.
We find it less likely that the psychological effects from the patients' own expectations had a significant impact on our results. That kind of anticipatory responses to the food ingestion should be compensated for by the randomized crossover design, and meal tests have been used to provoke symptoms in IBS in an experimental setting before and found to be reliable by reflecting the IBS symptom severity and proposed to be valid tests to study the effect of therapeutic approaches.29, 43 In addition, the objective measurements of methane and hydrogen, where no patient was a non-producer of one or both gases, strengthen that the negative outcome in GI symptoms is true.
A limitation of this study is the lack of sample size calculation. However, when the study was planned in 2010–2011, there were only a couple of similar studies done in healthy adults.22 There is reason to believe that IBS subjects with complaints of bloating and/or flatulence are more sensitive to the test meal used in the study than healthy adults. As we used the same type of crossover design as in previous studies evaluating the effects of α-galactosidase in adults and aimed at a sample size that was larger22 or of at least of similar size23 as in previous studies in order to maximize power, the statistical effect size in the measured parameters is such that it seems unlikely that the inclusion of more participants would have had the capability to show significant positive effects of α-galactosidase treatment with this study design.
To conclude, the present pilot study did not find evidence for a positive effect of short-term/acute treatment with α-galactosidase on GI symptoms in IBS patients with pre-existing complaints of bloating, abdominal distension, and flatulence after intake of symptom-provoking meals. Based on the available evidence, α-galactosidase cannot be recommended to adult IBS patients in order to alleviate acute GI symptoms caused by meals rich in incompletely absorbed carbohydrates. However, our study used an experimental design that prevents us from determining an accumulating positive effect of α-galactosidase used for a longer period of time and after meals with another composition of FODMAPs.
ACKNOWLEDGMENTS
This study was supported by an unrestricted research grant from Oy Verman Ab, Kerava, Finland, the Swedish Medical Research Council (grants 13409, 21691, and 21692), Marianne and Marcus Wallenberg Foundation, and by the Faculty of Medicine, University of Gothenburg.
CONFLICT OF INTEREST
Lena Böhn: None. Stine Störsrud: None. Hans Törnblom has received unrestricted research grants from Tillotts Pharma and served as a consultant/advisory board member for Almirall, Allergan, Danone Nutricia Research and Shire. Lukas Van Oudenhove has received unrestricted research grants from Nestlé and served as a consultant//advisory board member for Danone Nutricia Research. Magnus Simren has received unrestricted research grants from Danone Nutricia Research, Oy Verman Ab, Glycom, and Ferring Pharmaceuticals and served as a consultant/advisory board member for Danone Nutricia Research, Nestlé, Menarini, Biocodex, Genetic Analysis AS, Glycom, Arena, and Shire.
AUTHOR CONTRIBUTIONS
LB involved in acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. SS involved in study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript. HT involved in analysis and interpretation of data, statistical analysis, and critical revision of the manuscript. LVO involved in analysis and interpretation of data, statistical analysis, and critical revision of the manuscript.MS involved in study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript.