Quercetin relaxes human gastric smooth muscles directly through ATP-sensitive potassium channels and not depending on the nitric oxide pathway
Abstract
Background
Quercetin has recently become a remarkably popular subject of research due to its broad beneficial pharmacological properties. The goal of our study was to observe its effects on contractility of human gastric smooth muscles in reference to the NO pathway and direct influence of potassium channels.
Methods
Tissues were obtained from patients undergoing sleeve gastrectomy due to morbid obesity (n = 10 aged 24–56; BMI 47.16 ± 1.84). The following parameters were evaluated in the recordings: area under the curve (AUC), average baseline muscle tone, and relative change in muscle contraction.
Key Results
Quercetin induced noticeable, dose-dependent relaxation of the carbachol treated gastric strips. The substantial effect was noted at concentrations higher than 10−7 mol/L and maximal at 10−4 mol/L (81.82 ± 3.32%; n = 10; p < 0.0001) of the control. Neither NOS blockers nor guanylyl cyclase blockers had inhibitory effects on the relaxation of strips induced by examined polyphenol. Glibenclamide inhibited the relaxing effect of quercetin, significant at concentrations higher than 5⋅10−5 mol/L. Preincubation with charybdotoxin or apamin extended the relaxing effect of quercetin (from 10−6 mol/L). Tamoxifen, in turn, significantly increased muscle relaxation at all quercetin concentrations.
Conclusions & Inferences
In conclusion, the current study was the first to show that quercetin-induced relaxation of human gastric smooth muscle occurs directly through K+ATP channels and independently to NO pathways. The present results suggest that quercetin is a potential nutraceutical in the treatment of functional gastrointestinal dyspepsia and other minor gastric muscle motility disturbance.
Abbreviations
-
- AUC
-
- area under the curve
-
- BKCa
-
- high conductance Ca2+-dependent potassium channels
-
- cAMP
-
- 3'-5'-cyclic adenosine monophosphate
-
- cGMP
-
- cyclic guanosine monophosphate
-
- CTX
-
- charybdotoxin
-
- DMSO
-
- dimethyl sulfoxide
-
- GI
-
- gastrointestinal
-
- K+ATP
-
- ATP-sensitive potassium channels
-
- L-NAME
-
- NG-methyl-L-arginine
-
- L-NNA
-
- NG-nitro-L-arginine
-
- MAPK
-
- mitogen-activated protein kinase
-
- NANC
-
- non-adrenergic non-cholinergic
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthase
-
- ODQ
-
- 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one
-
- sGC
-
- soluble guanylyl cyclase
-
- SKCa
-
- small conductance apamin-sensitive Ca2+-dependent potassium channels
1 INTRODUCTION
Phenolic substances including flavonoids, for example, quercetin, have gained significant attention over the years for their potential health benefits. Quercetin is a naturally occurring, plant-derived polyphenol. It can be found in the form of a glycoside in various vegetables, fruits, nuts, seeds, red wine, and tea.1 Numerous biological effects of this substance have been published in a great number of studies based on animals and human experiments. Among others, quercetin is presumed to have antioxidant, anti-inflammatory, anticarcinogenic, antidiabetic, and immunoprotective effects..2-6 Moreover, promising experimental studies induced oral supplementation of quercetin in many clinical trials.3
Supplementary intake of quercetin was found to lower the blood pressure of patients suffering from hypertension, smokers, or type 2 diabetes mellitus patients.2-4 Furthermore, quercetin has been reported to have beneficial effects on neurodegenerative diseases, for example, Alzheimer's or Parkinson's disease.1, 5 In addition, it also inhibits fat accumulation in mature human fat cells and triggers apoptosis in existing fat cells.6 Moreover, quercetin has shown to have antiulcer effect due to decreased gastric acid secretion and increased gastric mucus production.7
Quercetin is known for its very broad spectrum of biological activities; however, little attention has been attributed to quercetin-induced relaxation of smooth muscle of the human gastrointestinal (GI) tract.8-10 Even though flavonoids are largely consumed in the diet, the plasma concentrations of flavonoids are lower than their effective therapeutic doses due to poor bioavailability. Human kinetic studies recovered total concentrations of quercetin (including free and sulfated/glucuronidated metabolites) reached plasma levels ~15–450 μg/L. Therefore, high concentrations of these nutraceuticals may exist especially within the digestive tract and express local rather than systemic effects after oral administration.11, 12
GI motility is defined by the contraction of the muscles of the digestive system, and the transit of the contents within it. It is regulated by a complex interactions of nervous and endocrine systems.13 Symptoms related to motility problems may have various causes, but the main is gastric motility dysfunction. What is more, sex hormones, estrogens in particular may contribute to GI disorder and irritable bowel syndrome.8-10 Quercetin is known to exert strong estrogenic14 and antiestrogenic15 effects; hence, this estrogen receptor regulator has been classified as a phytoestrogen.16 Moreover, quercetin has been reported to inhibit GI tract contractility in mice and guinea pigs.8, 10 Despite the increasing attention to the effect of nutraceuticals on smooth muscles, little is known about the mechanisms of these effects. Although the flavonol quercetin is among the most frequently investigated flavonoids, with both beneficial and harmful effects on diverse cell types being thoroughly described, its mechanism of action remains unclear.17, 18
Neurons that regulate the motor function of the GI tract are located in myenteric plexus between the inner circular and outer longitudinal layers. They undergo relaxant responses to non-adrenergic non-cholinergic (NANC)13 and purinergic co-neurotransmission.19 Inhibitory neuromuscular transmission in the GI tract therefore involves at least two inhibitory co-transmitters: a purine and nitric oxide (NO). NO is synthetized from L-arginine by neuronal isoform of NO synthase (nNOS). The classic target for NO is soluble gyanylyl cyclase (sGC), which produces a second messenger cyclic guanosine monophosphate (cGMP).20 Increased level of cGMP leads to activation of potassium channels: high conductance Ca2+-dependent (BKCa) and small conductance (SKCa) apamin-sensitive or ATP-sensitive K+ (K+ATP).10, 19, 21, 22 Furthermore, NO can also directly activate cGMP-independent K+-channels and inhibit L-type Ca2+-channels that cause smooth muscle relaxation.23, 24 In vitro studies show that quercetin may exert multiple actions on the NO-guanylyl cyclase pathway and causes both NO-dependent and NO-independent vasodilatation.25
Our study aimed to observe the effects of quercetin on the contractility of human gastric smooth muscles in reference to the NO pathway and direct influence of potassium channels. Additionally, the results may support the potential use of quercetin for the treatment of functional dyspepsia and other minor gastric muscle motility disturbances. A better understanding of the physiochemical properties of this bioactive nutraceutical would be beneficial for the development of an optimized system for oral administration.
2 MATERIALS AND METHODS
2.1 Sample processing
The study was conducted in accordance with the Helsinki Declaration principles, the International Conference on Harmonisation Guideline for Good Clinical Practice, and the laws and regulations of Poland, and with the approval from the Ethical Committee of Medical University of Białystok (No.R-I-002/304/2018). Tissues were obtained from patients undergoing sleeve gastrectomy due to morbid obesity (n = 10: women = 2, man = 8; aged 24–56; BMI 47.16 ± 1.84). The surgical procedure involved a longitudinal resection of the stomach on the greater curvature from the antrum up to the angle of His. 26 The experiment did not affect the course of the operation. Written informed consent was achieved from the patients before processing and analyzing the specimens.
Tissue samples were removed immediately after surgical resection of the stomach and cooled on ice cold Tyrode's buffer, bubbled with carbogen (95% O2 +5% CO2), and transferred directly to the laboratory wherein they were processed as previously described.27 After removal of mucosa and submucosa, muscle strips (10 × 3 × 1.5 mm) were cut in the direction of the longitudinal muscle layer and mounted to organ bath (20 ml) of isometric force transducer system. The temperature of bath solution was thermostatically maintained at 37°C and continuously bubbled with carbogen. Each strip was stretched passively to resting tension after 1∼1.5 hours of equilibration. The bath solution was replaced every 20 min of incubation. Contractile responses of the strip to the high K+ (80 mmol/L, 10 min) was then repeated two or three times until the responses were reproducible.
2.2 Experimental protocol
After the equilibration period, direct smooth muscle contractions were obtained by addition of carbachol (10−6 mol/L) to the organ bath, and then, the muscle strips contractions were subsequently checked. Only the strips exhibiting stable response to the administered agonist were used in further experiments. The contractile activity of strips incubated only with carbachol was considered as control after reaching the plateau. To examine concentration-response relationships, quercetin was added cumulatively to the organ chambers (range 10−7–10−4 mol/L) at 10-min intervals, and the isolated human muscle strips relaxations were measured. To eliminate the effect of quercetin solvent, a DMSO group was established. To verify the role of NOS in antispasmodic effect of quercetin, 10−5 mol/L of NG-nitro-L-arginine (L-NNA) and NG-Methyl-L-arginine (N-Nitroarginine methyl ester, L-NAME), NO synthase (NOS) blockers, and 10−6 mol/L cystamine,28, 29 and 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (soluble guanylyl cyclase blockers, sGCs) were used. Concentration-response curves to quercetin were also constructed in the absence and presence of: 10−7 mol/L charybdotoxin (CTX), an inhibitor of BKCa and intermediate (IKCa) conductance calcium-activated potassium channels; 10−6 mol/L SKCa blocker—apamin or 10−6 mol/L glibenclamide, an K+ATP channel blocker, and 10−6 mol/L tamoxifen—a selective estrogen response modifier, protein kinase C inhibitor. As far as possible, experiments were performed with strips from the same stomach sample and were studied in parallel. Appropriate controls were run under similar experimental conditions obtained from the same patient. In addition, vehicle-matched control experiments were conducted under similar experimental conditions with strips obtained from the patient.
2.3 Measurement of contraction parameters
Gastric muscle activity was recorded by an isometric force transducer with digital output (BIO-SYS-TECH, Białystok, Poland) and calculated with the DASYLab software unit (version 9.0; Laboratory Data Acquisition System, SuperLogics, Waltham, MA, USA). The following parameters were evaluated in the recordings: area under the curve (AUC), average baseline muscle tone, and relative change in muscle contraction. Values from three to four strips from each sample were averaged at each time point for each drug dose. The AUC reflected the total change over time representing the contractile activity of stomach muscle responses before and after the administration of the given drug. The AUC was measured as the area under all recorded contractions over a 10-min interval before the addition of each agonist or antagonist, respectively.30, 31 The AUC of contractions of each muscle strip over a 10-min interval before the addition of quercetin was treated as control (100%).
All results are expressed as mean ± SEM of experiments performed on muscle strips from different patients or as percentage change [(effect size - baseline contractile response)/baseline contractile response × 100]. When the same protocol was run on two strips from the same gastric specimen, the data were averaged. Mean concentration-response curves to the used drugs were analyzed by fitting to a four parameter logistic equation using nonlinear regression. The AUC was evaluated by calculating the integral of the appropriate section of the curve. Concentration-response curves were fitted to the logistic equation using nonlinear regression Y=Bottom+(Top-Bottom)/(1 + 10^((LogIC50-X)*HillSlope)) (PRISM 6.0, GraphPad Software Inc., San Diego, CA, USA). The maximal response (Emax) was expressed as a percentage of the contractile activity before administration of tested substances, whereas the concentrations of agents that resulted in a half-maximal effect were expressed as -log IC50.
2.4 Chemicals
Quercetin (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one) was purchased from abcr GmbH (Karlsruhe, Germany), while Carbamylcholine chloride ((2-Hydroxyethyl)trimethylammonium chloride carbamate; carbachol), N5-(Nitroamidino)-L-2,5-diaminopentanoic acid, NG-NO2-L-Arg (Nω-Nitro-L-arginine, L-NNA), NG-Methyl-L-Arg (Nω-Nitro-L-arginine methyl ester hydrochloride, L-NAME), cystamine, pinacidil, 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), apamin, charybdotoxin (CTX), glibenclamide, and tamoxifen were purchased from the Sigma Chemical Company, were purchased from Sigma (St. Louis, MO).
Quercetin, ODQ, and glibenclamide were dissolved in in dimethyl sulfoxide (DMSO) so that the final concentration of DMSO was never >0.1%, which had no effect on basal contraction. Dilution series of quercetin were prepared on the day of experiment and were sustained at room temperature during the period of the experiment.
Stock solutions of carbachol, L-NNA, L-NAME, cystamine, pinacidil, apamin, CTX, and tamoxifen were prepared using bidistilled water. All substances were added directly to the organ bath containing a Tyrode's solution composed of (mmol/L): NaCl 139.6; KCl 2.68; MgCl2 1.05; NaH2PO4 1.33; CaCl2 1.80; NaHCO3 25.0; and glucose 5.55.
2.5 Statistical analysis
All series of data were checked for consistency with a Gaussian distribution in accordance with the D'Agostino-Pearson normality test. Dose-response was determined using one-way ANOVA or the Kruskal-Wallis test, where appropriate. Statistically significant differences between means were determined by Tukey's post hoc or a nonparametric Mann–Whitney U test, where appropriate. Values were considered to be statistically significant at p < 0.05. All analyses were performed using Prism 6 for Windows.
3 RESULTS
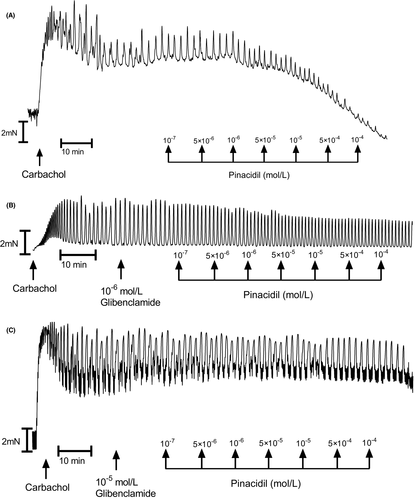
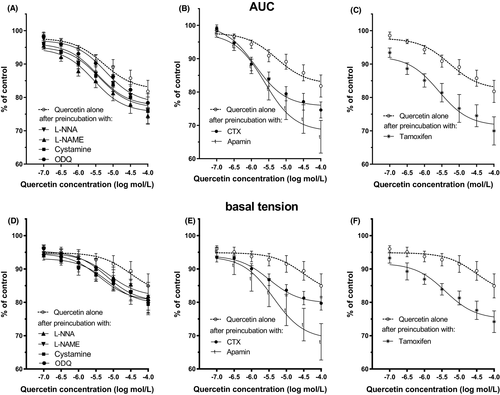
In muscle strips isolated from human gastric specimens, 10−6 mol/L carbachol induced a marked and long-duration muscle contraction (Figure 1A). Cumulatively applied quercetin induced a significant muscle relaxation of the carbachol treated gastric strips which was dose-dependent (Figures 2A and 4 and 5A and C). Typical tracings show the response to quercetin or pinacidil in body of stomach smooth muscle strips (Figures 2A). Quercetin caused substantial relaxation at concentrations higher than 10−7 mol/L and maximal at 10−4 mol/L (81.82 ± 3.32%; n = 10; p < 0.0001) (Figures 4A–C and 5A). This polyphenol also dose-dependently decreased the resting tension in all concentrations, and the reduction was significant in all utilized concentrations of quercetin (Figures 3 and 4D–F and 5C).
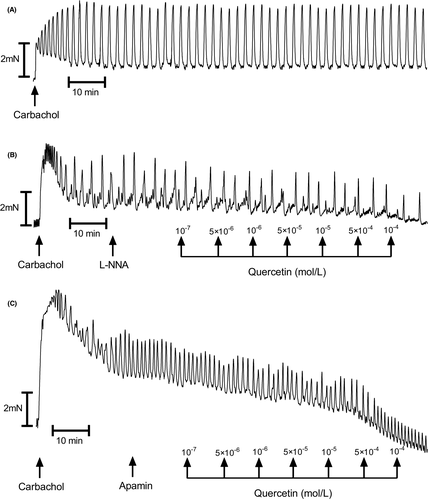
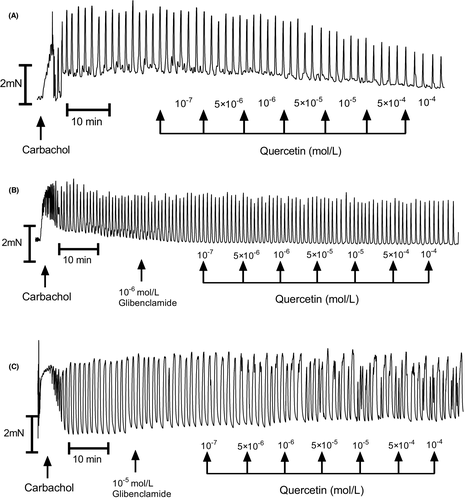
3.1 The influence of blockers on carbachol-induced contractility
Preincubation with a NOS inhibitor—L-NNA, a sGC inhibitor—ODQ, a SKCa channel blocker—apamin, a BKCa channel blocker—CTX, and ATP-sensitive K+ blocker—glibenclamide (10−6 mol/L) did not alter carbachol-induced muscle contractions. In turn, the other NOS inhibitor—L-NAME (95.23 ± 0.62; p < 0.0001) and an estrogen receptor blocker—tamoxifen (89.77 ± 0.96; p < 0.0001) significantly inhibited contractility induced by carbachol. A sGC inhibitor—cystamine significantly reduced AUC (96.55 ± 0.27; p < 0.0001) but did not influence basal tension of muscle strips. Preincubation with 10−5 mol/L glibenclamide substantially extended basal tension (103.3 ± 1.14; p = 0.0184) without affecting AUC (Figure 6).
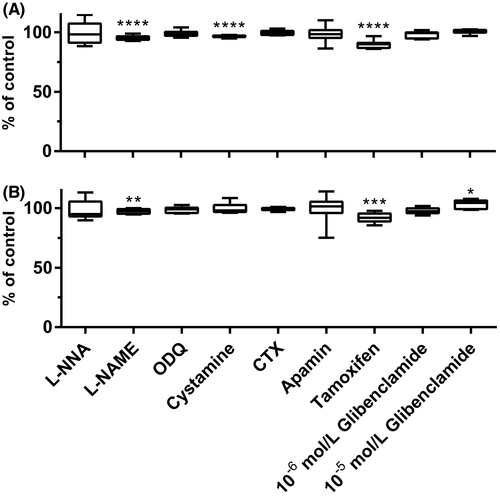
3.2 The influence of blockers on quercetin-induced relaxation
Blockers of the nitric oxide pathway (L-NNA, L-NAME, ODQ, or cystamine) had no inhibitory effects on the relaxation of muscle strips induced by quercetin. Moreover, no obvious effect of preincubation with L-NNA, L-NAME, ODQ, or cystamine on the decrease of basal tension was observed Figure 4A and D and Table 1.
| logEC50 | p | Emax (%) | p | |
|---|---|---|---|---|
| Quercetin alone | −5.27 ± 0.27 | 81.82 ± 3.32 | ||
| Quercetin after preincubation with | ||||
| L-NNA (10−5 mol/L) | −5.44 ± 0.28 | 0.1830 | 76.16 ± 3.95 | 0.3150 |
| L-NAME (10−5 mol/L) | −5.55 ± 0.21 | 0.0199 | 74.46 ± 2.54 | 0.1051 |
| ODQ (10−6 mol/L) | −5.21 ± 0.26 | 0.5977 | 78,37 ± 2.74 | 0.1763 |
| Cystamine (10−6 mol/L) | −5.40 ± 0.20* | 0.2466 | 75.85 ± 2.17 | 0.0892 |
| Apamin (10−6 mol/L) | −5.62 ± 0.22** | 0.0063 | 66.59 ± 4.91* | 0.0288 |
| CTX (10−7 mol/L) | −5.87 ± 0.18* | 0,0131 | 74.61 ± 2.41* | 0,0446 |
| Glibenclamide (10−6 mol/L) | −5.38 ± 0.56 | 0.5985 | 93.06 ± 1.81** | 0.0026 |
| Glibenclamide (10−5 mol/L) | −4.86 ± 0.54* | 0.0441 | 88.53 ± 3.60 | 0.1357 |
| Tamoxifen (10−6 mol/L) | −5.59 ± 0.29* | 0.0225 | 69.96 ± 4.22* | 0.0433 |
| Pinacidil alone | −5.36 ± 0.13 | 0.3798 | 56.31 ± 3.30*** | 0.0001 |
| Pinacidil after preincubation with | ||||
| Glibenclamide (10−6 mol/L) | −5.09 ± 0.19¥¥ | 0.0016 | 70.15 ± 3.83¥¥ | 0.0092 |
| Glibenclamide (10−5 mol/L) | −4.86 ± 0.53¥¥ | 0.0096 | 88.53 ± 3.60¥¥¥¥ | <0.0001 |
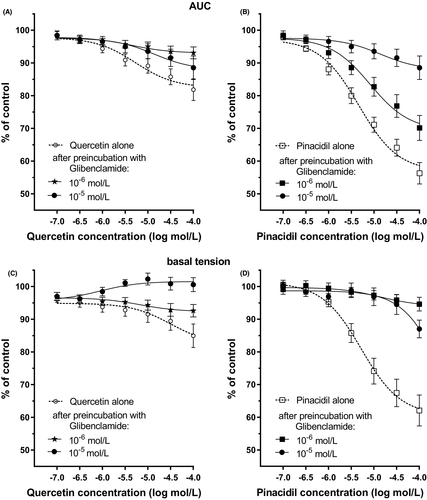
As shown in Figure 5A and C and Table 1, glibenclamide inhibited the relaxing effect of the tested polyphenol on human gastric muscle strips. Statistically significant effect was observed at concentrations of quercetin higher than 5⋅10−5 mol/L for 10−6 mol/L glibenclamide and higher than 5⋅10−6 mol/L for 10−5 mol/L glibenclamide.
Preincubation with CTX deepened the relaxing effect of quercetin observed as a decrease of AUC and basal tension substantial at quercetin concentrations, 10−6 mol/L and 10−6–5⋅10−4 mol/L, respectively (Figure 4B and E and Table 1). Apamin also extended the effects of cumulatively applied quercetin statistically significant at concentrations higher than 10−6 mol/L (AUC) and 5⋅10−5–10−4 mol/L (basal tension) (Figure 4B and E and Table 1).
Preincubation with tamoxifen substantially increased the relaxation of muscle strips observed as a decrease of AUC and their basal tension at all quercetin concentrations (Figure 4C and F and Table 1).
4 DISCUSSION
The relaxant property of polyphenols has been reported in a variety of organs including arteries,32 uterus,33 bladder,34 and GI tract.35, 36 The studies were in most cases carried out on the tissues of various animal species: rats, guinea pigs, rabbits, or mice. In regard to potential clinical aspects, some of the previously conducted interventional studies include the oral administration of quercetin in combination with one or more other possibly biologically active substances.11 It is worth noting that due to low oral bioavailability, quercetin in differently selected oral doses from 25 mg to even 5 g operates almost exclusively in the GI tract.12, 37, 38 Therefore, we have investigated the effect of this nutraceutical on the contractility of isolated human gastric smooth muscles.
We found that quercetin concentration dependently reduced the carbachol-induced contractions and the resting tension in all concentrations used in the isolated human gastric smooth muscle. This observation is consistent with those reported for the effects of flavonoids on GI smooth muscles of rats and guinea pigs.9, 10, 36, 39, 40
Modulation of NO metabolism is one of the possible mechanisms that may contribute to the explanation of polyphenol activity observed in vivo. In the stomach, polyphenols in the diet may not only promote the reduction of nitrites toward NO, but also deal with a complex network of chemical reactions to produce NO with its signaling functions. Therefore, in this sense, the local and systemic effects of NO can be caused in this sense by dietary flavonoids.41 NO is considered to be the most important neurotransmitter in the GI tract. The relaxation of smooth muscles is regulated by many signaling pathways, including NO, cAMP, and cGMP. In the classic concept of nitrergic or purinergic co-neurotransmission, NO produced from L-arginine by neuronal NO synthase (nNOS, NOS-1) in NANC neurons penetrates to the GI smooth muscle cells where its major target is sGC generating cGMP.19, 20 Previous studies have shown that quercetin-induced vasorelaxant activity was mainly associated with NO-related and NO-unrelated mechanisms and potassium channels.8, 25, 32 The role of NO is also not clearly defined in the literature on the influence of polyphenols on the GI tract. The inhibitory effect of flavonoids was also not significantly reduced in the presence of the NOS inhibitor L-NAME on the mouse stomach smooth muscles, suggesting that it is not related to NO production.39 In this study, we showed that quercetin-induced relaxation was not blocked by inhibition of NO with the NOS inhibitor L-NNA and L-NAME or the sGC inhibitor cystamine. Our data indicate that quercetin induces human gastric muscle fiber relaxation independently of NO pathway. This result suggests that a nitrergic or purinergic pathway may not be activated during the carbachol-induced contraction. The difference of quercetin-induced relaxation mechanisms between the rat, guinea pig, and human smooth muscle might be due to possible organ specificity. The ion channels may vary structurally according to the organ or may show different expression depending on the location of the smooth muscle, and more studies are needed to clarify the difference between them.
Potassium channels play an important role in regulating the excitability of the syncytium. Smooth muscle contraction depends on a balance of K+. The activation of membrane K+ channels leads to hyperpolarization of the plasma membrane and further inhibits Ca2+ influx into the cell, resulting in relaxation of smooth muscle.42 Inhibition of peristalsis through facilitating inhibitory enteric pathways by some flavonoids has been declared in guinea pig small intestine or rat colon.8, 35 To explore whether quercetin acts directly on the BKCa or SKCa channels, the effects of CTX or apamin on quercetin-induced relaxation were examined in this study. The usage of these blockers not only did not cause inhibition of induced by quercetin muscle relaxation; on the contrary, it deepened this effect. These results suggest that the majority of the response is independent of BKCa or SKCa channels or, alternatively, the involved BKCa or SKCa channels are apamin- or CTX-insensitive.19 Nevertheless, relaxation of the examined strips does not occur directly through the effects of quercetin on BKCa or SKCa channels. On the other hand, in future research, the nitrergic component should be isolated from the purinergic component by more appropriate pharmacological tools that allow to distinguish between neurotransmission.19, 43
However, in the presence of glibenclamide, the relaxant effect of quercetin was significantly attenuated, confirming that ATP-sensitive K+ channels are positively modulated by quercetin to block L-type Ca2+-channels indirectly. Moreover, the lack of effect on gastric tone by glibenclamide alone might indicate a low activity of K+ATP channels under the carbachol-induced condition. A number of studies have confirmed the presence of K+ATP channels in GI smooth muscle.44 Some research suggested that the inhibitory NANC neuromuscular transmission in GI smooth muscle does not involve the opening of K+ATP channels on the mouse gastric fundus or rat ileum.44 In turn, Yang et al. showed that the K+ATP channel opener, pinacidil, induced a dose-dependent gastric relaxation and glibenclamide completely and the NOS inhibitor L-NAME partially blocked the pinacidil induced relaxation effect.35 The difference may be attributed to species or regional differences in the activity and the regulation of K+ATP channels. Notably, this is the first study showing that quercetin can relax human gastric muscles directly via K+ATP channels. The present, in vitro study demonstrated that quercetin may have the ability to act as an opener of K+ATP channels and suggests the therapeutic possibility of this nutraceutical for GI motility disorders in vivo. Furthermore, its use as a potential K+ATP channel opener could explain important benefit as a myocardial protector, an antihypertensive vasodilator, a bronchodilator, a bladder relaxant, and an antiepileptic factor. The therapeutic implications of quercetin should also be considered in the treatment of hyperinsulinemia and type 2 and type 1 diabetes.45, 46
Since, the chemical structures of quercetin and other bioflavonoids and the synthetic estrogen diethylstilbestrol are similar, we used tamoxifen, as an estrogen receptor antagonist, to explore whether an estrogen receptor is involved in quercetin-induced relaxation of human gastric smooth muscles. Tamoxifen was not able to inhibit the investigated quercetin-induced relaxation, suggesting that the estrogen receptor may not play a significant role in regulating smooth muscles in the human stomach. Furthermore, Al-Shboul et al. demonstrated that, in rats, estrogen relaxes gastric muscle cells via a NO‑ and cGMP‑dependent mechanism.47 In turn, our results indicate that quercetin causes human gastric muscle relaxation regardless of the NO pathway. If the relaxing effect of estrogen also occurs in humans via the NO metabolic pathway, this may explain the lack of inhibition of quercetin-induced action despite blocking the estrogen receptor.
In addition to assessing the effect of quercetin, the influence of the used blockers on the contractile activity of the stomach muscles was also considered. Preincubation of the gastric muscle with L-NNA did not significantly alter its contraction parameters; therefore, NO does not appear to be tonically active in the gastric fundus, which is consistent with the observation of Holzer-Petsche and Moser.48 However, other researchers observed increases in amplitude and basal tension after incubation with L-NNA.49, 50 In turn, L-NAME significantly inhibited contractility induced by carbachol. In the literature, no such effect can be found48 and an increase in contractility is reported.51 The variety of the observed gastric muscle responses to L-NAME may be due to the fact that, despite its use as a NOS antagonist, there are numerous reports of L-NAME mediated reactions that are incompatible with NOS inhibition.52 Moreover, several electrophysiological studies have also been conducted showing that NO synthase inhibitors reduce or abolish NANC inhibitory induced by intestinal nerves.53 As for GC blockers we observed that ODQ did not affect carbachol-induced contractility while cystamine significantly reduced AUC but did not influence basal tension of muscle strips. Cystamine was investigated to induce vasodilation in rat mesenteric small arteries by inhibiting the receptor-coupled TG2, leading to the opening of Kv channels and reduction of intracellular calcium, and the activation of the pathway sensitive to inhibitors of mitochondrial complexes I and III.54
The blocker of an ATP-sensitive K+ channel—glibenclamide at the concentration of 10−6 mol/L did not significantly modify carbachol-induced muscle contractions, but at 10−5 mol/L—substantially increased the basal tension. Multiple in vitro studies have reported that glibenclamide (≤ 10−6 mol/L), which blocks K+ATP channels, increases muscle tone and depolarizes vascular and non-vascular smooth muscles.55 The attenuation of carbachol-induced muscle contraction by tamoxifen may be mediated by inhibition of the Ca2+—independent mitogen-activated protein kinase (MAPK) pathway. Park et al. (2003) demonstrated in a rat aortic smooth muscle study that tamoxifen inhibited the ET-1-induced ERK1/2 and p38 MAPK activity.56
To sum up, dietary polyphenols and/or their metabolites may exert valuable health effects directly during their passageway through the oral cavity and the entire GI tract at the local level. Many is known about possible therapeutic role of various polyphenols such as quercetin. However, due to their low bioavailability, main pharmacodynamics and/or adverse effects may affect the GI motility in first place. Thus, taking into account the physiology of the digestive system, a further interpretation of the effects of quercetin should be proposed. The presence of polyphenols in the lumen of the stomach and further parts of the GI tract leads to the assumption that these compounds may exert their beneficial effects in situ. However, currently biochemical modifications of quercetin have led to the formation of new semi-synthetic derivatives with improved bioavailability and solubility. These new compounds are still being studied.3 Therefore, nutraceuticals that enhance gastric relaxation to treat functional dyspepsia are worth studying.
5 CONCLUSIONS
In conclusion, the current study was the first to show that quercetin-induced relaxation of human gastric smooth muscle occurs directly through K+ATP channels and independently to NO pathways. The present results suggest that quercetin is a potential nutraceutical in the treatment of functional gastrointestinal dyspepsia and other minor gastric muscle motility disturbance. Furthermore, its use as a potential K+ATP channel opener could explain important benefit as a myocardial protective agent, antihypertensive vasodilator, bronchodilator, bladder relaxant, islet cell protector, and antiepileptic drug.
ACKNOWLEDGMENTS
This work was supported by Grant No. N/ST/ZB/18/002/1116 from the Medical University of Białystok, Białystok, Poland.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
BM and KD conceptualized the study and involved in writing—original draft preparation. BM, PP, HRH, and TK involved in methodology. BM and TK validated and investigated the study. BM involved in informal analysis.BM, HRH, and KD collected resources. BM, AK, KK, KD, and AC involved in data curation. BM, KD, and TK involved in writing—review and editing, and funding acquisition. BM, KK, and AK visualized the study. BM and HRH supervised the project. BM, PP, and HRH involved in project administration. All authors have read and agreed to the published version of the manuscript.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.