Cabozantinib-induced renal thrombotic microangiopathy
CASE REPORT
A 61-year-old woman, affected by medullary thyroid cancer with multiple metastases (lymph nodes, liver, pancreas) treated with surgical intervention, started cabozantinib therapy with a good response. After 3 years she developed oedema, proteinuria and acute kidney injury. Blood tests showed: serum creatinine 132 μmol/L, haemoglobin 9.1 g/dL, platelet count 326 000/mm3, LDH 200 UI/L, proteinuria 3 g/day. A renal biopsy was performed to rule out malignancy-induced nephropathy, calcitonin amyloidosis or drug-related glomerulopathy.1 The biopsy showed thrombotic microangiopathy (TMA) (Fig. 1). Due to the good oncological response, cabozantinib dosage was slightly lowered with a consequent reduction of serum creatinine to normal value and of proteinuria down to 2 g/day.
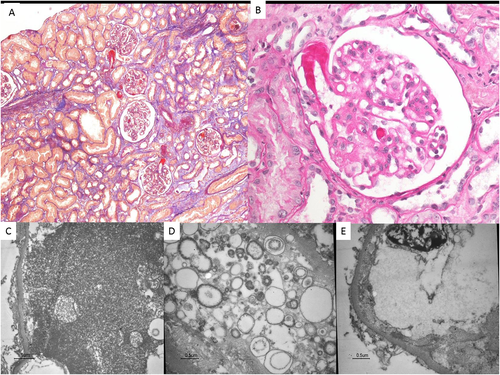
DISCUSSION
In 2012, cabozantinib was approved for treatment of metastatic medullary thyroid cancer.2 Subsequent clinical trials have been conducted in various malignant neoplasms, in particular gastric, renal cell, pancreatic, and prostate cancer previously treated with other therapy or strategies.3, 4 The most common adverse events included diarrhoea, nausea, palmar–plantar erythrodysesthesia syndrome, hypertension and proteinuria.1, 2 Cabozantinib (RTKI) is a small molecule inhibitor of the tyrosine kinase c-Met and VEGFR2 that are also expressed in podocytes and glomerular endothelial cells.2, 5 The inhibition of different VEGF-signalling pathways by RTKI such as cabozantinib, frequently results in minimal change nephrotic syndrome or focal and segmental glomerulosclerosis, whereas the association with TMA was only observed in an experimental model.5
To the best of our knowledge, this is the first histological description of TMA localized in the kidney induced by cabozantinib therapy.
An open challenge in the management of this patient is the decision whether to stop or continue the therapy. In some cases, dose reduction might represent a good strategy, and it is feasible that the combination of cabozantinib with a complement inhibitor might represent a new effective treatment strategy.
DISCLOSURE
The authors declare there are no conflicts of interest.




