Microplastics in the menu of Mediterranean zooplankton: Insights from the feeding response of the calanoid copepod Centropages typicus
[Correction added on 20 October 2023, after first online publication: The copyright line was changed.]
Abstract
Microplastic input into the ocean represents an increasing threat to marine biota and may endanger the functioning of marine ecosystems, especially in semi-enclosed basins, such as the Mediterranean Sea. The size spectrum of microplastics overlaps with that of nano-microplankton (2–200 μm), thus potentially misleading suspension-feeding zooplankton, which represent a key trophic link in pelagic food webs. We investigated the effects of microplastics on the feeding performance of the copepod Centropages typicus in laboratory experiments. Adult females were incubated in natural prey assemblages under different conditions: without and with nutrient enrichment, and in the presence and absence of microplastics (20–1000 μm). Non-significant changes were recorded in either copepod ingestion rates or daily rations upon microplastic addition. However, the copepod diet shifted significantly in the presence of microplastics, as the copepods fulfilled their individual carbon requirements by ingesting different protistan prey. The number of microplastic particles found in copepod guts (0.9 particles ind.−1) and fecal pellets (2.1 particles pellet−1) was low and particles occurred mostly in the lower size range (∽20 μm). Overall, the exposure of C. typicus to microplastics did not affect the copepod daily intake of food, likely due to the avoidance of microplastics and flexible feeding habits.
1 INTRODUCTION
Concern has been mounting in recent decades regarding the impact of microplastics (<5 mm in size) on aquatic ecosystems and their inhabitants (Barnes et al., 2009; Gall & Thompson, 2015; Ryan et al., 2009; Ryan & Moloney, 1993). These particles may favour the dispersal and bioavailability of persistent organic pollutants (Guerrini et al., 2021) and act as vectors for non-indigenous species (Avio et al., 2017; Barnes, 2002; UNEP/MAP, 2015). Plastic particles are spread in the global ocean from the poles to the equator (Ajith et al., 2020) and occur from the surface to the deepest waters (Peng et al., 2018). The concentration of surface microplastics spans over at least three orders of magnitude, being higher near the coasts (Desforges et al., 2014) and within estuaries, where the numbers increase up to five orders of magnitude (Dris et al., 2020). The concentration and potential harmfulness of microplastics increase in semi-enclosed regions with high riverine loads and long seawater residence time, such as the Mediterranean Sea (UNEP/MAP, 2015). The concentration of surface microplastics in Mediterranean sub-basins has recently been estimated to range between 0.18 (±0.16) and 2.93 (±9.11) items m−3 with high heterogeneous distribution and the lowest and highest concentrations in the Levantine Sea and in the Tyrrhenian Sea, respectively (Pedrotti et al., 2022). In the same survey, the quantitative analysis of microplastics and co-occurring zooplankton highlighted a high potential risk of contamination of marine fauna (Fabri-Ruiz et al., 2022).
In particular, zooplankton are exposed to microplastics (Cole et al., 2011; Sun et al., 2017, 2018), especially in the upper layers of the water column where they feed and where low-density polymers (polypropylene and polyethylene) are highly concentrated (Ajith et al., 2020; Faure et al., 2015). Particles with a specific density of <1 g cm−3 and size between 10 and 70 μm float in the surface layer (Wright et al., 2013) and are available as food to a wide variety of organisms (Bai et al., 2021; Moore et al., 2001) because they overlap in size with the natural protistan prey of most mesozooplankters (Botterell et al., 2019; Cole et al., 2013; Reisser et al., 2014). In addition, zooplankton are food for several larger consumers and hence are potential vehicles of microplastic particles to other zooplankton and fish populations via direct predation (Setälä et al., 2014) and coprophagy (Cole et al., 2016). The transfer of microplastics throughout pelagic food webs can lead to the bioaccumulation and biomagnification of the ingested contaminants (Costa et al., 2020; Miller et al., 2020), posing serious risks to marine biodiversity (Secretariat of the Convention on Biological Diversity and the Scientific and Technical Advisory Panel—GEF, 2012), biogeochemical cycling (Cole et al., 2016) and ecosystem services (Avio et al., 2017; UNEP/MAP, 2015).
Our present knowledge of the distribution and effects of microplastics on planktonic communities and trophic webs remains limited (GESAMP, 2020). With the aim of understanding the extent of microplastic-plankton interactions and cascading effects on the whole pelagic ecosystem, a multidisciplinary project (INPUT, INtegrative Pelagic ecosystem response to nUtrients fertilization and microplasTics addition) was carried out in the inner Gulf of Naples (Tyrrhenian Sea, western Mediterranean Sea) in June 2016. Here, we present the results of a side experiment that was conducted in parallel with the INPUT mesocosm experiment with the aim of elucidating the possible impact of microplastic exposure on the feeding activity of target zooplanktonic species, i.e., the calanoid copepod Centropages typicus Krøyer, 1849. This omnivorous temperate eurythermal species is highly abundant in neritic zones of the Mediterranean Sea (Mazzocchi et al., 2007) and the North Atlantic Ocean (Bonnet et al., 2007), and it is one of the most abundant copepod species in the coastal waters of the Gulf of Naples (Mazzocchi et al., 2012).
We conducted grazing experiments to ascertain the effect of the presence of microplastics on C. typicus feeding performances. We estimated the clearance and ingestion rates and daily ration of C. typicus fed on a natural protistan prey assemblage and characterised its diet and selectivity in two different trophic (nutrient-poor vs. nutrient-rich) conditions and in absence versus presence of microplastics, to test the hypothesis that microplastic ingestion negatively impacts C. typicus feeding by reducing the acquisition of natural food.
2 MATERIALS AND METHODS
2.1 Preparation of microplastics
The microplastics used in this study were prepared at Universitá Politecnica delle Marche following the protocol described in Corinaldesi et al. (2021). All procedures were performed under a laminar fume hood, using previously sterilised tools. The microplastic particles were produced by milling plastic objects used in everyday life (i.e., bottles, cups, and containers) and represented the plastic polymers commonly found in seawater samples, namely polyethylene (PE), polyvinylchloride (PVC), polypropylene (PP), polyethylene terephthalate (PET) and polystyrene (PS). The milled particles were then sieved through different meshes, to obtain three size classes for each polymer: 20–200 μm, 200–500 μm and 500–1000 μm. The resulting particles were spherical, triangular, or irregularly rectangular in shape, regardless of the polymer type, and each polymer had a distinct colour to facilitate the identification under a microscope (PE: blue, PVC: green, PP: yellow, PET: orange; PS: pale pink). The specific density was highest for PET (1.375 g cm−3) and lowest for PP (0.885 g cm−3). Each polymer stock was weighed on a precision scale (AS, RADWAG, accuracy ±0.001 g), and the microplastic particles contained in the subsample were enumerated under a stereomicroscope (Zeiss Stami 2000 at 50× magnification). A working stock of the microplastic mixture used in the mesocosm experiments was prepared by combining a known amount of microplastics from each polymer type. The relative contribution of each polymer to the mixture (20%) and size distribution were confirmed by enumeration and observation under a stereomicroscope, revealing a very similar abundance of particles of the three size classes (variation coefficient <10%) (Corinaldesi et al., 2021).
2.2 Experimental set-up and sampling
The timeline of the two grazing experiments, the mesocosm structure and treatments, and the copepod sample processing for microplastic analysis are schematically illustrated in Figure 1a–c. Briefly, six mesocosms placed in the inner Gulf of Naples (M1-M6) in the afternoon of the first day (T0) were enriched with nutrients to maintain nutrient replete conditions throughout the experiment. Final concentrations of inorganic nutrients after the addition were as follows: 0.40 ± 0.19 μM PO4, 20.08 ± 6.22 μM SiO4, 1.08 ± 0.86 μM NO3. Immediately after nutrient addition, microplastic particles were added only to mesocosms M4-M5-M6 to reach a target concentration of 100 particles L−1 (20 particles for each plastic type), while M1-M2-M3 were kept as only nutrient-rich controls. Copepods for the grazing experiments were collected 200 m off the coastline towing a WP2 net (200 μm mesh size) in the upper 50 m of the water column the day before the start of the mesocosm experiment (T-1) and 6 days later (T5). The samples were promptly transferred to coolers screened from direct light and transported to the laboratory within half an hour.
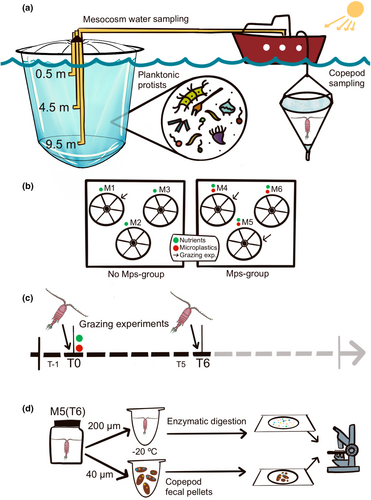
For the first experiment, the water for acclimation was taken at T-1 in the mesocosms M1 and M4 and that for the grazing experiment from the same mesocosms in the morning of the day after (T0) before any additions. For the second experiment, the water for acclimation was taken from M1 and M5 at T5 and that for the grazing experiment at T6. In the mesocosms, the water for the experiments was taken at three depths (0.5, 4.5 and 9.5 m) and mixed.
2.3 Grazing experiments
The first experiment represented the pre-treatment conditions, i.e., with the protistan community as prey before any additions, while the second experiment represented the conditions achieved after 6 days from nutrients and microplastic addition. Both experiments followed exactly the same procedures and were conducted in a light- and temperature-controlled room, at 12:12 h light:dark photoperiod and 20°C (corresponding to the depth-averaged temperature in the upper 50 m of the water column).
In the laboratory, adult females of Centropages typicus from the net samples were sorted and observed under a dissecting microscope (Wild M5-48552 at 12-50x magnification) to check their conditions. Only healthy and intact individuals were selected and placed in two 5 L acclimation glass jars that were covered with a cling film. The acclimation water was prepared by gently combining 3 L of water collected at each of the three sampling depths and passed through a 200 μm mesh. Extreme caution was taken when filtering the water and filling the jars to minimise protistan mortality.
Among the protistan prey cells (<200 μm), phytoplankton (strict autotrophs), mixoplankton (mixotrophs), and protozooplankton (strict heterotrophs) comprised the natural food environment for copepods, hereafter referred to as “planktonic protists” for simplicity. The acclimation phase lasted 24 h.
For the incubation phase, eight glass jars (1130 mL) per mesocosm were carefully filled by sequentially pouring water after gentle thorough mixing, to ensure similar communities in all the jars. Two jars served as initial snapshots of the protist concentration and taxonomic composition, whereas three control jars (without copepods) and three jars with C. typicus were used to estimate copepod feeding rates by applying the food removal method (Frost, 1972). From each start jar, 250 mL subsamples were fixed with acid Lugol's iodine solution (2% final concentration) and stored in dark glass bottles in darkness at 4°C for subsequent estimation of planktonic protist abundance and taxonomic composition. C. typicus females sorted from the acclimation jars were gently distributed into the copepod jars (10 females per jar). All jars were then sealed with a cling film, closed with screw caps, and placed end over end on a plankton wheel at 0.2 rpm to prevent cell sedimentation. The incubation phase lasted 24 h.
After 24 h of incubation and gentle mixing, 250 mL subsamples were taken from each jar for plankton counts. The subsamples were immediately fixed with acid Lugol's iodine solution (2% final concentration) and stored in dark glass bottles in darkness at 4°C for 3–6 months before the analyses. Before subsampling, all the copepods were recovered using a wide-bore glass pipette and separately kept in small cups with GF/F-filtered seawater; they were counted and examined under a dissecting microscope (12–50x magnification) to check their conditions and the presence of gut content. Thereafter, the copepods from each jar (we did not observed mortality during the two experiments) were picked up one by one and placed in Eppendorf vials with a few drops of GF/F-filtered seawater. The vials were immediately frozen at −20°C until further processing. The fecal pellets present in the copepod jars were concentrated on a 40 μm mesh, placed in small glass vials, preserved in 96% ethanol, and kept at 4°C until microplastic particles were counted (see Section 2.6).
2.4 Planktonic protists
The abundance and diversity of protist assemblages were assessed following the Utermöhl method (Edler & Elbrächter, 2010) using an inverted microscope (Zeiss Axiovert 200) at 200–400x magnification according to the size of the species. Depending on cell concentration, subsamples of variable volume (3–50 mL) were allowed to settle in sedimentation chambers. Only cells ≥5 μm were counted, as smaller prey are quite uncommon for C. typicus to ingest (Calbet et al., 1999; Hansen et al., 1994) and, when possible, identified at the species level. Otherwise, identification was performed at the genus or group level, or size categories. Prey cells spanned a large size range (5–200 μm) in our samples, and their relative abundance led to the establishment of different counting strategies.
The individual cell C content (μg C cell−1) was multiplied by the cell concentrations of each taxonomic category detected in a sample to obtain the total amount of C.
2.5 Copepod feeding performance
After the incubation step, alive copepods that had remained in the acclimation jars were compacted in small pellets, oven-dried (60°C; 24 h), weighted on a micro-balance (Sartorius CP225D) and analysed using a CHN analyser (Thermo Scientific FlashEA 1112 Elemental Analyzer) for their elemental composition. Daily ration was expressed as the percentage contribution of the food C ingested per day to copepod body C (ingested C (body C)−1 d−1). Daily rations were calculated only when the ingestion rates were positive.
2.6 Microplastic ingestion
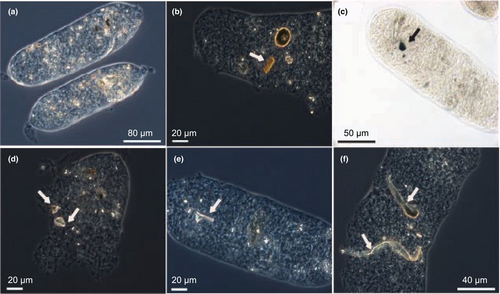
The 30 copepods retrieved from the Experiment 2 in presence of microplastics (with seawater collected from M5 at T6) were unfrozen, observed for gut fullness, photographed, and checked to exclude external adhered plastic particles. Thereafter, the samples were processed to determine their microplastic content (Figure 1d). The enzymatic digestion protocol developed by Cole et al. (2014) was applied to copepod tissues to ensure the degradation of copepod organic matter without affecting the shape and colour of the microplastics. After inspection, the unfrozen copepods were oven-dried (60°C; 24 h), transferred to three small glass jars (10 copepods each) containing a buffer homogenising solution (400 mM Tris–HCl pH 8.00, 60 mM EDTA pH 8.00, 5 M NaCl, SDS 1%), stirred with a syringe, incubated at 50°C for 15 min and treated with Proteinase K (1 mg mL−1) in a 3 mL reaction volume. The samples were then dried for 2 h at 50°C, repeatedly stirred after removal from the oven, re-incubated at 60°C for 20 min, and sonicated on ice (1–2 min) to prevent excess heat in the sample. Preparation of the homogenizing solution and copepod manipulation were performed under a fume hood, and precautions were taken to avoid contamination from external plastic sources. After digestion, the microplastic-containing suspensions were placed in an Utermöhl chamber (3 mL), finally covered with a glass slide. Microplastics were examined under an inverted microscope (Zeiss Axiovert 200), counted, measured, and categorised by colour (associated with the polymer type) when possible. Microplastics were also analysed in the fecal pellets retrieved from the same copepod jars (M5 at T6) by examining each pellet under an inverted microscope at 100–200x magnification (Figure 1d).
2.7 Statistical analysis
The results obtained from the grazing experiments were statistically tested (software Prism 5.0) by applying an unpaired two-tailed t-test to compare protistan biomass, community composition and copepod feeding responses between the two pre-treatment conditions (M1 and M4 at T0) and between treatments (M1 and M5 at T6), separately. In case of comparisons between samples of reduced size, the non-parametric unpaired two-tailed Mann–Whitney test was applied. Differences were considered statistically significant at p < .05. Graphical exploration of the results was performed using Rstudio software (version 3.6.0, RStudio, Inc.). The results are presented as average ± standard deviation.
3 RESULTS
3.1 Planktonic protist biomass and community composition
The initial C concentration available to copepods during Experiment 1 (pre-treatment conditions, T0) was 79 ± 1 μg C L−1 in M1 and 187 ± 57 μg C L−1 in M4, but the difference was not statistically significant (Mann–Whitney test, p > .05) due to reduced sample size and large variability between replicates. In Experiment 2 (6 days after the nutrient and microplastic addition, T6), the C concentration was 360 ± 18 μg C L−1 in M1 and 283 ± 4 μg C L−1 in M5, without significant differences between these two mesocosms (Mann–Whitney test, p > .05) (Figure 2a). We categorised the food environment for copepods as composed of diatoms, dinoflagellates, flagellates and ciliates. Under pre-treatment conditions, no significant differences were found between M1 and M4 in the relative contribution of planktonic protist groups to the total community biomass (Mann–Whitney test, p > .05) (Figure 2b). In M1, flagellates accounted for the highest C percentage (68%), followed by dinoflagellates (22%) and diatoms (8%), whereas ciliates were poorly represented (2%). In M4, dinoflagellates dominated (48%), followed by flagellates (40%), diatoms (7%), and ciliates (6%) (Figure 2b). At T6, a shift to diatom-dominated C occurred; this group accounted for 77% and 80% of the total C in M1 and M5, respectively. Dinoflagellates (10% and 12%), flagellates (11% and 3%) and ciliates (1% and 5%) followed in a rank order of contribution (Figure 2b). Overall, diatoms increased by 70% in the community C biomass in both M1(T6) and M5(T6) at the expense of dinoflagellates (−24%), flagellates (−46%), and ciliates (−1%) with respect to the starting pre-treatment conditions (T0).

The full list of 88 protist taxa (5–120 μm ESD) found in the food landscape for Centropages typicus in the experiments is reported in Table 1. Our protist size classification included three dimensional ranges: small-sized (5–15 μm), intermediate-sized (15–30 μm), and large-sized (>30 μm) prey cells. In pre-treatment mesocosms, the highest contribution to total biomass (Figure S1) was given by the ichthyotoxic species Pseudochattonella cf. verruculosa (Dictyochophyceae), followed by intermediate-sized athecate dinoflagellates. Small athecate dinoflagellates and flagellates (both <10 μm and >10 μm) were relevant to total C only in M1, whereas large (>30 μm) athecate dinoflagellates and thecate dinoflagellates (15–30 μm) prevailed in M4. At T6 in M1 (with nutrients only), the C biomass was dominated by colonial pennate (Thalassionema nitzschoides, Pseudo-nitzschia fraudulenta) and centric diatoms (Cerataulina pelagica, Leptocylindrus cf. danicus, Pseudosolenia calcar-avis, Chaetoceros spp.) spanning all size classes; smaller contributions were due to large-sized athecate dinoflagellates and P. cf. verruculosa. In M5 (with nutrients and microplastics), the total C available was mainly supplied by small and large Chaetoceros species, followed by intermediate- and large-sized athecate dinoflagellates.
| Functional group | Taxa | Size (ESD) | Trophic mode |
|---|---|---|---|
| Diatoms | Actinocyclus sp. | 29 | Autotrophic |
| Cerataulina pelagica | 24 | Autotrophic | |
| Chaetoceros affinis | 16 | Autotrophic | |
| Chaetoceros brevis | 15 | Autotrophic | |
| Chaetoceros costatus | 12 | Autotrophic | |
| Chaetoceros curviseutus | 12 | Autotrophic | |
| Chaetoceros danicus | 16 | Autotrophic | |
| Chaetoceros decipiens | 19 | Autotrophic | |
| Chaetoceros protuberans | 17 | Autotrophic | |
| Chaetoceros pseudocurvisetus | 16 | Autotrophic | |
| Chaetoceros simplex | 7 | Autotrophic | |
| Chaetoceros spp. (small) | 4.9 | Autotrophic | |
| Chaetoceros spp. (large) | 30 | Autotrophic | |
| Chaetoceros tenuissimus | 4 | Autotrophic | |
| Chaetoceros throndsenii | 5 | Autotrophic | |
| Cyclotella sp. | 7 | Autotrophic | |
| Cylindrotheca closterium | 6 | Autotrophic | |
| Dactyliosolen fragilissimus | 37 | Autotrophic | |
| Guinardia striata | 35 | Autotrophic | |
| Eucampia zodiacus var. cylindricornis | 18 | Autotrophic | |
| Hemiaulus sp. | 25 | Autotrophic | |
| Lauderia annulata | 30 | Autotrophic | |
| Leptocylindrus convexus | 11 | Autotrophic | |
| Leptocylindrus cf. danicus | 11 | Autotrophic | |
| Lithodesmium variabile | 23 | Autotrophic | |
| Pseudo-nitzschia cf. delicatissima | 6 | Autotrophic | |
| Pseudo-nitzschia fraudulenta | 12 | Autotrophic | |
| Pseudo-nitzschia galaxiae | 5 | Autotrophic | |
| Pseudo-nitzschia multistriata | 7 | Autotrophic | |
| Pseudo-nitzschia cf. pseudodelicatissima | 7 | Autotrophic | |
| Pseudosolenia calcar-avis | 85 | Autotrophic | |
| Skeletonema pseudocostatum | 7 | Autotrophic | |
| Thalassionema nitzschioides | 10 | Autotrophic | |
| Thalassiosira rotula | 25 | Autotrophic | |
| Undetermined pennate diatom | 13 | Autotrophic | |
| Dinoflagellates | Alexandrium pseudogonyaulax | 47 | Mixotrophic |
| Alexandrium sp. | 22 | Mixotrophic | |
| Blepharocysta splendor-maris | 21.8 | Autotrophic | |
| Dinophysis sacculus | 23.8 | Mixotrophic | |
| Gyrodinium sp. 1 | 25 | Heterotrophic | |
| Gyrodinium sp. 2 | 60 | Heterotrophic | |
| Heterocapsa sp. | 13.9 | Mixotrophic | |
| Kofoidinium velleloides | 120 | Heterotrophic | |
| Lessardia elongata | 8.8 | Heterotrophic | |
| Lingulodinium polyedra | 40.7 | Mixotrophic | |
| Micracanthodinium sp. | 16.6 | Autotrophic | |
| Oxytoxum scolopax | 19.3 | Heterotrophic | |
| Phalacroma sp. | 27.1 | Heterotrophic | |
| Phalacroma rotundatum | 45 | Heterotrophic | |
| Pronoctiluca spinifera | 28 | Heterotrophic | |
| Prorocentrum compressum | 24.9 | Autotrophic | |
| Prorocentrum dentatum | 18.5 | Autotrophic | |
| Prorocentrum gracile | 24.7 | Mixotrophic | |
| Prorocentrum redfieldii | 13.8 | Autotrophic | |
| Protoperidinium cf. longipes | 60 | Heterotrophic | |
| Protoperidinium diabolus | 35.4 | Heterotrophic | |
| Protoperidinium oblongum | 85 | Heterotrophic | |
| Protoperidinium sp. | 30.5 | Heterotrophic | |
| Pyrophacus horologium | 64 | Autotrophic | |
| Scrippsiella sp. | 25 | Mixotrophic | |
| Tripos furca | 47.9 | Mixotrophic | |
| Tripos pentagonus | 55.6 | Autotrophic | |
| Tripos sp. | 41 | Mixotrophic | |
| Undetermined athecate dinoflagellates <15 μm | 12.5 | Multiple | |
| Undetermined athecate dinoflagellates 15–30 μm | 22 | Multiple | |
| Undetermined athecate dinoflagellates >30 μm | 40 | Multiple | |
| Undetermined thecate dinoflagellates <15 μm | 12.5 | Multiple | |
| Undetermined thecate dinoflagellates 15–30 μm | 22 | Multiple | |
| Undetermined thecate dinoflagellates >30 μm | 40 | Multiple | |
| Flagellates | Chrysochromulina sp. | 6.7 | Mixotrophic |
| Diplostauron pentagonium | 6.6 | Autotrophic | |
| Dictyocha sp. | 15.1 | Mixotrophic | |
| Leucocryptos sp. | 6.6 | Mixotrophic | |
| Oltmannsiellopsis viridis | 8.1 | Autotrophic | |
| Pseudochattonella cf. verruculosa | 18.5 | Autotrophic | |
| Pyramimonas sp. | 5.3 | Autotrophic | |
| Tetraselmis sp. | 7.5 | Mixotrophic | |
| Undetermined Cryptophyceae | 5.9 | Mixotrophic | |
| Undetermined Euglenophyceae | 13.8 | Mixotrophic | |
| Undetermined flagellates 5–10 μm | 7.5 | Multiple | |
| Undetermined flagellates 10–15 μm | 12.5 | Multiple | |
| Ciliates | Mesodinium rubrum | 17 | Mixotrophic |
| Tontonia sp. 1 | 50 | Mixotrophic | |
| Tontonia sp. 2 | 22 | Mixotrophic | |
| Undetermined aloricate ciliates <15 μm | 10 | Heterotrophic | |
| Undetermined aloricate ciliates >30 μm | 40 | Heterotrophic | |
| Undetermined Tintinnidae | 90 | Heterotrophic |
3.2 Feeding rates
The copepod clearance rates (Table S1) in pre-treatment conditions at T0 did not differ between M1 and M4 (t-test, p > .05), but they were significantly different between M1 and M5 under treatment conditions at T6 (t-test, p < .05). Despite differences in the clearance rates and cell-specific ingestion rates (Table S1), the C ingestion rates did not differ significantly between treatments within each experiment. In fact, the C ingested by C. typicus in pre-treatment conditions (T0) was similar in the two mesocosms, being 1.2 ± 0.5 μg C ind.−1 d−1 in M1 and 1.9 ± 0.4 μg C ind.−1 d−1 in M4 (Figure 3a, t-test, p > .05). After nutrient addition (T6), the ingestion rates were 4.2 ± 1.2 μg C ind.−1 d−1 in M1 and 3.6 ± 0.5 μg C ind.−1 d−1 in M5 containing microplastics, without significant differences (Figure 3a, t-test, p > .05). At T0 (Figure 3b), dinoflagellates accounted for most of the C. typicus diet (50% in M1 and 48% in M4), followed by flagellates (34% in M1; 16% in M4). Diatoms contributed to the ingested C similarly in M1 (13%) and M4 (11%), whereas ciliates were responsible for higher C ingestion in M4 (26%) than in M1 (3%). At T6 (Figure 3b), C. typicus incubated in water from only-nutrient enriched M1, fed more on diatoms (61%) than on dinoflagellates (25%) and flagellates (13%), while the feeding on ciliate C was negligible (2%). In the diet with food from microplastic-supplied M5, ciliates dominated (43%), diatoms and dinoflagellates contributed less but similarly (30% and 27%), while flagellates were not detected (0%).
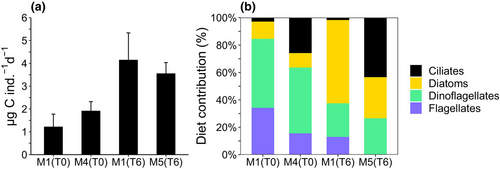
Under pre-treatment conditions (T0), the species in the copepod diet were the same that contributed to the environmental C biomass (Figure S1). In particular, in M1 the main supply to the C. typicus diet was due to P. cf. verruculosa, intermediate- and large-sized athecate dinoflagellates, and small and large thecate dinoflagellates. In M4, the C source was mainly represented by large and intermediate athecate dinoflagellates, intermediate thecate dinoflagellates, P. cf. verruculosa, flagellates >10 μm, and large spherical and elliptical ciliates. In treatment conditions (T6), in M1 the ingested C was dominated by diatoms, in different proportion compared to the diatom abundance in the environment. The most notable taxa were colonial diatoms, such as T. nitzschiodes, large Chaetoceros spp., C. affinis, and Leptocylindrus cf. danicus, but also athecate dinoflagellates contributed to the ingested C. In M5, dietary C was mainly acquired through the least abundant taxa, i.e., large spherical and conical ciliates, large athecate dinoflagellates and intermediate- and large-sized diatoms organized in colonies (Chaetoceros spp. and C. affinis).
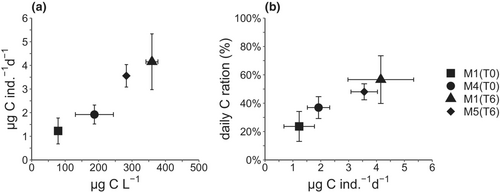
The ingestion rates of C. typicus increased with the C concentration following a linear relationship (p = .0016, R2 = .77, on log-transformed data considering all replicates) in all mesocosms (Figure 4a). The daily C ration did not differ significantly between the pre-treatment mesocosms (t-test, p > .05), increasing from 24 ± 11% in M1 to 37 ± 8% in M4 (Figure 4b). Under the treated conditions, the daily C ration accounted for 57 ± 17% in M1 enriched with only nutrients and 48 ± 6% in M5 enriched also with microplastics. However, the difference in the daily ration between the two latter mesocosms was not significant (t-test, p > .05) and reflected the C biomass present in the initial prey composition.
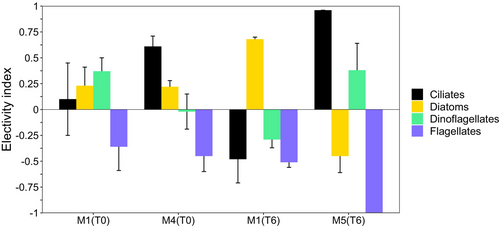
The electivity index (Figure 5) showed that in pre-treatment conditions (T0) in M1 C. typicus selected dinoflagellates, diatoms and ciliates (+0.37, +0.23, and + 0.1, respectively), whereas they avoided flagellates (−0.4). In M4, ciliates (+0.61) and diatoms (+0.22) were selected, whereas flagellates were not preferred (−0.4) and only dinoflagellates were ingested according to their presence (−0.02), in significantly different fashion from M1 (t-test, p < .05). The differences in electivity for the other prey types between the two pre-treatment mesocosms were not statistically significant (t-test, p > .05). After nutrient addition (T6), in M1 diatoms were preferred (+0.7) to dinoflagellates, ciliates and flagellates (−0.3, −0.5, −0.5, respectively). In the presence of microplastics in M5, significantly different electivity emerged with respect to the only-nutrient counterpart (M1). In fact, a remarkable preference for ciliates (+0.96; t-test, p < .001) and dinoflagellates (+0.38; t-test, p < .05) was observed, together with negative selection of diatoms (−0.48; t-test, p < .001) and total avoidance of flagellates (−1.0; t-test, p < .001).
3.3 Microplastic ingestion
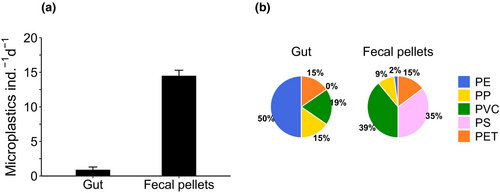
Microplastics present inside the C. typicus individuals after the enzymatic digestion accounted for 0.9 ± 0.4 particles ind.−1 (Figure 6a). Fecal pellets contained 2.1 ± 2.2 particles each, and since we observed a mean production of 6.7 fecal pellets ind.−1 d−1 (data not shown), we estimated that each copepod egested nearly 14 plastic particles per day (Figure 6a). PE was the most common plastic type found in C. typicus gut (50%), followed by PP (19.2%), and PVC, and PET (both 15.4%) (Figure 6b). PS particles were not detectable under the microscope because of their faint colour (light violet-transparent) and optical reflection, similar to that of chitinous C. typicus remains. PP and PS were the most abundant polymers in fecal pellets (39% and 35.2%, respectively), followed by PVC (14.8%), PET (9%), and PE (2%) (Figure 6b). Some plastic types were not evenly distributed between the gut and fecal pellets (e.g., PE, Figure 6b). Occasionally, fibres were also found in the fecal pellets, likely present as contaminants in the seawater, but they accounted for a low proportion (6.7%) of the total microplastics ingested. The microplastics found in the fecal pellets ranged from 0 to 6 particles pellet−1 and measured an average of 20 μm in length (Figure 7).
4 DISCUSSION
4.1 Feeding responses of Centropages typicus to natural food and microplastics
A change in protist biomass and composition available as food for C. typicus occurred between the first and second experiments of our study: small flagellates and dinoflagellates present at T0 were replaced by diatoms after nutrient addition at T6 in both treated mesocosms as well as in the open waters (D. Sarno, unpublished data). However, in the presence of microplastics, the diatom community biomass was dominated by smaller-sized species. It is possible that differential herbivory by nano- and micrograzers in the different mesocosm groups (M1 vs. M4 at T0) might have induced a trophic cascade (Calbet et al., 2012), thus shaping a different community composition in M1 and M5 at T6. Although functionally different across treatments, the experimental food environments at T6 were comparable in terms of C, thereby providing C. typicus with similar food biomass.
C. typicus ingested a daily amount of C in proportion to the available food C regardless of the prey spectrum. Our estimates of clearance rates and daily rations are in agreement with previous studies that investigated the feeding rates of C. typicus on natural protist communities (Broglio et al., 2004; Calbet et al., 2007; Smith & Lane, 1988). The food concentration available to copepods throughout the experiments (79–360 μg C L−1) and the resulting daily rations (24–57%) indicate that copepods were in non-saturating food conditions (<1000 μg C L−1) (Olivares et al., 2020; Smith & Lane, 1985; Traboni et al., 2021) neither before nor after the nutrient addition. No significant difference in C intake was observed in the presence of microplastics, likely because of the ability of the omnivorous C. typicus to exploit different prey items through selection and switch from suspension feeding to ambush strategies, thus increasing the chance of prey intake (Alcaraz et al., 2007; Calbet et al., 2007). Ambush feeding can be more suitable when rheotactic prey are in the proximity of the predator, whereas non-motile prey (e.g., diatoms), which generate no hydrodynamic disturbance, are more likely to be captured through suspension feeding mode (Almeda et al., 2018). Switching feeding modes enable copepods to meet their metabolic needs by diversifying their prey types according to the particle environment. The limited encounter probability with prey experienced by ambush feeders is usually balanced by the higher nutritional quality of their prey, which is often represented by large dinoflagellates or ciliates with quick escape responses (Kiørboe et al., 2010). Although small (∽5 μm) diatoms (Chaetoceros spp.) constituted the highest fraction of C biomass in microplastic-enriched water, they are too small for an effective capture by mesozooplanktonic grazers, including C. typicus (Calbet et al., 1999; Hansen et al., 1994). In this study, C. typicus C intake increases for larger sized prey (15–60 μm), which explains the negligible ingestion of abundant small centric diatoms prevailing in seawater supplied with microplastics.
The dominance of small flagellates observed in the pre-treatments (M1 and M4 at T0) and of small diatoms after microplastic addition (M5 at T6) might have driven the establishment of a multilevel and less efficient food web. In these conditions, mixoplankton and protozooplankton are known to play a major role as grazers of small protists and bacteria (Landry & Calbet, 2004; Pomeroy, 2001; Sherr & Sherr, 2002). In contrast, in productive nutrient-rich waters, copepods are the dominant consumers of phytoplankton, which are often represented by larger bloom-forming diatoms (Saiz & Calbet, 2011). In response to the change in the composition of the food environment, the diet of C. typicus changed accordingly.
Different food spectra characterised the C. typicus diet throughout the experiments. As expected, the diverse and less abundant food available in pre-treatment conditions determined a mixed and rather unselective diet. In contrast, in the only-nutrient treatment (M1 at T6), C. typicus met its metabolic requirements with highly abundant large diatoms. In line with our observations, C. typicus and congeneric species have been shown to be unselective when nutritional stimuli are scarce (Cowles & Strickler, 1983; Vincent & Hartmann, 2001), according to the optimal foraging theory (DeMott, 1989, 1995). However, when exposed to microplastics (M5), C. typicus strongly preferred the least numerous prey groups (i.e., large ciliates and dinoflagellates, ~70% of C ingested), showing a less diversified, but highly selective food intake. In the presence of multiple prey types, C. typicus has been reported to prefer food in the following order: ciliates > dinoflagellates > diatoms > flagellates (Calbet et al., 2007). This preference order also emerged in our study and it was likely related to the ciliate energetic value (Calbet & Saiz, 2005). A ciliate-based diet provided C. typicus with the highest daily ration (Broglio et al., 2004; Wiadnyana & Rassoulzadegan, 1989) yielding optimal developmental performance, growth, and egg production (Bonnet & Carlotti, 2001). Diet change towards ciliates and feeding compensation mechanisms may also be linked to the stoichiometric and biochemical fingerprints of the prey in relation to the consumer's needs (Calbet et al., 2007; Traboni et al., 2021). During some mesocosm studies, ciliates became more important in the diet of Temora longicornis as soon as the morphology and/or size of the dominant phytoplankton became less suitable for the copepods (Löder et al., 2011). Another study highlighted that the copepod Acartia tonsa showed a tendency to feed on the prey conferring the highest intake of the limiting nutrient (Meunier et al., 2016). The preference for ciliates we observed is also strictly linked to the motility of this group, benefitting raptorial predators, such as C. typicus (Kiørboe & Saiz, 1995; Klein Breteler et al., 1999; Vincent & Hartmann, 2001). This may explain our observed negative selection for very small diatoms (Chaetoceros spp., ~5 μm), far below the lower size limit of the prey size spectrum (>10 μm) for C. typicus (Calbet et al., 1999). As previously mentioned, this diet remodulation can be supported by the well-known ability of this calanoid to alternate between different feeding behaviours, thus posing no impairment to the daily C ration even in the presence of microplastics.
4.2 Factors affecting microplastic ingestion and egestion
Less than 1 microplastic particle was found in each copepod after enzymatic digestion, and the number of microplastics identified and counted in fecal pellets was only slightly higher, indicating that microplastics were ingested and then egested (defecated). Considering a clearance rate of 133 mL ind.−1 d−1 (Table S1), a C. typicus individual was expected to ingest ~14 microplastics per day. Copepods were exposed to ~120 particles in the total incubation volume (1130 mL), together with 2.56 × 107 cells L−1 of natural food. Hence, it seems that C. typicus neglected plastics, either because recognised as non-valuable food source or because plastics were too diluted with respect to the natural prey cells, despite the fact that the concentration of microplastics used in our study was five orders of magnitude higher than that estimated from Manta samples in the Tyrrhenian Sea (2.93 items m−3 corresponding to about 3 × 10−3 items L−1, Pedrotti et al., 2022). The concentration we used was lower than those used in other laboratory studies, where clear effects of microplastics on C. typicus have been reported on ingestion, respiration, and egestion (Cole et al., 2016; Svetlichny et al., 2021). Although the microplastics added to the mesocosms during our study ranged from 20 to 1000 μm (including the full prey size spectrum for C. typicus), we mainly detected the smallest-sized particles in fecal pellets (~20 μm) with some rare exceptions (50–100 μm fibres). The microplastics found in C. typicus fecal pellets were therefore channelled to the mouth through the feeding appendages, likely during the creation of feeding currents (Price & Paffenhöfer, 1985). The possible avoidance of larger microplastics, the switching feeding mode and the selective nature of this copepod species may all explain the absence of impaired feeding, gut blockage and reduced reproductive potential (Y. Carotenuto, pers. comm.) in this study.
Other calanoids have been reported to deliberately avoid PS spheres when offered as food (Xu et al., 2022), especially when natural prey cells were added as a nutritional source (Huntley et al., 1983). However, the copepod feeding strategy and the size of the plastics are important factors to consider (Bai et al., 2021). Prey of the same size range of flagellates are more easily cleared and ingested than larger ones e.g., fast-escaping ciliates, by suspension feeders, whereas non-motile plastics will not be detected hydrodynamically by ambush feeders. Similarly to our study, recent field experiments have shown that T. longicornis does not ingest microplastics, but the size range of the plastic analysed was >200 μm, which is perhaps too large for active copepod uptake via feeding currents (Outram et al., 2020). The feeding-current feeder T. longicornis rejected up to 80% of the microplastic particles offered when feeding in the presence of natural food, and showed no reduced grazing on the natural protistan population (Xu et al., 2022). In contrast, a decrease in clearance rate was reported in Calanus pacificus (Huntley et al., 1983) and Acartia clausi (Ayukai, 1987) when PS beads (15–17 μm) were provided at increasing concentrations together with diatoms. However, in both cases, the authors reported that the copepods preferred large dinoflagellates when their diet was supplemented with plastic beads. Similarly, it can be hypothesised that, when microplastics are present together with ciliates and dinoflagellates, as observed in the present study, the ingestion of these nutritional prey may compensate for any microplastic particle ingested by C. typicus. Recent evidence supports our findings in terms of food selection and ingested microplastic size. Calanus helgolandicus fed on a mixture of Thalassiosira rotula (24 μm), Prorocentrum micans (35 μm) and Dunaliella tertiolecta (11 μm) showed lower species-specific clearance rate and changed preference for the prey offered based on their resemblance in shape and size to the microplastic fibres/particles offered (Coppock et al., 2019). Moreover, the authors reported that the preferred size range of ingested-egested PE microplastics was 10–20 μm, probably unselectively entrained within copepod feeding-currents (Coppock et al., 2019).
Microplastic age and conditions (e.g., presence of biofilm) play important roles in the likelihood of being ingested by consumers, many of which detect prey based on mechano- or chemoreception (Bai et al., 2021; Carbery et al., 2018). Bacterial and microalgal coated surface on microplastics may interfere with the discrimination between natural food and microplastic beads, leading to the ingestion of aged PS particles (10–15 μm) on the basis of their microbial flavour as it emerged in freshwater (Kerfoot & Kirk, 1991) and marine calanoids (Calanus finmarchicus and Acartia longiremis) (Vroom et al., 2017). It may be that the plastics offered to C. typicus were not weathered enough and colonized to be perceived as a natural prey, leading the copepods to ignore and/or reject them when confronted with abundant natural food. Furthermore, ingestion and rapid regurgitation of bacterially-coated latex beads (15 μm) have been reported in Eurytemora affinis (Powell & Berry, 1990). We cannot rule out that, in our study, C. typicus might have captured but promptly rejected some of the microplastics of the larger size class, fully ingesting and defecating only the smallest ones.
5 CONCLUSIONS
The present study on Centropages typicus grazing contributes to improving our understanding of the impact of microplastics on the feeding of a common and abundant marine copepod. Despite the increasing number of physiological and ecotoxicological effects reported in zooplankton exposed to microplastics, and particularly copepods (Bai et al., 2021; Botterell et al., 2019) including C. typicus (Cole et al., 2013; Svetlichny et al., 2021), the present results do not reveal any significant impairment to C. typicus feeding performances or survival in the short term (24–48 h exposure), in agreement with previous observations (Beiras et al., 2018).
In summary, the results of our incubation experiments show that: (1) in lower nutrient load, the copepods fed on natural plankton assemblages in proportion to food availability, likely through suspension feeding; (2) in more eutrophic conditions, copepods had a wider food choice in terms of quality and abundance and the majority of diet C was supplied by large and colonial diatoms; (3) the presence of abundant microplastics of wide type and size range (20–1000 μm) did not affect quantitatively neither the feeding rates in terms of ingested C nor the daily ration. However, in the presence of nutrients and microplastics, the available food was dominated by small diatoms, but they were neglected by C. typicus, which selected dinoflagellates and ciliates, likely acquired by raptorial behaviour. We hypothesize that the switching feeding modes and broad-spectrum diet enabled C. typicus to perform efficiently also in the presence of microplastics. (4) Finally, the number and the small size of microplastics recovered in the copepod gut (ingested) and fecal pellets (egested) suggest avoidance of larger particles and passive capture of the tiny particles that are closer to the optimal prey size for this copepod species.
Our findings highlight the ability of this copepod species to adapt to variable food environments and cope with disturbances, at least within a short time interval and at the microplastic concentration we tested.
AUTHOR CONTRIBUTIONS
MGM designed the experiment and shared equal contribution during the experimental procedure with CT. CT conducted the data acquisition, data analysis, graphical representation and writing of the manuscript. MGM, DS, and MR participated in data analysis, data interpretation, discussion and revision of the manuscript.
ACKNOWLEDGMENTS
We thank C. Brunet (Stazione Zoologica Anton Dohrn) for conceiving and directing the mesocosm experiment of the INPUT project, allowing us to conduct this parallel study. We acknowledge E. Rastelli (Università Politecnica delle Marche) for supplying the microplastics used in this study. We are grateful to I. di Capua, F. Margiotta and V. Manna for helping with sampling, copepod elemental analysis and microplastic concentration estimates. We thank E. Saiz (Institut de Ciències del Mar-CSIC) for the support with the statistical analysis. We are grateful to the reviewers for the constructive comments and suggestions.
FUNDING INFORMATION
This work was funded by Stazione Zoologica Anton Dohrn.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. This study did not involve any endangered or protected species.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available upon manuscript acceptance.




