From metabarcoding time series to plankton food webs: The hidden role of trophic hierarchy in providing ecological resilience
Roberta Congestri and Domenico D’Alelio contributed equally.
Abstract
The advent of metabarcoding (metaB) in aquatic ecology has provided a huge amount of information on plankton biodiversity worldwide. However, the large datasets obtained with that approach are still partially explored, especially for what concerns the study of trophic interactions and food webs. In this study, we analysed a metaB time series from the Long-Term Ecological Research station MareChiara (LTER-MC) in the Gulf of Naples, Mediterranean Sea, Italy, to describe the link between plankton diversity and food-web structure. We derived co-occurrence networks from metaB time series, identified putative trophic interactions among co-occurrences based on biological information (body size and trophic habit) available for planktonic organisms detected by metaB, and converted co-occurrence networks into conceptual models of food webs. The latter showed structural properties resembling ecological processes, because network modularity (the presence of semi-independent sub-networks) paralleled trophic hierarchy (the dimensional difference between predator and prey). We also analysed the role of planktonic organisms in maintaining network modularity. The largest predators occupied distinct modules, suggesting niche partitioning, whereas the smallest preys worked as fundamental connectors between larger predators (and different modules). Overall, the presence of trophic hierarchy and modularity shown herein supports the view of the high ecological resilience of plankton, pursued via food-web rewiring, to environmental shifts.
1 INTRODUCTION
Plankton are aquatic organisms floating passively with currents (Hensen, 1887). They include dimensionally and phylogenetically distant species (Boyce et al., 2015; Ruggiero et al., 2015), spanning from unicellular organisms to metazoans, which however acquired similar functions in the course of evolution (Delmont et al., 2022). Intertangled by ecological interactions, plankton play a crucial role in several ecological processes, such as carbon and nutrient storage (Chakraborty et al., 2020; Falkowski, 2012; Worden et al., 2015) and the transfer of matter and energy to higher trophic levels (e.g., fishes; Lomartire et al., 2021; Worden et al., 2015).
Plankton diversity studies have traditionally relied on sample collection and direct observation. However, “traditional” biomonitoring cannot detect all species due to the low abundance of most plankters and the damage caused by preservation methods that may hamper their correct taxonomic identification (Stoeck & Stock, 2010; Stoecker et al., 1994). This is particularly true for the microbial component of plankton, such as protists, whose ecology is usually less explored compared with zooplankton (e.g., Persad & Webber, 2014; Vassallo et al., 2022; Webber et al., 2015). The development of omics tools like metabarcoding (metaB) analysis of water samples has improved the detection of even the smallest planktonic organisms and the study of their biodiversity (e.g., Louca et al., 2016; Sunagawa et al., 2015). DNA metaB is defined as high-throughput sequencing of marker genes, allowing multi-taxon identification from the total DNA of an environmental sample (Taberlet et al., 2012). However, the large datasets obtained with this approach are still underexploited in ecological studies, such as those aiming to reconstruct planktonic food webs.
Food webs, that is, the tangled ensemble of trophic interactions, also known as trophic networks, are theoretical and numerical models, offering a powerful tool for integrating biodiversity and ecosystem functioning (Thompson et al., 2012). They represent a particular type of ecological network where species (network nodes) establish trophic interactions (network links, or edges, directed from prey to predators; Cohen, 1977; Ings et al., 2009; Moya-Laraño, 2011). The arrangement of nodes and edges in a food web (i.e., the food-web “structure”) follows common rules in both terrestrial and aquatic systems which are less noisy at higher level of aggregation (Giacomuzzo & Jordán, 2021). Among the main functional rules, food-web structure can be characterized by a positive relationship between body size and trophic levels (TLs), that is, the so-called trophic hierarchy (for marine systems, see Riede et al., 2011; Russo, Casella, et al., 2022; Sommer et al., 2018; Woodward et al., 2005).
Food webs also show modularity, that is, a property of networks that consists of the non-random distribution of edges leading to the formation of modules (Newman, 2006). In a modular network, groups of nodes, or modules, are more connected with each other than with nodes belonging to other modules. In the ecological context, modularity can increase the stability of interaction networks (Grilli et al., 2016) by reducing the cascade propagation of negative effects arising from environmental shifts and species extinction (Montoya et al., 2015; Stouffer & Bascompte, 2011). Food-web modularity stems from dimensional, habitat, and behavioral differences between predators and preys (Guimerà et al., 2010), reflects ecological and evolutionary processes (Delmas et al., 2018; Dormann et al., 2017), and can enhance food-web resilience (Grilli et al., 2016; Montoya et al., 2015; Stouffer & Bascompte, 2011).
In this study, we tested the power of metaB to link plankton diversity and food-web structure in the Gulf of Naples (GoN, Tyrrhenian Sea, Mediterranean Sea). This is relevant because diversity is thought to play an important role in keeping plankton communities ecologically stable and resilient (D’Alelio et al., 2016; Mazzocchi et al., 2012; Zingone et al., 2019). In the GoN, abundance and biomass data were already successfully used to provide an approximate view of plankton food webs (D’Alelio et al., 2015, 2016) and their changes under different environmental conditions (D’Alelio et al., 2019), analogously to other systems (e.g., Trombetta et al., 2020). However, those studies investigated plankton by considering a limited number of groups, which were aggregated based on morpho-functional and taxonomical criteria, leading to potentially biased results (Giacomuzzo & Jordán, 2021). In fact, the use of metaB data would allow a better description of community composition, leading to a more effective inference of potentially trophic interactions and, therefore, of the structure of the trophic network.
Herein, we studied time-series data from the Long-Term Ecological Research site MareChiara (LTER-MC, 40°48.5' N, 14°15' E) in the GoN, where plankton are collected and analysed at high taxonomical resolution, weekly, with microscopy methods (Ribera d'Alcalà et al., 2004; Zingone et al., 2019, 2022). We analysed a metaB dataset obtained by high-throughput sequencing (HTS) of PCR amplicons of the V4 and V9 regions of the 18S (small subunit) ribosomal RNA gene, allowing a high taxonomic resolution at the genus level, from 48 samples collected over a three-year period at LTER-MC, between January 2011 and December 2013. Different subsets of this dataset were already investigated in other studies. Eight out of the 48 samples mentioned above were analysed for protist biodiversity by Piredda et al. (2017); the mesozooplankton V4 fraction of the whole dataset was studied by Di Capua et al. (2021); the dinoflagellate and diatom V4 fractions were analysed by Mordret (2018) and by Caracciolo et al. (2022), respectively; diatom parasites were analysed by Reñé et al. (2022); and, finally, the V4 of the diatom family Chaetocerotaceae was analysed in Gaonkar et al. (2018).
Our aim was to analyse the metaB dataset mentioned above and integrate it within an ecological framework. Specifically, we: (1) derived new libraries of amplicon sequence variants (ASV) from raw data and assigned them taxonomically; (2) correlated the time-course of these ASVs; (3) aggregated multiple correlations into co-occurrence networks; (4) among co-occurrences detected, we identified all the putative trophic interactions based on already published biological information, that is, body sizes and trophic habits of planktonic organisms; (5) integrated putative interactions into a conceptual model for the planktonic food web; and (6) tested the planktonic food web for the presence of trophic hierarchy and modularity. Finally, we characterized the roles played by planktonic organisms in keeping food-web structure, stability, and resilience in the GoN.
2 METHODS
2.1 Data generation processing
Surface water samples for environmental DNA extraction were collected at the LTER-MC station in the GoN on 48 sampling dates from January 2011 to December 2013. Methods for sample filtration and DNA extraction are detailed in Piredda et al. (2017). PCR amplification of 18S V4 and V9 regions, library preparation, and sequencing were performed as described in Piredda et al. (2017). Briefly, the V4 region of 18S SSU rRNA gene was amplified in a first-round PCR using modified BioMarKs primers (Piredda et al., 2017; Stoeck et al., 2010), with 5' tails corresponding to Nextera transposase primer and 1U of Phusion High-Fidelity DNA polymerase (New England Biolabs Inc) in 25 μl reactions. Purified amplicons were used as templates for the second round PCR using Nextera dual-indexed primers and Illumina P5 and P7 primers. After purification and quantification of second-round PCR products, amplicons were pooled and paired-end sequenced on a MiSeq platform (2 × 250 bp). The same procedures were followed for the V9 region.
2.2 Denoising and taxonomic assignment
After assessment of read quality with FastQC (Andrews, 2010), primers were trimmed from demultiplexed reads using cutadapt (Martin, 2011). Contig assembly and denoising were performed using the dada2 R library (Callahan et al., 2016), using the default parameters described in the MiSeq tutorial, except for the number of accepted mismatches in the overlap region (8 for V4 and 9 for V9; default no mismatches). This allowed to improve the sequence quality in the overlap regions and decrease the number of reads discarded due to the presence of ambiguities (Ns).
Amplicon sequence variants (ASVs) derived from the denoising procedure were classified with BLAST against the PR2 v4.12 reference database (Guillou et al., 2012), integrated with 169 additional sequences, mostly from taxa regularly found in the GoN; BLAST was set to return a single match at >90% similarity; 15 ASVs deemed of particular interest for the present study, but assigned to reference sequences of low taxonomic resolution, were re-assigned with BLAST against the GenBank NR nucleotide database at >95% similarity, setting no limits on the number of matches returned. The highest-scoring matches with the best taxonomic resolution were then selected among the returned results.
Before deriving the co-occurrence networks, the dataset underwent some filtration steps to remove: (1) unassigned ASVs (n = 3,538 and 13,941, accounting for 9% and 42% of V4 and V9, respectively); (2) contaminating ASVs assigned to the benthic Anellida Hydroides elegans (236 and 10 ASVs removed for V4 and V9, respectively); and (3) the ASVs with a taxonomical resolution lower than the genus level, that is, the taxonomical level used in the networks (n = 24,204 and 11,595, accounting for 62% and 35% of V4 and V9, respectively).
In order to avoid the over-representation of zero values (no presence; Röttjers & Faust, 2018), the two matrices were further filtered by selecting for the analysis only the ASVs with reads occurring in more than 30% of samples (Connor et al., 2017; Lentendu & Dunthorn, 2021). Lastly, the most frequent genera in the V4 and V9 datasets were further checked to remove terrestrial and strictly benthic genera.
2.3 Co-occurrence network generation
Co-occurrence networks were derived using the CoNet app available in Cytoscape and following standard methodology (Shannon et al., 2003; Faust & Raes, 2016). The V4 and V9 matrices were log-transformed, and each column was normalized by its sum to avoid the inference of spurious correlations caused by differences in the sequencing depth of the samples (Faust & Raes, 2016; Röttjers & Faust, 2018). Two correlation methods (Spearman and Pearson) and two dissimilarity distances (Bray-Curtis and Kullback-Leibler) were employed. The combination of different methods was used to reduce the erroneous correlation prediction associated to the similarity metrics, which are influenced by the compositionality and the presence of double zeros, and the dissimilarity metrics, which are sensitive to outliers (Faust & Raes, 2016; Friedman & Alm, 2012). Only the positive and negative interactions supported by all metrics and with a merged p-value < .05, after Benjamini-Hochberg's correction and testing through 1000 permutations, were retained in the final V4 and V9 co-occurrence networks (Tables S1 and S2).
2.4 Biological properties of nodes
We characterized the biological properties of all genera included in the final V4 and V9 co-occurrence networks mentioned above by acquiring information from about 450 scientific articles and from the World Register of Marine Organisms (Costello et al., 2013). We associated with each node: (1) a body size (reported as median values of those found in the literature) subdivided into four different size classes, that is, pico- (< 2 μm), nano- (2–20 μm), micro- (20–200 μm) and mesoplankton (200-2000 μm; Sieburth et al., 1978); (2) a lifestyle (either holo- or meroplanktonic); and (3) a trophic and dietary habit. Furthermore, combining the above-mentioned biological properties, we defined five functional groups: holo- and meroplankton (metazoa), heterotrophic, mixotrophic and autotrophic protists (see Table S3 for further details on the information collected and their bibliographic sources). Based on this background knowledge, we analysed all co-occurrences and identified those representing putative predatory interactions, obtaining two trophic interaction lists, from the V4 and V9 datasets. Moreover, as the two datasets were obtained from the same water samples (they referred to the same community), we combined the respective trophic edge lists shared by V4 and V9 to obtain the final food web. This choice allowed us to consider the whole plankton biodiversity retrieved with the V4 and V9 markers, which can detect different planktonic taxa (Choi & Park, 2020; Tragin et al., 2018).
The food web was built considering the whole V4 and V9 edges lists without separating negative and positive links, that is, using the absolute values of correlations. This choice was guided by the impossibility of associating uniquely the verse of interactions with specific biological processes (D’Alelio et al., 2015; Faust & Raes, 2012; Fuhrman et al., 2015; Posch et al., 2015). Networks were visualized using adjacency matrices produced with R version 4.2.1 (R Core Team, 2017).
2.5 Network hierarchy and modularity
where DCij was the proportion of prey j in the diet of species i, TLj was the trophic level of prey j, and N was the number of prey nodes.
We also explored the node's Degree (D), that is, the total number of edges with other nodes. The Degree metrics describe the centrality of a node in terms of its positional importance based on its direct interactions (Jordán, 2009). This information is ecologically useful and allows to predict the amplitude of the trophic niche of a taxon, because specialist and generalist taxa will show, respectively, lower and higher D values (Kortsch et al., 2019; Scotti & Jordán, 2010). Being the food web composed of directed edges (i.e., interactions goes from preys to predators), the value of D included the inDegree (inD) and outDegree (outD), which represented the number of interactions that a node established as predator or prey, respectively (Koutrouli et al., 2020).
where L(M) was the trajectory of the random walk; m was the number of modules within a network; H(Q) and H(ρi) represented the entropy of the movement between and within modules, respectively; was the rate at which a random walker entered and exited each module; was the fraction of time a random walker spent in each module.
From an ecological perspective, the modules identified by infomap can be associated with the trophic compartments of a community, each of which includes a group of organisms establishing similar trophic interactions (D’Alelio et al., 2019). By means of linear regressions carried out using R (R Core Team, 2017), we analysed the relationship between the mean TL and mean body size weighted by the proportion of functional groups in each module.
2.6 Trophic roles of plankton
To characterize the trophic roles of plankton and their influence in maintaining network structure and trophic hierarchy, we focused on the identification of biological properties relating to each node and the ecological role each node played in a trophic network. To do that, we estimated specific metrics, based on a rationale already applied to plankton (D’Alelio et al., 2019; Liu et al., 2022; Russo, Casella, et al., 2022).
where Dw was the Degree of a node within its module, Dt was the average Degree of all nodes in all of the modules, and σt was the standard deviation of Dt (Deng et al., 2012; Guimerà et al., 2007).
where Dw was the Degree of a node within its module, D was the total Degree of a node, and Nm was the number of modules in the network (Deng et al., 2012; Guimerà et al., 2007).
- peripheral nodes (Z < 2.5 and P < .62), which established few interactions, mainly or totally within their module;
- module hubs (Z > 2.5 and P < .62), which were mainly or totally involved in within-module interactions;
- connectors (Z < 2.5 and P > 0.62), which were highly linked to other modules;
- network hubs (Z > 2.5 and P > 0.62), which were the most central nodes in the network being both module hubs and connectors.
From an ecological perspective, peripheral nodes should be considered as specialists, network hubs, and module hubs as generalists, whereas connectors could be either generalists or specialists (Deng et al., 2012; Guimerà et al., 2007). Network hubs, module hubs, and connectors are important in maintaining network structure and are considered key nodes (Deng et al., 2012; Liu et al., 2022). In particular, module hubs are important in maintaining the stability of network structure by stabilizing their own module, whereas connectors have a crucial role in the energy transfer among different network compartments (Liu et al., 2022). The removal of connectors, which are highly linked with different modules, could exert a larger impact on fluxes within the whole network compared with the removal of module hubs whose effects are more restricted within their module (Guimerà et al., 2007).
All the metrics illustrated above to investigate trophic roles in plankton consider only direct interactions of nodes with their neighbors. However, it was demonstrated that indirect effects can also be important and keystone species can be found also among nodes involved in a few direct interactions (Jordán, 2009). Thus, to consider the importance of indirect effects spreading from plankton nodes to distant nodes too, we calculated topological importance (TI) and trophic overlap (TO).
TO is a measure of trophic specialization, with lower TOs corresponding to higher trophic uniqueness (Jordán et al., 2009). In this way, TO, following an early definition of keystone species, helps to identify those specialist nodes that cannot be easily replaced by other nodes in a food web (D’Alelio et al., 2019). Taking into account the indirect effects alike TI, the TO indicator derives the positional uniqueness of a node expressed as a function of n-steps and a threshold t for interaction strength of am,ij values that separate the strong interactors from the weak ones (Jordán et al., 2009). The sum of all the single values of TO between a node and the others provided the final TO values for the node. TI and TO metrics were estimated with the CoSBiLab Graph software (Valentini & Jordán, 2010) considering n-step length = 3 and a threshold t = 0.02.
3 RESULTS
A total of 21,799,137 and 22,841,380 reads were available for the 18S V4 and V9, respectively, of which 20,698,551 and 21,503,286 reads passed quality filtering. Reads counts at each step of processing for all samples are shown in Tables S4 and S5. The denoising procedure resulted in a total of 39,078 and 33,305 ASVs for the V4 and V9 regions, respectively. The total number of read pairs that could be taxonomically assigned was 17,923,133 and 16,154,208, respectively. After having removed all the unassigned and contaminant ASVs and those with a low taxonomical resolution, the V4 and V9 datasets resulted, respectively, in 11,100 and 7,759 ASVs that were then aggregated to the genus level, obtaining a total of 1,108 genera for the V4 and 1,377 for the V9 region. Lastly, after further filtration of all the genera with a frequency minor or equal to 30% of the samples and those that were not planktonic, we obtained a final input datasets of 163 ASVs aggregated at the genus level in the V4 dataset and 206 in the V9 dataset (available in Russo, Zampicinini, et al., 2022).
Co-occurrence networks derived from the V4 and V9 datasets were composed mainly of protists, accounting for more than 90% of the total nodes. The remaining nodes were zooplankton, which included mainly holoplankton and only one meroplanktonic genus retrieved in both datasets, that is, Mytilus, here assumed to be in its planktonic stage (Figure S1). After revising all the statistically significant co-occurrences, based on the biological information found in the literature for each genus, we found that 51% and 47% of the edges, respectively, in the V4 and V9 networks, were putative trophic interactions (Figure S1). All these edges were congregated in the final food web (Table S6). Within the latter, we found the presence of trophic hierarchy, that is, a parallel increase in the TLs and the body size of the nodes in the whole network (Figure 1a). Moreover, we found the highest concentration of trophic links between nodes of intermediate body sizes (nano- and microplankton; Figure 1a).
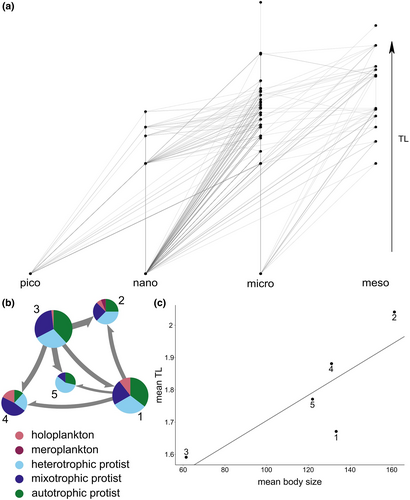
The observed trophic hierarchy was reflected in the modularity of the food web, which showed five different modules (M1–M5) including nodes that established inter-module connections (from a minimum of 8 edges between M1 and M5 to a maximum of 70 connecting M2 and M3; Figure 1b). Such modularity appeared as influenced by the body size and trophic habits of nodes. We found a positive (even though not statistically significant) relationship between the mean TL and the mean body size of the nodes of the modules, with a net difference between M3 (mean body size = 61.28 μm) and M2 (mean body size = 161.03 μm), which presented the lowest and highest mean TLs (1.59 vs. 2.04), respectively (Figure 1c). Other modules with intermediate TLs and body size showed different mean TLs (1.67, 1.88 and 1.77, for M1, 4, and 5, respectively), but their mean body size was almost equal with a slight variation of only 10 μm (Figure 1c).
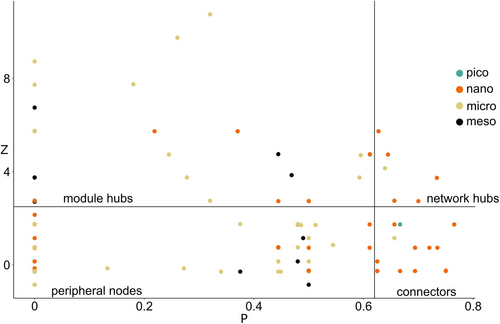
From the analysis of the within-module (Z) and among-module connectivity (P), we detected six network hubs: Biecheleriopsis, Hemiselmis, Lotharella (autotrophic protists), Paragymnodinium, Ansanella, and Lepidodinium (mixotrophic protists). A total of 18 nodes (10%) were connectors, whereas 31 nodes were module hubs (17%). We found that 20 out of 24 nodes presenting a P > 0.62, that is, connectors and network hubs, were nanoplanktonic taxa, 14 of which being strictly autotrophs. No mesozooplanktonic taxa were retrieved among the connector nodes but some of them were module hubs: Acartia, Penilia, and Oithona in M1, Acanthocolla in M2 and Noctiluca in M5. The other module hubs included almost entirely mixotrophic and heterotrophic protists, with a large presence of microplankton (about 50% of the total module hubs; Figure 2 and Figure S2).
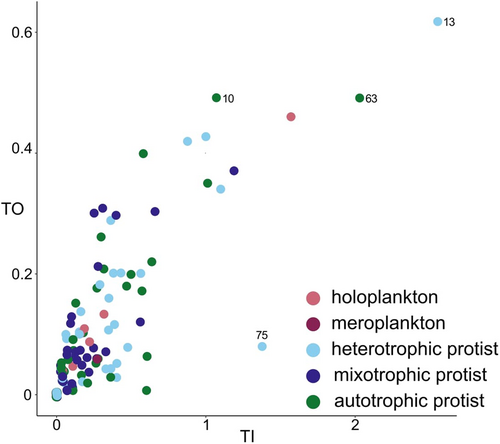
Lastly, based on the positional importance and specialization (TI-TO metrics), we found a large trophic homogeneity within the trophic network, with the presence of a high number of nodes showing a high uniqueness (low TO) and a low topological importance (low TI), with a slight difference between the heterotrophic protists and the mixotrophic ones, which were less important but more unique (Figure 3 and Table S7). Nonetheless, we highlight the presence of some nodes (e.g., Fragilariopsis, node #10; Aplanochytrium, #13 and Amphidoma, #63), showing both high TI and TO and one showing a high TI and a relatively low TO (Helicostomella, #75; Figure 3 and Table S7).
4 DISCUSSION
The advent of metabarcoding (metaB) in aquatic ecology has provided a huge amount of information on plankton biodiversity worldwide. However, the large datasets obtained with that approach are still partially explored, especially for what concerns the study of trophic interactions and food webs. We analysed co-occurrences derived from a metaB time series to build a conceptual model for the plankton food web in the GoN. In the final food-web model, we included the whole 18S V4 and V9 edges lists without separating negative and positive co-occurrences. This not only helped us avoid problems related to the interpretation of ecological interactions, but also focus on the most important associations, either positive or negative. On the one hand, negative co-occurrences may be suggestive of predator–prey relationship (D’Alelio et al., 2015; Faust & Raes, 2012; Fuhrman et al., 2015). On the other hand, positive co-occurrences between nodes may indicate balanced trophic relationships where predator and prey increase simultaneously due to the effect of a favorable environmental condition or in presence of a tri-trophic chain, where the prey involved in the positive links is itself a predator (D’Alelio et al., 2015).
Half of the total co-occurrences were representative of putative trophic interactions, involving mainly unicellular organisms. The arrangement of links among nodes in the final co-occurrence network appeared mainly guided by dimensional differences between predators and preys (Figure 1a and Figure S1), resulting in a characteristic trophic hierarchy like in: (1) other marine communities investigated without biomolecular methods (Pérez-Matus et al., 2017; Rezende et al., 2009; Riede et al., 2011; Scotti et al., 2009), and (2) the Monterey Bay community studied by metaB technique but with a lower taxonomical resolution (taxa studied at the family level; Russo, Casella, et al., 2022).
Moreover, the plankton food web from the GoN was modular and such feature was driven by the body size and trophic habits of planktonic taxa rather than by their seasonality or habitat preference, as shown in other systems (Krause et al., 2003) and, for what concerns the time-aggregation of taxa within specific seasons, in the GoN (Di Capua et al., 2021; Piredda et al., 2017; Ribera d'Alcalà et al., 2004). Our results may have been influenced by the methodology used to derive co-occurrence networks (e.g., filtration of the less frequent taxa to avoid spurious correlations) and by the aggregation at genus level, as the aggregation criterion can affect the results obtained (Giacomuzzo & Jordán, 2021; Jordán et al., 2018). Nonetheless, we highlight that congeneric species often show similar (if not the same) trophic characteristics (body size and diets, among all) and the genus-aggregated metaB data allowed us studying at greater depth the potential inter-dependence of planktonic taxa showing significantly different body sizes and dietary behaviors.
We also stress that our focus was not on the taxa per se but on the links between them and, from our analysis, the assembly of those links was not only ruled by the seasonal succession. As our analysis was based on taxonomical data aggregated at genus level, we found that most planktonic players were always present during the three years of our study, because at this higher level of aggregation, patterns are generally more consistent. A similar result was observed from the metaB data of Helgoland Roads, North Sea, where, behind a recognized seasonality of the local community described at species level, there was evidence that most of the planktonic organisms were present all over the year (Käse et al., 2021).
4.1 Trophic hierarchy behind modularity
Body size and diets are fundamental biological attributes influencing trophic interactions and roles (Polis, 1991), giving origin to the compartmentalization (or modular organization) of a food web (Arim et al., 2010; Borthagaray et al., 2014; Guimerà et al., 2010; Kenitz et al., 2019). The relationships between modularity, body size, and trophic habits in food webs were explained by the large effect of organismal dimension in influencing trophic interactions and consequently inducing niche partitioning within communities (Basset & Angelis, 2007). For instance, it was observed that greater diversity in the body size of predators is reflected in a more diversified trophic niche partitioning, with each predator showing its own optimal prey size (García-Comas et al., 2016; Ye et al., 2013). This condition can play a crucial role in ecosystem stability (Rakowski et al., 2021). By constraining the negative effect of perturbation, propagating them mainly within rather than between modules, modularity provides stability and resilience to food webs (Grilli et al., 2016; Thébault & Fontaine, 2010).
In the plankton food web from the GoN, we found a positive relationship between TL and body size. Moreover, M3 (the food-web module composed of the smallest organisms at the base of the food web) and M2 (the module composed of the top-level consumers with the largest size) established different inter-module interactions: the former included only preys as connectors between modules, the latter only predator nodes, as shown by directional arrows, from preys to predators, in Figure 1b. Yet, the other modules showed less concordance between TL and body size (e.g., M1 and 4): this is not surprising, because plankton encompass a wide range of organisms that can present similar size and trophic habit leading to competitive interactions (Russo, Casella, et al., 2022; Stukel et al., 2021).
Plankton also include organisms presenting similar size but different trophic habit leading to trophic niche partitioning (Latorre et al., 2021; Zamora-Terol et al., 2020). In our work, such case was found in M1 and M4, which showed the same mean body size, but different mean TL. This pattern was driven by the different “cores” of each module, which were made of autotrophic (TL = 1) and mixotrophic protists in M1 and M4, respectively. Mixotrophs, being able to prey on other organisms (Flynn et al., 2019), present TLs > 1, which increased the mean TL of M4. Overall, the diversified presence of resources in different modules has important effects on the plankton consumers. For instance, due to their high nutritional quality (Glibert & Mitra, 2022), the presence of more mixotrophic protists could directly improve the trophic transfer to the highest TLs (Traboni et al., 2021).
4.2 Trophic roles as drivers of modularity
Our study highlighted the contribution of network nodes in keeping food-web structure, including modularity. Nodes of different body sizes played different roles in the network: (1) micro- and mesoplankton played mainly as intra-modular hubs; (2) nanoplankton were more important in connecting different modules.
The first result provides evidence of niche partitioning between top-level planktonic consumers, which tend to occupy different modules (e.g., Rezende et al., 2009). More specifically, the largest plankton, that is, predators occupying the highest TLs, mainly eat a selected group of preys belonging to their own module, thus reducing trophic competition with predators in other modules. We observed examples of this type of trophic niche partitioning in all modules, for instance, the microplanktonic dinoflagellate Akashiwo (a top predator in M3) shared only one prey (Pelagomonas) with other top consumers of other modules (the microplanktonic dinoflagellate Lepidodinium and mesoplanktonic dinoflagellate Noctiluca, M4 and M5, respectively); similarly, the dinoflagellate Gyrodinium in M1 shared only two preys (Gymnodinium and Biecheleria) with Lepidodinium from M4; finally, the dinoflagellate Torodinium (M2) did not present any prey in common with other top consumers in other modules. This apparently non-random arrangement of trophic links contributed to shape the modularity of the plankton network.
The second result—that nanoplankton were more important in connecting different modules—suggests a central role played by autotrophic protists within planktonic networks in connecting a large number of nodes with each other, as already observed by Kruk and Paturej (2020). In our food web, nanoplanktonic connectors were either generalists, establishing a high number of interactions (Biecheleriopsis, Hemiselmis) or specialists, sustaining few interactions which however allowed inter-modular connections (Phaeomonas, Lauderia, and Bacterosira; see Table S7).
4.3 Are there keystones in plankton food webs?
From our analysis, some nodes were topological important while establishing few interactions (high TI, low D): They were the autotrophic protists Fragilariopsis and Amphidoma (Figure 3, nodes #10 and 63, respectively; Lundholm & Hasle, 2010; Tillmann et al., 2012), and the heterotrophic protists Helicostomella (#75) and Aplanochytrium (#13; Dolan et al., 2002; Hamamoto & Honda, 2019), which interacted only as predator and prey, respectively, in our network. These “specialist” nodes were highly central in the network and played as “indirect interactors,” having a large influence not only on their direct neighbors but also on distant ones (Jordán, 2009). Indeed, they could be considered network keystone (sensu D’Alelio et al., 2019). This result remarks the potential importance of protists in driving food-web structure in plankton, in line with previous observations (D’Alelio et al., 2019; Russo, Casella, et al., 2022).
However, network nodes included in the plankton food web of the GoN showed largely similar roles (i.e., similar TI-TO values). An exception was provided by mixotrophic protists which appeared less topologically important (low TI) but more unique (low TO) compared with heterotrophic ones. This slight difference can be explained considering the more specific trophic behavior of mixotrophic protists (Flynn et al., 2019; Leles et al., 2019; Mitra et al., 2016; Schneider et al., 2020), showing a restricted size range of edible preys compared with the preys of heterotrophic protists. For example, in the mixotrophic dinoflagellates Alexandrium and Heterocapsa, the predator-to-prey-size ratio accounts for 2.5 and 1.5, respectively, whereas in heterotrophic Gyrodinium, it goes down to 0.5 (Jeong et al., 2010).
All these observations remark the importance of selecting informative metrics to describe the trophic roles of species in ecological networks, by choosing those metrics that consider not only the whole spectrum of interactions that a node sustains but also their qualitative aspects (Jordán, 2009; Lai et al., 2015). This is even more important in any attempt to exploit biodiversity data obtained with biomolecular methods and investigate the ecological properties of plankton communities.
ACKNOWLEDGMENTS
The authors thank Adriana Zingone for providing raw data of amplicon reads analysed in this study. The authors are grateful to the associated editor Adriana Zingone and anonymous reviewers for their helpful comments and suggestions for improving the quality of the manuscript.
FUNDING INFORMATION
This research is part of the PhD thesis in Evolutionary Biology and Ecology of the University of Rome “Tor Vergata” of Luca Russo. Gianpaolo Zampicinini was supported by “ASSEMBLE PLUS” funded by the European Union's Horizon 2020 research and innovation program under grant agreement No 730984. Daniele Bellardini was supported by the project “DemerstEm” funded by the European Union and “PO FEAMP 2014/2020 (Misura 2.51)” funded by Regione Campania, Italy.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Raw metabarcoding reads used in this study were submitted to the European Genome-Phenome Archive EBI-ENA under the Bioproject number PRJEB56637. The ASV dataset used to derive co-occurrence networks for the plankton community of the GoN was deposited into the Harvard Dataverse database and is freely available at https://doi.org/10.7910/DVN/JMW4NY.




