A Prospective, Blinded Study of Symptom Prevalence and Specificity of Porphyrin Precursors in Carriers of Acute Hepatic Porphyria
Funding: The Porphyrias Consortium (PC) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). PC is funded under grant number U54DK083909 as a collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Mohsen Merati and Nanditha Jayakumar contributed equally to this work.
Handling Editor: Dr. Luca Valenti
ABSTRACT
Background and Aims
This study aimed to characterise symptoms and assess the prevalence of elevated urine porphyrin precursors in first-degree relatives of acute hepatic porphyria (AHP) patients who have never experienced acute attacks and had no previous AHP genetic or biochemical testing.
Methods
149 first-degree relatives of confirmed AHP patients, previously unscreened for the family mutation, were recruited. All underwent genetic analysis, with 143 completing a study questionnaire and 118 undergoing urine analysis for delta aminolevulinic acid (ALA) and porphobilinogen (PBG). The questionnaire focused on symptoms, medical and family history, and quality of life.
Results
The study included 79 AHP mutation carriers and 70 non-carriers. Carriers had significantly higher ALA (6.98 vs. 2.21 mg/g creatinine) and PBG levels (9.17 vs. 1.31 mg/g creatinine) than non-carriers. Female carriers showed higher ALA (9.29 vs. 3.07 mg/g creatinine) and PBG levels (12.71 vs. 3.16 mg/g creatinine) than male carriers. Porphyria-related symptoms were reported by 27% (21/77) of carriers compared to 15% (10/66) of non-carriers, with carriers more likely to report dark urine and prolonged symptoms. Finally, 30.8% of carriers were asymptomatic high excreters (ASHE) with PBG levels exceeding four times the upper limit of normal (ULN).
Conclusions
Significant differences in porphyrin precursor excretion and symptom profiles were found between AHP mutation carriers and controls, as well as between female and male carriers. Female carriers are more likely to excrete porphyrin metabolites above the normal range. A larger than expected number of undiagnosed carriers are ASHE with levels greater than four times the ULN.
Abbreviations
-
- AHP
-
- acute hepatic porphyria
-
- ALA
-
- delta aminolevulinic acid
-
- ASHE
-
- asymptomatic high excreter
-
- HMBS
-
- hydroxymethylbilane synthase
-
- PBG
-
- porphobilinogen
-
- PROMIS-57
-
- Patient-Reported Outcomes Measurement Information System-57
-
- UCSF
-
- University of California San Francisco
-
- ULN
-
- the upper limit of normal
Summary
- This study examined individuals who carry genetic mutations for acute hepatic porphyria (AHP) but have never had an acute attack or been diagnosed.
- We found that these carriers had significantly higher levels of porphyrin precursors in their urine than non-carriers, with female carriers showing higher levels than male carriers.
- Some carriers reported symptoms like dark urine, while others had extremely high porphyrin levels despite having no symptoms.
- These findings highlight the importance of screening individuals with a family history of AHP, even if they have never experienced an acute attack.
1 Introduction
Acute hepatic porphyrias (AHP) are rare metabolic diseases due to genetic defects of enzymes in the heme biosynthesis pathway [1]. The three most common AHP are acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria, which are all inherited in an autosomal dominant fashion. In the setting of triggering factors that increase heme production primarily in the liver, AHP present with identical episodes of acute neurovisceral symptoms, called acute porphyria attacks, due to abnormal accumulation of pathway intermediates delta-aminolevulinic acid (ALA) and porphobilinogen (PBG) [2]. The AHP are genetic diseases with low clinical penetrance. While the combined prevalence of symptomatic AHP is estimated to be between 1 and 5 per 100 000 [3, 4], the prevalence of pathologic mutations is much higher, between 1 in 1300 and 1 in 1785 based on population studies [5, 6].
The diagnosis of AHP is difficult because few medical providers have seen a case, and the symptoms of acute attacks are non-specific. As a result, AHP patients generally experience a long delay from the time they develop symptoms to when they are diagnosed [7]. That said, clinical experience has pointed to features that may distinguish AHP from more common causes of unexplained abdominal pain [8]. For example, the symptoms of AHP typically progress from mild to severe over several days, not hours. Pain that comes and goes within a day is likely not due to AHP. However, these features have never been validated.
Another question concerns the presence of subacute and often chronic symptoms in AHP. In general, the focus has been on symptoms during acute attacks and the neurological residue afterwards [9, 10]. Recent studies, however, have shown that chronic symptoms occur between attacks in recurrent acute porphyria patients and in symptomatic high excreters who are several years removed from acute attacks and that such symptoms impact a patient's quality of life [11-15]. Although the nature of these symptoms and their prevalence remain to be determined, it has been reported recently that symptomatic high excreters may benefit from treatment with Givosiran [14].
A subset of AHP has persistently high urine porphyrin precursors but has not experienced acute attacks. These asymptomatic high excreter (ASHE) mutation carriers have recently been more clearly defined [15]. They may be at higher risk of experiencing acute attacks and may experience chronic sequelae related to elevated levels of ALA and PBG [16]. The prevalence of ASHE within the genetic carrier population is unknown.
The present study explores these issues with a prospective, blinded design. Although genetic screening is recommended for all first-degree relatives of an index case, this is often not done in clinical practice. This study consisted of first-degree family members who have never undergone genetic testing for AHP or urine testing for ALA, PBG and porphyrins. Participants provided samples for the AHP screening tests and took a questionnaire, which was done before the results of genetic testing were known. In the analyses, two groups were compared: carriers of an AHP mutation vs. non-carriers.
2 Methods
2.1 Study Design
This was a prospective, multi-center, observational study conducted from 2012 to 2020 at six sites of the Porphyrias Consortium of the National Institute of Health Rare Diseases Clinical Research Network. It was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board at all six participating sites. Written informed consent was obtained from participants after meeting the inclusion criteria.
2.2 Study Population and Assessments
First degree relatives of individuals with genetically confirmed AHP were contacted for enrollment. Only individuals ≥ 15 years of age, who were never screened before for the family mutation, and who have never had symptoms consistent with acute attacks were enrolled. Those with a history of alarm symptoms (gastrointestinal bleeding, anaemia with haemoglobin < 10 g/dL, hematuria, unintentional weight loss and dysphagia) were excluded. All 149 included subjects underwent genetic testing to determine their mutation status (carrier or control).
The questionnaire that was completed before genetic and biochemical testing sought information on socio-demographics, medical and surgical history, menstrual and reproductive history for female subjects and concomitant medications. Symptoms experienced by subjects that were relevant to porphyria were recorded. We collected data on specific treatments for or prophylactic measures taken to prevent acute attacks. Other covariates examined were the age of first symptoms, number of hospitalisations, precipitating factors, and quality of life. The Patient-Reported Outcomes Measurement Information System (PROMIS-57) scale was utilised to assess patient-reported health status across various domains, including physical, mental and social health. Following the completion of the questionnaires, saliva or blood samples were collected for genetic testing and urine samples were gathered for analysis of porphyrin precursors. Porphyrin precursors were normalised to urine creatinine and reported in mg/g creatinine.
The AHP clinical severity of 64 index patients, all of whom were patients in the Porphyrias Consortium Longitudinal Study [7], was examined to identify any correlation of their clinical severity to biochemical results of their corresponding first-degree relatives enrolled in the study. These 64 index patients were first-degree relatives of 99 study subjects out of 102 reported cases. Three study subjects (case 55, 63 and 66), each reported two first-degree index patients (Table S5). Index patients all had confirmed AHP diagnosis by urine biochemistry and genetic testing. The index patients were classified based on whether they reported a history of acute porphyria attacks (no attack or attacks) in the Longitudinal Study database. For index patients with a history of attacks, those with > 4 hospitalizations within 1 year, use of scheduled prophylactic hemin, or treatment with givosiran were classified as ‘Recurrent attacks’, they were classified as ‘Sporadic attack’ patients (Table S4).
3 Measures
3.1 Mutation Status
Mutation carrier status was analysed as a binary variable with categories, ‘carrier’ and ‘control.’
3.2 Symptom Profile and Manifestations
Symptom profile was analysed as a binary variable with categories, ‘symptomatic’ and ‘asymptomatic.’ Individual symptom manifestations were also analysed as binary (yes/no) variables.
3.3 Duration of Symptoms
Duration of symptoms was analysed as a binary variable with categories, ‘A few minutes-hours to 2 days’ and ‘Several days-weeks/months.’
3.4 Porphyrin Precursor Levels
Porphobilinogen (PBG) and delta-aminolaevulinic acid (ALA) were analysed as continuous measures (normal range of PBG and ALA: 0–2 mg/g creatinine and 0–7 mg/g creatinine respectively). All urine was analysed at the University of Texas Medical Branch Health Porphyria Center in Galveston, Texas.
3.5 High Excreters
High excreters were defined as subjects with urine PBG levels greater than four times the upper limit of normal (ULN), measured in mg/g creatinine (≥ 8) after unit conversion. In cases where unit conversion was not possible, we included one subject (case 39) with 27.6 mg/L (> 8 mg/L) and another (case 36) with 19.2 mg/24 h (> 10.8 mg/24 h), using four times the ULN values in their respective units, based on recent consensus definitions [16]. (Table S3).
3.6 Statistical Analysis
Categorical subject characteristics were summarised as counts and percentages, and continuous measures were summarised using descriptive statistics including mean (SD) and median (range). Symptom manifestations were analysed across carrier and control groups. PBG and ALA values were presented based on carrier status, sex and symptom profile. The prevalence of high excreters overall and within each sex and symptom profile subgroup was reported and represented as percentages. A subgroup analysis of PBG among high excreters was also conducted.
Differences between groups for continuous outcome measures were tested using the Welch's two sample t-test of means, and the Wilcoxon rank sum test of medians was used for comparison of subgroups within the high excreter population given the smaller sample sizes. The Fisher's exact test was used for the categorical symptom measures. All hypothesis tests were two-sided. The significance threshold was set to 0.05 for Welch's two sample t-test of means and the Wilcoxon rank sum test of medians, while a significance threshold of 0.10 was used for Fisher's exact tests in the subgroup of participants who reported non-specific symptoms. Statistical analyses were performed using R software (Version 1.4.1106 2009–2021 RStudio).
4 Results
The study enrolled a total of 149 first degree relatives of confirmed AHP patients. None of the enrolled subjects had prior genetic testing, porphyrin precursor biochemical testing, or experienced acute attacks. The mean age was 44.25 years and 95 (63.8%) of the subjects were females (Table 1). The subject selection and the study assessments conducted are shown in (Figure 1). Genetic testing of AHP genes identified 79 mutation carriers (53%). The remaining 70 subjects constituted the control population.
| Characteristics | Females | Males | Total |
|---|---|---|---|
| n (%) | n (%) | N | |
| Subjects | 95 (63.75) | 54 (36.24) | 149 |
| Mean age | 42 | 48 | 44.25 |
| Age range | 16–75 | 15–77 | 15–77 |
| With a mutationa | 49 (62) | 30 (38) | 79 |
| Without a mutation | 46 (65.71) | 24 (34.30) | 70 |
| With symptoms | 25 (80.64) | 6 (19.35) | 31 |
| Without symptoms | 65 (58) | 47 (42) | 112 |
| With mutation and symptoms | 18 (85.71) | 3 (14.3) | 21 |
| Without mutation and symptoms | 7 (70) | 3 (30) | 10 |
- a Of the 79 mutation carriers, 72 were found to be carriers of Acute Intermittent Porphyria (AIP), 1 of Hereditary Coproporphyria (HCP) and 6 of Variegate Porphyria (VP).
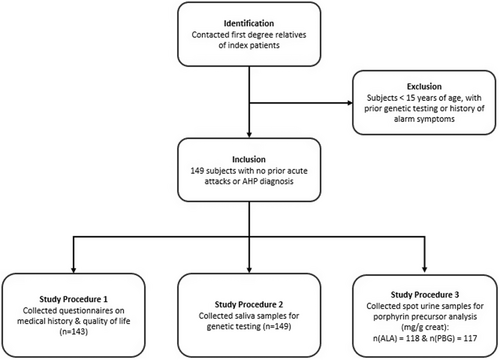
Of the 149 subjects, 143 completed the questionnaire, 118 and 117 submitted their urine samples in the unit of mg/g creatinine for porphyrin precursors delta aminolevulinic acid (ALA) and porphobilinogen (PBG), respectively (normal range: PBG; 0-2 mg/g creatinine and ALA; 0-7 mg/g creatinine) (Figure 1, Tables S1–S3).
4.1 Mutation Carriers and Female Carriers Excrete Significantly Higher Urine Porphyrin Precursors Than Non-Carriers and Male Carriers
We examined the distribution of urine ALA and PBG from 62 carriers and 56 controls. Urine ALA and PBG were significantly higher in carriers than controls (mean ALA 6.98 vs. 2.21 mg/g creatine; mean PBG 9.17 vs. 1.31 mg/g creatinine Figure 2a). Twenty-three carriers (35%) had PBG levels above the ULN (5.9–89.2 mg/g creatinine) compared to only 6 (11%) controls (2.1–5.7 mg/g creatinine. Seventeen (27.4%)) carriers had ALA above the ULN (7.2–48.3 mg/g creatinine) while all controls except one case (13 mg/g creatinine) had levels within the normal range.
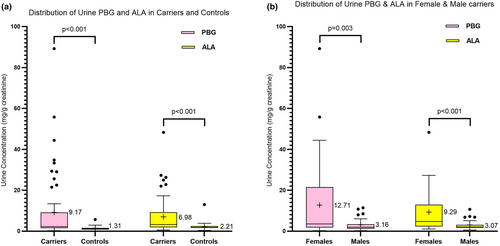
As women are far more likely than men to have symptomatic acute attacks [17], we examined potential sex differences in porphyrin precursor levels. Female carriers had significantly higher ALA and PBG levels compared to male carriers (mean ALA: 9.29 vs. 3.07 mg/g creatinine; mean PBG: 12.71 vs. 3.16 mg/g creatinine Figure 2b). Additionally, 66.6% of female carriers had abnormal PBG values (exceeding its ULN (2 mg/g creatinine)), compared to 39.1% of male carriers (Table S3).
4.2 Carriers Are More Likely to Report Dark Urine and Experience Longer Duration of Symptoms
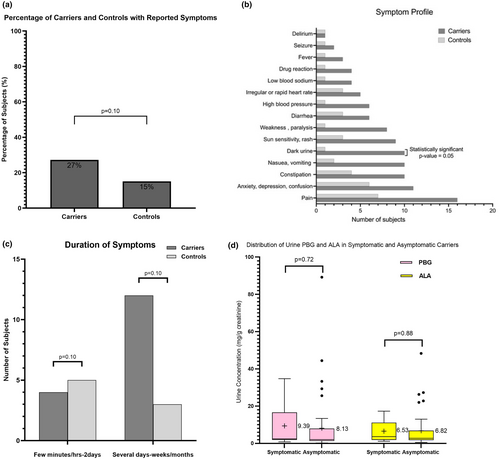
We characterised symptom manifestations in undiagnosed relatives of AHP patients to see if any correlate with AHP carrier status. Of the 143 subjects that completed the questionnaire, 31 (21.7%) reported non-specific symptoms. In these subjects, 21 (68%) were carriers for the family mutation comprising 19 AIP and 2 VP subjects, indicating that 27% (21 of 77) of all carriers that completed questionnaires reported symptoms, compared to 15% (10 of 66) of non-carriers (Figure 3a). Dark urine was the only symptom significantly more likely to be reported by carriers than controls (p < 0.05 Figure 3b). AHP carriers were more likely to experience symptoms for several days to weeks or months than controls (Figure 3c). We compared the urine porphyrin precursor levels in 61 symptomatic and symptom-free carriers but did not find a statistically significant difference (Figure 3d).
4.3 Female Carriers and Symptomatic Carriers Are More Likely to Be Asymptomatic High Excreters (ASHE) With Urine PBG > 4times the ULN
A finding of considerable interest in our carrier population was the high prevalence of AHP carriers with urine PBG excretion greater than 4 times the ULN [16]. We focused on PBG levels due to the increased sensitivity and specificity in identifying not only acutely symptomatic patients but also latent carriers and those in remission [18]. Of the 65 carriers who provided urine samples for porphyrin precursor analysis, 20 (30.8%) had PBG greater than 4 times the ULN, with a median of 22 (range 8.5–89.2 mg/g creatinine) (Figure 4a). Of note, none of the controls had PBG greater than 4 times the ULN. More than 38% of female carriers were high excreters, compared to 17.39% of male carriers (Figure 4b). All male high excreters had urine PBG levels between 8.0–11.4 mg/g creatinine, while more than 70% of female high excreters had PBG > 15 mg/g creatinine (Figure 4b).
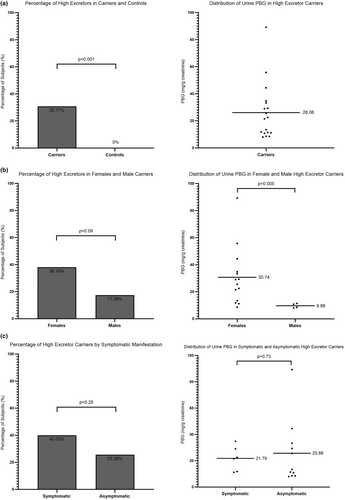
Similarly, we looked at 20 carriers who reported non-specific symptoms and found 8 (40%) to be high excreters, compared to 11 of 43 (25.6%) asymptomatic carriers (Figure 4c). We did not see a statistically significant difference in urine PBG levels between symptomatic and asymptomatic high excreters (Figure 4c), which is likely due to the presence of two asymptomatic carriers with more than a 20-fold increase in urine PBG levels (44.4 and 89.2 mg/g creatinine, respectively). Of note, only AIP carriers excreted high levels (> 8 mg/g creatinine) of PBG in urine in this study population.
4.4 No Correlation Existed Between the Genotype and Phenotype in Carriers
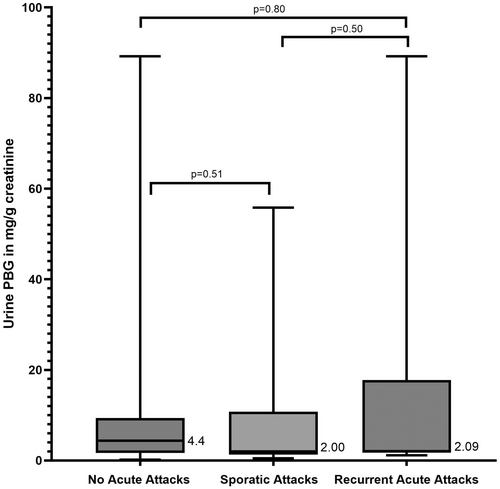
We asked if the increased proportion of ASHE in our study population can be attributed to the severity of disease of their corresponding index cases. We could identify first-degree index patients for 99 out of 149 study subjects, representing 64 unique index patients (Table S5). All index patients are enrolled in the US Porphyrias Consortium Longitudinal Study. We characterised their AHP disease severity to look for a potential relationship with urine PBG excretion in corresponding study subjects. Fifteen index patients have never experienced acute attacks, 37 had sporadic attacks, and 12 had frequent recurrent attacks based on recent definitions [15]. We compared the disease severity of index patients and the urine PBG in their first-degree study subjects who are mutation carriers. Twenty-two study subjects with urine PBG levels had index patients who were sporadic attack patients (Table 2), 8 study subjects had index patients who were recurrent attack patients (Table 3), and 14 study subjects had index patients who were asymptomatic (Table 4). We found no significant association between study subject urine PBG levels and the clinical severity of their index patients (Figure 5).
| Case | Age | Gender | PBG (mg/g creatinine) | 1st degree AHP type | 1st degree gender | Index patient case number |
|---|---|---|---|---|---|---|
| 47 | 26 | Female | 29.00 | AIP | Female | 155 |
| 43 | 57 | Female | 1.70 | AIP | Female | 156 |
| 17 | 15 | Male | 0.85 | AIP | Female | 163 |
| 30 | 19 | Female | 6.00 | AIP | Female | 167 |
| 31 | 39 | Female | 55.80 | AIP | Female | 168 |
| 33 | 29 | Female | 25.60 | AIP | Female | 169 |
| 52 | 43 | Female | 1.48 | AIP | Female | 173 |
| 51 | 54 | Male | 1.90 | AIP | Female | 174 |
| 66 | 29 | Female | 1.30 | AIP | Female | 174 |
| 54 | 21 | Female | 3.20 | AIP | Female | 177 |
| 58 | 45 | Male | 1.30 | VP | Female | 179 |
| 68 | 22 | Female | 6.60 | AIP | Female | 184 |
| 70 | 55 | Female | 1.70 | AIP | Female | 184 |
| 67 | 25 | Male | 2.10 | AIP | Female | 185 |
| 74 | 46 | Male | 1.90 | AIP | Female | 187 |
| 105 | 41 | Female | 11.18 | AIP | Female | 196 |
| 107 | 17 | Female | 0.88 | AIP | Female | 196 |
| 108 | 21 | Male | 11.43 | AIP | Female | 197 |
| 45 | 51 | Female | 2.20 | AIP | Male | 202 |
| 59 | 40 | Male | 10.80 | AIP | Female | 203 |
| 86 | 64 | Female | 2.00 | AIP | Female | 207 |
| 69 | 31 | Female | 0.50 | AIP | Female | 211 |
| 66 | 29 | Female | 1.30 | AIP | Female | 213 |
| Case | Age | Gender | PBG (mg/g creatinine) | 1st degree AHP type | 1st degree gender | Index patient case number |
|---|---|---|---|---|---|---|
| 89 | 32 | Female | 6.60 | AIP | Female | 152 |
| 76 | 39 | Female | 21.50 | AIP | Female | 153 |
| 79 | 51 | Male | 1.70 | AIP | Female | 153 |
| 14 | 63 | Female | 2.13 | AIP | Female | 160 |
| 63 | 26 | Female | 89.20 | AIP | Female | 165 |
| 77 | 71 | Female | 1.90 | AIP | Female | 182 |
| 98 | 18 | Female | 1.16 | VP | Female | 195 |
| 116 | 53 | Male | 2.05 | AIP | Male | 198 |
| Case | Age | Gender | PBG (mg/g creatinine) | 1st degree AHP type | 1st degree gender | Index patient case number |
|---|---|---|---|---|---|---|
| 83 | 21 | Female | 1.80 | AIP | Male | 150 |
| 84 | 17 | Female | 33.30 | AIP | Female | 151 |
| 12 | 51 | Male | 6.06 | AIP | Female | 161 |
| 101 | 27 | Female | 2.00 | AIP | Female | 166 |
| 37 | 65 | Male | 8.00 | AIP | Female | 171 |
| 46 | 61 | Male | 0.20 | AIP | Male | 171 |
| 63 | 26 | Female | 89.20 | AIP | Female | 171 |
| 48 | 36 | Male | 1.20 | AIP | Female | 175 |
| 49 | 19 | Female | 2.40 | AIP | Female | 176 |
| 56 | 33 | Female | 8.60 | AIP | Male | 178 |
| 57 | 33 | Female | 5.90 | AIP | Male | 180 |
| 80 | 67 | Male | 2.90 | AIP | Female | 186 |
| 82 | 63 | Male | 1.40 | AIP | Female | 186 |
| 95 | 33 | Female | 11.80 | AIP | Female | 206 |
5 Discussion
In this study, index cases were patients enrolled in the longitudinal study of porphyrias in the Porphyrias Consortium and were more likely to have had acute attacks than the general population of AHP patients [7]. We found that first-degree relatives of this population who carry the family mutation have significantly higher ALA and PBG levels than family members without the mutation. This is consistent with previous reports [7, 19, 20]. Within mutation carriers in our study, females had higher porphyrin precursor levels than did males. There are few data comparing porphyrin precursors in female and male carriers without a history of acute attacks. Our findings are consistent with the importance of sex hormones in AHP [7, 17, 18].
We were surprised to find that 30.7% of the mutation carriers in our study were asymptomatic high excreters with urine PBG levels greater than 4 times the upper limit of normal (Figure 4a). This is significantly higher than earlier studies [16]. A population study with 43 asymptomatic carriers identified only ~9% (n = 4) subjects with PBG 10 times the reference range [21]. Recent population-based genetic screening studies have shown that the prevalence of AHP mutations is much higher than previously thought: approximately 1 in 1600 people [5, 6]. Our data suggest that a large number of AHP mutation carriers may have significantly elevated ALA and PBG levels. While it is unclear whether ASHE carriers are at risk for long-term complications of AHP, recent data showing increased HCC risk in AHP patients with elevated porphyrin precursor levels, independent from a history of attacks, suggest that ASHE carriers may require more monitoring [16].
Our study also allowed us to characterise the presence of symptoms in mutation carriers. Symptoms were reported by 27% of mutation carriers but only 15% of non-carriers. They were more likely to be prolonged in carriers than in non-carriers, suggesting that AHP may underlie minor but recurrent symptoms in carriers who have never experienced an acute attack. However, this association will need to be validated in larger patient cohorts. Dark urine can be seen during acute attacks in AHP patients, though this is typically due to increased levels of porphyrins in the urine, as the porphyrin precursors ALA and PBG are themselves colourless. This was the only symptom to be significantly increased in carriers in this study, though this is likely due in part to the small sample size, as several other symptoms showed a trend towards carriers. Surprisingly, the presence of symptoms was not correlated with urine ALA or PBG levels. We looked for correlations between elevated urine ALA and PBG in carriers to disease severity in the index cases within their families. While no correlation was found, our analysis was limited by the small sample size.
In conclusion, we identified significant differences in porphyrin precursor excretion levels and symptom profiles between carriers and controls, and between females and males. Female carriers have a higher tendency to not only excrete porphyrin metabolites above the normal range but also to be high PBG excreters with levels greater than 4-times the ULN. Carriers identified in families through genetic analysis require biochemical testing to determine if previous unexplained gastrointestinal, neurological, psychiatric, or autonomic symptoms could have been due to porphyria and to predict the future risk of developing acute attacks.
Author Contributions
Manisha Balwani, Karl E. Anderson, Herbert L. Bonkovsky, Robert J. Desnick, John Phillips, and Bruce Wang designed the project. Mohsen Merati, Nanditha Jayakumar, Yuvraaj Kapoor, Hetanshi Naik, Manisha Balwani, Karl E. Anderson, Herbert L. Bonkovsky, Robert J. Desnick, and Brendan McGuire performed data collection. Mohsen Merati, Nanditha Jayakumar, and Bruce Wang analysed data and wrote the paper. Bruce Wang supervised the project.
Acknowledgements
We would like to thank Amy Shui (Department of Epidemiology and Biostatics of University of California, San Francisco, USA) for her advice and assistance with the original statistical analysis.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest. Nanditha Jayakumar: No conflict of interest to declare. Yuvraaj Kapoor: No conflict of interest to declare. Hetanshi Naik: No conflict of interest to declare. Manisha Balwani: M.B. is a consultant for Alnylam Pharmaceuticals, Disc Medicine, and Mitsubishi Tanabe Pharma. Karl E. Anderson: K.E.A. is a consultant for Disc Medicine, Mitsubishi Tanabe Pharma, and Recordati Rare Diseases. Herbert L. Bonkovsky: KLB is a consultant for Alnylam Pharmaceuticals, Disc Medicine, Eiger Biopharma, Mitsubishi Tanabe Pharma, and Protagonist Therapeutics. Robert J. Desnick: R.J.D. is a consultant for Alnylam Pharmaceuticals, CRISPR Therapeutics, Disc Medicine, Mitsubishi Tanabe, Recordati Rare Diseases, and Protasonist Therapeutics. Brendan McGuire: No conflict of interest to declare. John Phillips: No conflict of interest to declare. D. Montgomery Bissell: No conflict of interest to declare. Bruce Wang: B.W. is a consultant for Alnylam Pharmaceuticals, Disc Medicine, Mitsubishi Tanabe Pharma, and Recordati Rare Diseases; he received grant support from Alnylam Pharmaceuticals and Mitsubishi Tanabe Pharma.
Open Research
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author upon request.




