HFD Exacerbates Hepatic Lipid Metabolism Disorders After Cholecystectomy by Regulating the Bile Acid and Neutrophil Recruitment
Funding: This work was supported by the Capital Health Research and Development of Special (No. CFH2024-1-4081 to LYL, and No. CFH2024-4-4089 to XJ), the National Natural Science Foundation of China (No. 82370537, 82341228 to LYL, and 82370555 to XJ), the Beijing Municipal Natural Science Foundation (No. 7232196 to LYL), and Peking University People's Hospital Scientific Research Development Funds (No. RDJP2023-22 to XJ).
Yanting Xu and Feng Zhang contributed equally to this study.
Handling Editor: KyeongJin Kim
ABSTRACT
Background and Aims
Previous studies have suggested that cholecystectomy may lead to an elevated risk of metabolic dysfunction-associated fatty liver disease (MAFLD). However, there is a dearth of knowledge concerning the precise effects and underlying mechanisms through which cholecystectomy influences hepatic lipid metabolism, necessitating further investigation.
Methods
Post-cholecystectomy (PC) model mice were established under both normal and high-fat diet conditions. Liver lipid metabolism, inflammation, bile acid profiles, and immune cell alterations were evaluated. Hepatocytes mimicking a high-fat state were exposed to distinctive bile acids to investigate the chemotactic effect on neutrophils. Co-culture experiments of hepatocytes and neutrophils evaluated lipid accumulation in hepatocytes and the expression levels of lipid metabolism-related genes.
Results
Cholecystectomy under a high-fat diet markedly disrupted hepatic lipid metabolism and exacerbated inflammation, while cholecystectomy under a normal diet did not. The bile acid profiles in the ileum and liver of PC mice underwent notable changes, along with modifications in bile acid receptors and transporters associated with enterohepatic circulation. Notably, deoxycholic acid (DCA) and tauroursodeoxycholic acid (TDCA) were identified as distinctive bile acids. Furthermore, hepatocytes in PC mice with a high-fat diet showed an elevated level of neutrophil infiltration. Hepatocytes showed a significant increase in releasing CXCL1 and CXCL2 when administered with DCA or TDCA, suggesting an intensified chemotactic response for neutrophils. Co-culturing neutrophils and hepatocytes exacerbated hepatocyte lipid deposition and reduced the expression of genes associated with lipid oxidative degradation.
Conclusions
Cholecystectomy alongside a high-fat diet aggravates hepatic lipid metabolism disorders and inflammation by modulating hepatic bile acid profiles and promoting neutrophil recruitment.
Summary
- Cholecystectomy links to MAFLD, with effects potentially tied to metabolic/dietary factors, but exact mechanisms remain elusive.
- Cholecystectomized mice exhibited altered ileal/liver bile acid profiles and modified enterohepatic circulation-associated bile acid receptors/transporters, with DCA and TDCA identified as distinct differential bile acids.
- Cholecystectomy alongside a high-fat diet aggravates hepatic lipid metabolism disorders and inflammation by modulating hepatic bile acid profiles and promoting neutrophil recruitment.
1 Introduction
Cholecystectomy is a common treatment for benign gallbladder diseases. Some studies suggest that cholecystectomy may elevate the risk of metabolic dysfunction-associated fatty liver disease (MAFLD) [1-3], with potential variations depending on metabolic state and dietary patterns [4-6]. On the other hand, several studies suggest that cholecystectomy does not affect hepatic lipid metabolism, highlighting the ongoing debate in this field [7-9]. Furthermore, there is a scarcity of animal studies examining the impact of cholecystectomy on hepatic lipid metabolism disorders, and the results obtained so far are inconclusive [10, 11]. Thus, additional animal studies are essential to determine if cholecystectomy aggravates hepatic lipid metabolic disturbances in MAFLD and to explore the underlying mechanisms.
Bile acids (BAs) have been identified as pivotal signalling molecules in the pathogenesis of MAFLD, exerting a crucial influence on metabolic pathways and immune homeostasis [12-14]. The loss of rhythmic function of the gallbladder as a bile reservoir and contraction pump following cholecystectomy leads to alterations in bile acid metabolism and recycling in the liver and intestines, subsequently affecting the bile acid–bile acid receptor axis [15, 16]. Changes in enterohepatic circulation and bile acid distribution can impact lipid homeostasis systemically and in local organs, suggesting that bile acid transporters may be closely related to MAFLD. However, research on transporters is relatively sparse, and studies on their interaction with MAFLD pathogenesis are inconsistent [17, 18]. Therefore, due to the lack of clarity regarding the overall coordination of enterohepatic circulation, including the bile acid profile, relevant receptors, and transporters, there is a necessity for extensive studies at a macro level to analyse the changes in enterohepatic circulation following cholecystectomy.
Various immune cells play crucial roles in the initiation and progression of MAFLD [19]. Interactions between immune cells, as well as cross-talk between immune cells and hepatocytes, play significant roles in the onset and progression of MAFLD [20]. Post-cholecystectomy (PC), there are notable alterations in BAs, which have been demonstrated to influence liver inflammation and immunity. These alterations can either stimulate hepatocytes or immune cells through receptor-dependent or independent mechanisms, thereby influencing the advancement of MAFLD [21-23]. Furthermore, our previous research has demonstrated that secondary BAs accumulated after cholecystectomy exhibit notable immunoregulatory effects, and alterations in microbial species involved in bile acid metabolism have been discovered [24-27]. Consequently, alterations in hepatic BAs following cholecystectomy may contribute to the development of hepatic lipid metabolic disorders and inflammation by causing an imbalance in the liver's immune system.
In summary, this study will focus on the relationship between cholecystectomy and MAFLD, with particular emphasis on the changes in bile acid profiles following cholecystectomy and their impact on hepatic lipid metabolism and immune inflammation. To evaluate the impact of cholecystectomy on hepatic lipid deposition and inflammation, a PC mouse model will be created and exposed to different dietary conditions, along with analysing both intestinal and hepatic bile acid profiles, evaluating alterations in hepatic immune cells, and elucidating the role of BAs and immune cells in MAFLD pathogenesis. Valuable insights and evidence are presented in this study, highlighting the metabolic risks that come with cholecystectomy.
2 Methods and Materials
2.1 Animal Experiments
C57BL/6J male mice (6–8 weeks old) were provided by SPF (Beijing) Biotechnology Co. Ltd. All mice were raised in a specific pathogen-free (SPF) facility of the Animal Experiment Center of Peking University People's Hospital with a temperature of 21°C ± 2°C, relative humidity of 40%–60%, and normal access to food and water. A 12-h light/dark cycle was maintained. Following a 6-h fasting period, the mice were euthanized via cervical dislocation. This study was approved by the Institutional Medical Ethics Review Board of Peking University People's Hospital (document no. 2021PHE023).
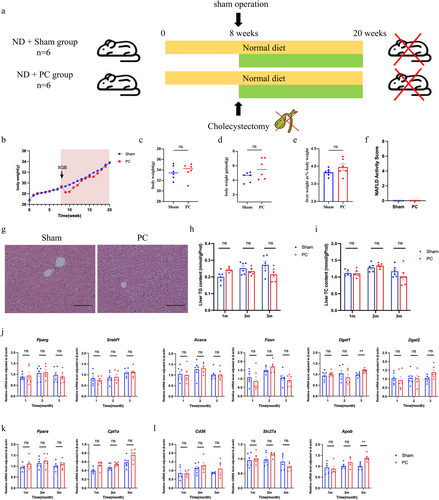
After a 1-week acclimation period, all mice were fed either a normal diet (ND) or a high-fat diet (HFD) for 4 or 8 weeks, based on the specific research requirements. The HFD composition contained 60% fat, 20% carbohydrate, and 20% protein by energy content, with a caloric density of 5.24 kcal/g (formulated by Research Diets Inc., New Brunswick, NJ, USA). Following the dietary intervention period, mice were randomly assigned to two groups: the sham-operated group (Sham) and the post cholecystectomy group (PC). Following an 8-h fast, mice underwent either cholecystectomy or sham surgery. Three months later, the mice were euthanized, and tissues including the liver and ileum were dissected and preserved. Detailed research procedures and group schematic are illustrated in Figures 1a and 2a.
2.2 Haematoxylin and Eosin (H&E) Staining
After euthanizing the mice, liver tissue specimens were fixed in 4% paraformaldehyde solution for at least 24 h, subjected to gradient ethanol dehydration, and then embedded in paraffin to produce 4 μm sections. The liver tissue sections were stained with H&E and examined under a microscope. NAFLD activity scores (NAS) for each group of mice were assessed by two observers using an optical microscope [28], with the scoring criteria detailed in Table S1.
2.3 Liver Triglyceride and Total Cholesterol Measurement and Bradford Assay for Determining Liver Protein Concentration
Liver samples (50 mg) were homogenised in 1 mL lysis buffer using a tissue homogeniser (Bio-Gen Pro200). The homogenate was incubated at room temperature for 10 min, and the supernatant was transferred to a fresh tube. The extract was heated at 70°C for 10 min, followed by centrifugation at 2000 rpm for 5 min (room temperature). The resulting supernatant was collected for analysis according to the instructions provided with the respective kits: The High Fatty Sample Triglyceride (TG) Content Assay Kit (Applygen Technologies Co. Ltd., Beijing, China), The High Fatty Sample Cholesterol Total (CHOL) Content Assay Kit (Applygen Technologies Co. Ltd., Beijing, China), and the Bradford Protein Concentration Assay Kit (Beyotime Biotechnology Co. Ltd., Shanghai, China) to measure liver triglyceride, total cholesterol, and tissue protein content.
2.4 Determination of Serum Triglycerides (TG), Total Cholesterol (TC), Alanine Aminotransferase (ALT), and Aspartate Aminotransferase (AST) Levels
Serum or cell supernatant levels of TG, TC, ALT, and AST were determined according to the instructions provided with the following kits: The Liquid Sample TG Content Assay Kit (Applygen Technologies Co. Ltd., Beijing, China), The Liquid Sample TC assay kit (Applygen Technologies Co. Ltd., Beijing, China), The Liquid Sample ALT Activity Assay Kit (Applygen Technologies Co. Ltd., Beijing, China), and The Liquid Sample AST Activity Assay Kit (Applygen Technologies Co. Ltd., Beijing, China).
2.5 RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted from liver tissue or HepG2 cells using TRIzol reagent (Thermo Fisher Scientific). Subsequently, mRNA from liver tissue was reverse transcribed into cDNA using the RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific). qRT-PCR was performed on an Applied Biosystems StepOne Plus Real-Time PCR System, employing SYBR Green Master Mix (Thermo Fisher Scientific). Primer sequences are provided in Tables S2 and S3.
2.6 BAs Detection
All BAs standards were obtained from Steraloids and TRC Chemicals Inc., or synthesised in the Metabolomics Laboratory (China). Ten stable isotope-labelled standards were obtained from C/D/N Isotopes (Canada) and Steraloids (USA) Internal standard (IS) concentrations were maintained consistently across all calibration points. BAs were quantitatively analysed using an ultra-high performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) system (ACQUITY UPLC Xevo TQ-S, Waters), with assistance from Shanghai Metware Biotechnology Co. Ltd. Raw data files from UPLC-MS/MS bile acid assays were processed using MassLynx software (v4.1, Waters, USA) for peak integration, calibration, and quantification of each metabolite. Data analysis was performed using SIMCA 14.1 (32-bit) (Umetrics).
2.7 Flow Cytometry Analysis
Mouse liver tissue was isolated and mechanically homogenised in 15 mL of precooled PBS. The homogenate was filtered through a 70 μm cell strainer, washed with PBS, and centrifuged at 500 g for 5 min. Cells were resuspended in PBS and aliquoted into tubes, with 100 μL added to each tube and stained with flow cytometry antibodies as per the manufacturer's instructions (Anti-mouse CD16/CD32, BioLegend, San Diego, CA, USA). Staining was performed at 4°C in the dark for 30 min, followed by the addition of 1 mL of PBS and centrifugation at 700 g for 5 min. The cell pellets were resuspended in 200 μL of PBS, and the proportion of B and T lymphocytes was analysed by flow cytometry. All data were acquired on a Gallios flow cytometer (Beckman Coulter Inc., Brea, CA, USA) and analysed using FlowJo 10.8.1 (BD Biosciences, San Jose, CA, USA) or Kaluza Analysis Software 2.1 (Beckman Coulter Inc., Brea, CA, USA).
2.8 Cell Isolation and Culture
HepG2 cells, a human liver cancer cell line, were obtained from the Cell Resource Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Cell revival, culture, and subculturing were performed according to the ATCC cell culture guidelines. Hepatocytes were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (10,000 U/mL penicillin, 10 mg/mL streptomycin). The culture medium was replaced every 48 h, and cells were passaged at a 1:3 ratio every 3–4 days. All cultures were maintained in a humidified incubator at 37°C with 5% CO. Human neutrophils used in this study were isolated from the peripheral blood of healthy volunteers. The isolation process, based on the referenced literature [29], is summarised as follows: 5 mL of Polymorphprep lymphocyte separation medium was added to a 15 mL sterile centrifuge tube. 5 mL of peripheral blood was carefully layered on top of the separation medium and centrifuged. The white cell layer (1–1.5 mL) was aspirated into a new centrifuge tube, resuspended in RPMI 1640 medium, centrifuged again, and the supernatant was discarded while the pellet was retained. A 10 μL aliquot of the cell suspension was diluted 10-fold, and cell counts were performed using a haemocytometer under a microscope. Based on the cell count, the cell concentration was adjusted to 2 × 106 cells/mL for subsequent experiments.
2.9 Oleic Acid (OA) and Palmitic Acid (PA) Treatment of Hepatocytes to Simulate a High-Fat State
To determine the optimal ratio of OA to PA, HepG2 liver cells were divided into six groups and treated with varying concentrations (0–1200 μM) of OA and PA for 24 h. The cell supernatants were collected to measure ALT levels. Cells were also collected for TG content analysis and Oil Red O staining.
2.10 Bile Acid Exposure Experiment
The HepG2 liver cells were divided into 8 groups (Figure 6a). For the bile acid groups, cells were treated with deoxycholic acid (DCA) or taurodeoxycholic acid (TDCA). The OA and PA treatment groups received 1 mM OA and PA solution for 24 h. Subsequently, in the bile acid exposure groups, cells were treated with different concentrations of individual BAs for 24, 48, and 72 h. The control and non-bile acid groups were treated with complete culture medium under the same conditions. Cell supernatants were collected, and CXCL1 and CXCL2 levels were measured according to the instructions of the respective ELISA kits (Human CXCL1/KC ELISA Kit, MultiSciences Biotech Co. Ltd., Hangzhou, China; Human CXCL2/GRO-β ELISA Kit, MultiSciences Biotech Co. Ltd., Hangzhou, China). Following cell collection, chemokine and Farnesoid X receptor (FXR) expression levels were detected using qRT-PCR as described above, with primers listed in Table S3.
2.11 Neutrophil Chemotaxis Assay and Co-Culture Experiments With Hepatocytes and Neutrophils
Neutrophil chemotaxis assays and co-culture experiments with hepatocytes and neutrophils were performed using Transwell plates (24-well, 3.0 μm, Corning Incorporated, America). Detailed experimental procedures and group assignments are outlined in Figure 7a. For the OA and PA treatment groups, cells were exposed to 1 mM OA and PA solution for 24 h. In the bile acid exposure groups, cells were stimulated with 100 μM deoxycholic acid for 48 h and 200 μM TDCA for 72 h. Control and non-bile acid groups were treated with a complete culture medium under the same conditions. Cell supernatants were collected for subsequent neutrophil chemotaxis assays, and hepatocytes were used for co-culture experiments with neutrophils.
In the neutrophil chemotaxis assay, cell supernatants were added to the lower chambers of 24-well Transwell plates, while the upper chambers received 200 μL of neutrophil suspension (2 × 106 cells/mL). The plates were incubated in a cell culture incubator (37°C, 5% CO2) for 2 h. The Transwell chambers were then carefully removed, fixed with 4% paraformaldehyde, and cells on the membrane surface were gently removed with a cotton swab. The chambers were stained with 0.1% crystal violet, and images were captured under a microscope. Cell counting was performed using ImageJ software.
For the hepatocyte and neutrophil co-culture experiments, HepG2 cells were plated in 24-well plates, and Transwell inserts with 3 μm pore size were placed in the 24-well plates. The upper chambers were filled with 200 μL of neutrophil suspension (2.5 × 106 cells/mL) and incubated in a cell culture incubator (37°C, 5% CO2) for 20 h. Afterward, the lower chamber supernatants were aspirated, and the wells were washed twice with 1× PBS. Oil Red O staining and qRT-PCR experiments were then conducted to assess lipid accumulation in hepatocytes and the expression levels of lipid metabolism-related genes. Primers are listed in Table S3.
2.12 Statistical Analysis and Data Visualisation
All data were represented as mean ± Standard error. GraphPad Prism 9.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis. Independent sample t-tests were used to compare means between two groups, one-way ANOVA was employed for comparing means across multiple groups, and pairwise comparisons were conducted using the LSD method. Non-parametric tests were used for data that did not meet normality assumptions. In correlation analyses, quantitative variables were tested for normality. Pearson correlation was used for normally distributed data, while Spearman correlation was applied for non-normally distributed data. GraphPad Prism 9.0 software was utilised for plotting, calculating correlation coefficients (r values), and determining p values, with p < 0.05 considered statistically significant.
3 Results
3.1 Cholecystectomy Does Not Cause Abnormalities in Hepatic Lipid Metabolism Under Normal Dietary Conditions
To investigate the impact of cholecystectomy on hepatic lipid accumulation and inflammation, a PC mouse model was established. The mice were kept on a ND for the entire duration of the study (Figure 1a). There were no significant differences between the two groups in absolute body weight or weight gain compared to the week of surgery at 3 months post-cholecystectomy (Figure 1b–d). Hepatic lipid accumulation was assessed by comparing liver-to-body ratio between the two groups, with no significant differences observed (Figure 1e). H&E staining was performed to analyse hepatic tissue, specifically for hepatic steatosis, ballooning degeneration, and inflammation. The NAFLD activity scores (NAS scores), depicted in Figure 1f,g, revealed no variations among the groups. The levels of hepatic TG and TC were measured to assess hepatic lipid accumulation. Interestingly, there were no significant differences observed at 1, 2, or 3 months post-cholecystectomy (Figure 1h,i). These results suggest that cholecystectomy under normal dietary conditions does not affect hepatic lipid content or induce liver inflammation.
To further confirm the impact of cholecystectomy on hepatic lipid metabolism, including lipid synthesis, degradation, and transport, the expression of relevant genes was measured using real-time quantitative PCR (RT-qPCR). The majority of genes involved in triglyceride synthesis were expressed without significant differences between the groups, except for an increase of Dgat1 in the PC group at 3 months after modelling (Figure 1j). No significant differences in Cpt1 and Pparα expression were observed between the PC and Sham groups under ND conditions, indicating similar liver fatty acid β-oxidation levels (Figure 1k). Moreover, genes related to lipid transportation, except for Apob at 3 months, were similarly expressed between the two groups (Figure 1l). These results suggest that under normal dietary conditions, cholecystectomy has no significant effect on hepatic lipid metabolism or inflammation.
3.2 Cholecystectomy Exacerbates Hepatic Lipid Metabolism Disorders Under a High-Fat Diet
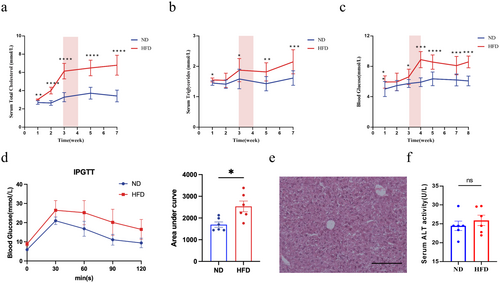
To determine the timing when mice exhibit systemic glucose and lipid metabolic disorders without hepatic lipid deposition, mice were initially fed with either a ND or a HFD, with dynamic monitoring of glucose and lipid metabolism as well as hepatic lipid accumulation. After 1 week on the HFD, mice in this group showed significant increases in blood TG, TC, and fasting blood glucose (FBG) compared to the ND group. Among these, TC levels continued to rise with prolonged high-fat feeding, resulting in a stable and significant difference between the two groups by week 3 (Figure 2a). In contrast, differences in blood TG and FBG between the groups emerged after 1 week on the high-fat diet but receded by week 2; significant differences reappeared by week 3 and remained stable and significant by week 4 (Figure 2b,c). To further clarify the metabolic state at this time, glucose tolerance tests were conducted to assess insulin resistance, revealing significant insulin resistance and impaired glucose tolerance in the HFD group after 4 weeks compared to the ND group (Figure 2d). Concurrently, dynamic monitoring of serum ALT levels and comprehensive liver pathology examination showed no significant difference in ALT levels and no signs of hepatic steatosis or liver damage under microscopy in the HFD group compared to the ND group after 4 weeks (Figure 2e,f), indicating the absence of hepatic lipid deposition and hepatocyte damage at this stage. Based on these findings, we decided to perform cholecystectomy on the HFD group after 4 weeks of high-fat diet feeding, and then to sacrifice the mice and collect blood and liver tissues after 3 months (Figure 2g).
To investigate the impact of cholecystectomy on hepatic lipid metabolism and inflammation under conditions of pre-existing insulin resistance and systemic glucose and lipid metabolic disturbances, the PC mouse model was established after 4 weeks on a HFD, with continuous high-fat diet administration throughout the study. Under HFD conditions, cholecystectomy resulted in a faster rate of weight gain in the PC group compared to the Sham group (Figure 3a). The absolute body weight of the PC group was higher than that of the sham-operated group at 3 months post-surgery (Figure 3b). The body weight gain, and the liver/body ratio of the PC group at 3 months post-surgery was significantly greater than that of the Sham group (Figure 3c,d). Both groups exhibited marked hepatic steatosis and ballooning degeneration; however, the PC group also displayed scattered inflammation within liver lobules. NAS scores were elevated in the PC group compared to the Sham group (Figure 3e,f). Circulating and hepatic TG and TC content were used to evaluate systemic metabolic disturbances and liver lipid accumulation, respectively; and circulating ALT and AST levels were assessed to evaluate liver injury. All of these parameters were significantly higher in the PC group relative to the Sham group (Figure 3g,h), suggesting that cholecystectomy under a HFD contributes to increased hepatic lipid accumulation and inflammation and may also impact systemic metabolic disorders.
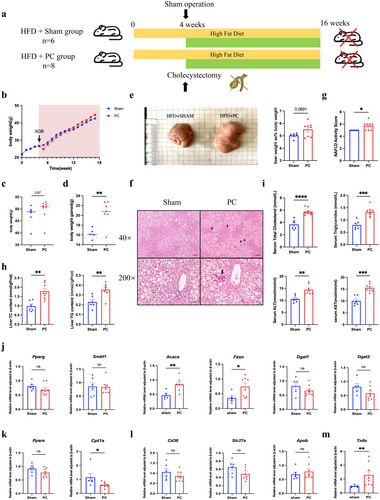
The expression of key genes involved in hepatic triglyceride synthesis was significantly elevated in the PC group (Figure 3i). Additionally, the expression levels of Ppara and Cpt1a were measured to evaluate the rate of fatty acid β-oxidation in the liver. Results indicated that Cpt1a expression was significantly reduced in the PC group compared to the Sham group (Figure 3j). Additionally, there were no significant differences in the expression of lipid transport genes between the PC and Sham groups (Figure 3k); while Tnfα, the inflammatory cytokine, was significantly higher in the HFD-fed PC mice (Figure 3l). These results suggested a disbiotic situation for HFD-dieted PC animals in hepatic triglyceride synthesis, metabolism, and liver inflammation.
3.3 Impact of Cholecystectomy on the Gut-Liver-Bile Acid Axis and Transport Systems in Mice
Orthogonal partial least squares discriminant analysis (OPLS-DA) was initially employed to observe the overall structural differences in BAs between the Sham group and the PC group in mouse ileal contents and liver tissues. The results show that bile acid distributions in the ileal contents and liver tissues of the two groups are markedly different (Figure 4a,f) indicating distinct characteristics in the bile acid profiles of the gut and liver between the groups.
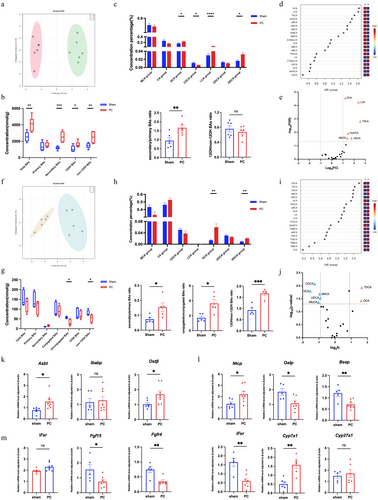
Quantitative analysis of bile acid concentrations in the ileal contents revealed a significant increase in total bile acid levels in the HFD-fed PC mice. Additionally, the ratio of secondary to primary BAs was significantly elevated in the PC group, attributable to the increased absolute concentration of secondary BAs, while no significant difference was observed in the absolute concentration of primary BAs between the two groups. Both 12α-hydroxylated (12-OH) and non-12-OH BAs were significantly increased in both groups under the high-fat diet, although the ratio of these two types of BAs did not differ significantly between the groups (Figure 4b). As to the bile acid contents in the liver tissues, no significant difference exists in total bile acid levels between the Sham and PC groups under a high-fat diet. Although the absolute concentrations of primary and secondary BAs were not significantly different between the groups, the ratio of secondary to primary BAs was significantly higher in the PC group compared to the Sham group. Additionally, the ratio of conjugated to unconjugated BAs was markedly elevated in the PC group due to a significant decrease in unconjugated BAs. Furthermore, the concentration of non-12-OH BAs was significantly lower in the PC group, resulting in a significantly higher ratio of 12-OH to non-12-OH BAs compared to the Sham group (Figure 4g).
Subsequently, we categorised the BAs in the distal ileum contents of the two groups of mice according to their types and assessed the changes in the concentrations of each bile acid. The results showed that, under a high-fat diet, compared to the Sham group, the proportions of DCA, lithocholic acid (LCA, and hyodeoxycholic acid) HDCA were significantly increased, while the proportion of chenodeoxycholic acid (CDCA) was significantly decreased in the PC group (Figure 4c). Categorization of BAs in liver tissues showed no significant differences in the proportions of Muricholic Acid (MCA), CA, CDCA, UDCA, and LCA between the PC and Sham groups, except for DCA and HDCA (Figure 4h).
Differential BAs were identified based on their concentrations. Using the VIP values from the first principal component of the OPLS-DA model, we identified differential gut BAs as DCA, LCA, TDCA, muroCA, alloCA, and HDCA, all of which were upregulated in the HFD-dieted PC mice (Figure 4d,e). For liver BAs, TDCA, DCA, CDCA, UDCA, βCA, βMCA and alloCA were revealed as differential liver BAs. Among these, TDCA and DCA were upregulated, while CDCA, UDCA, βCA, βMCA and alloCA were downregulated (Figure 4i,j).
The findings suggest that a high-fat diet following cholecystectomy induces substantial changes in bile acid profiles in both the intestine and liver, potentially affecting receptors and transporters associated with the enterohepatic circulation of BAs. Therefore, an evaluation was conducted on the expression of primary bile acid receptors and transporters involved in the enterohepatic circulation.
Intestinal bile acid transporters, such as the apical sodium-dependent bile acid transporter (ASBT), cooperating with the ileal bile acid binding protein (IBABP) and the organic solute transporter alpha/beta (OST α/β), mediated BAs transportation from the intestinal lumen to the portal vein. We found the mRNA levels of ASBT and OST β are significantly elevated in the PC group, whereas IBABP levels do not show a significant difference (Figure 4k). Hepatic bile acid transporters, including the sodium taurocholate cotransporting polypeptide (NTCP) and organic anion transporting polypeptides (OATP), and the bile salt export pump (BSEP), are responsible for transporting BAs from the portal vein into hepatocytes and bile ducts. Data showed that, compared to the Sham group, mRNA levels of NTCP and BSEP are significantly increased in the PC group, whereas OATP levels are significantly reduced (Figure 4l).
Other genes involved in bile acid metabolism were also evaluated, including bile acid receptors, signalling molecules, and synthesising enzymes, such as the intestinal Farnesoid X receptor (iFxr), fibroblast growth factor 15 (Fgf15), fibroblast growth factor receptor 4 (Fgfr4), liver FXR (lFxr), cholesterol 7α-hydroxylase (Cyp7a1), and cholesterol 27α-hydroxylase (Cyp27a1). Analysis of the mRNA expression levels of these genes revealed that, following cholecystectomy under a high-fat diet, the gene expression levels of the intestinal Fxr-Fgf15-Fgfr4 signalling axis were significantly downregulated. Additionally, the gene expression level of the key bile acid synthesis enzyme Cyp27a1 did not show significant variation, whereas the expression of Cyp7a1 was notably upregulated (Figure 4m).
These findings indicate that cholecystectomy under a high-fat diet markedly influences the bile acid profiles in both the intestine and liver, as well as the enterohepatic circulation of BAs. Given the essential role of BAs in energy metabolism and inflammatory responses, these findings suggest that changes in bile acid levels are likely involved in the development of hepatic lipid metabolic disorders and inflammation following cholecystectomy on a high-fat diet.
3.4 Impact of Cholecystectomy on Hepatic Immune Cells in Mice
The flow cytometry was performed to elucidate the underlying mechanisms of hepatic lipid metabolism disorders and exacerbated inflammation following cholecystectomy under high-fat diet conditions. The results demonstrated that, 3 months after cholecystectomy under ND, there was a significant increase in the proportion of B220+ B cells in the liver of the PC compared to the Sham group (Figure 5a). No significant differences were observed in the proportions of other immune cell types between the groups, including CD11b+F4/80+ macrophages (Figure S2a), CD11b+Ly6G+ neutrophils (Figure S2b), CD11c+ dendritic cells (Figure S2c), CD4+ helper T cells (Figure S2d), and CD8+ cytotoxic T cells (Figure S2e). These findings indicated that, under normal dietary conditions, cholecystectomy results in a notable increase in hepatic B cell proportions 3 months after modelling, while the proportions of other immune cell types do not exhibit significant changes.
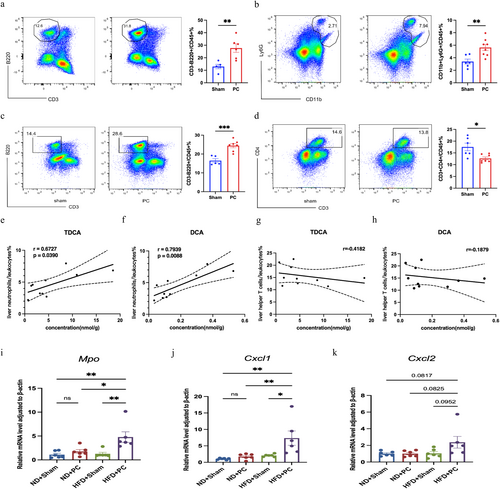
In HFD-fed PC mice, there was a significant increase in the proportions of CD11b+Ly6G+ neutrophils and B220+ B cells in the liver (Figure 5b,c). Conversely, CD4+ helper T cells were significantly reduced (Figure 5d). No significant changes were observed in the proportions of CD11c+ dendritic cells (Figure S2f), CD11b+F4/80+ macrophages (Figure S2g), and CD8+ cytotoxic T cells (Figure S2h) between the PC and Sham groups. These findings indicate that, under high-fat dietary conditions, cholecystectomy results in increased hepatic proportions of B cells and neutrophils, and a decrease in CD4+ helper T cells after 3 months.
3.5 Correlation Between Differential BAs and Immune Cell Changes
Based on the aforementioned results, since cholecystectomy exacerbated liver lipid metabolism disorders exclusively under high-fat diet conditions, this suggests that immune cells, specifically neutrophils and CD4+ T cells, may be involved in the progression of liver lipid metabolism disorders and inflammation. Therefore, correlations between differential BAs, that is, DCA and TDCA, and the proportion of neutrophils and CD4+ T cells in the liver were analysed in both PC and Sham groups. The results indicated that differential BAs exhibited strong correlations with changes in liver neutrophil proportions (Figure 5e,f), rather than CD4+ T cells (Figure 5g,h). This suggests that neutrophils likely play a significant role in the exacerbation of liver lipid metabolism disorders and inflammation following bile acid changes post-cholecystectomy.
3.6 Alterations in Neutrophil Markers and Chemokine Levels in Liver Tissue
To further validate changes in neutrophil numbers and functions in liver tissue following cholecystectomy under a high-fat diet, the gene expression levels of neutrophil-associated markers myeloperoxidase (Mpo) and the chemokines CXC motif ligand 1 (Cxcl1) and CXC motif ligand 2 (Cxcl2) were assessed. The results indicated that, under a ND, the mRNA expression levels of Mpo, Cxcl1, and Cxcl2 were similar between the PC and Sham groups. In contrast, under a high-fat diet, the PC group exhibited significantly elevated mRNA expression levels of Mpo and Cxcl1 in liver tissue (Figure 5i–k). These findings revealed that in mice on a high-fat diet, undergoing cholecystectomy resulted in increased expression of markers and chemotactic factors that attract neutrophils to the liver, indicating changes in both the quantity and function of neutrophils.
3.7 1 mM OA: PA (2:1) Solution Simulates Hepatocytes Under High-Fat Conditions
When HepG2 hepatocytes were exposed to mixtures of OA and PA at various concentrations (ranging up to 1000 μM), there was a clear dose-dependent increase in both intracellular TG levels and the size of lipid droplets, as observed through Oil Red O staining (refer to Figure S1a,b). In addition, it should be noted that there was no significant rise in ALT levels in the cell supernatant at concentrations up to 1000 μM. Nonetheless, a notable increase was detected at 1200 μM (Figure S1c). The results indicate that treatment of hepatocytes with an OA: PA (2:1) solution leads to lipid deposition within the cells without causing cellular damage. With adherence to the animal experiments, our aim was to simulate a state of lipid metabolism disorders that does not involve cellular injury and investigate the consequences of bile acid alterations on hepatocytes after cholecystectomy. For this purpose, we chose a specific concentration (1 mM) of an OA: PA (2:1) solution that leads to visible lipid deposition without causing any harm to the cells for the following experiments.
3.8 Enhanced Chemokine Release and Decreased FXR Expression From Liver Cells Following Stimulation With DCA or TDCA
The liver cells were divided into two groups: one treated with OA and PA solutions to simulate a high-fat state, and the other left untreated to represent a normal state. The cells were subjected to various concentrations of DCA and TDCA (0, 50, 100, and 200 μM) for different time periods (0, 24, 48, 72 h). The expression level of key chemokines, CXCL1 and CXCL2, was measured at both the mRNA and the protein levels (Figure 6a).
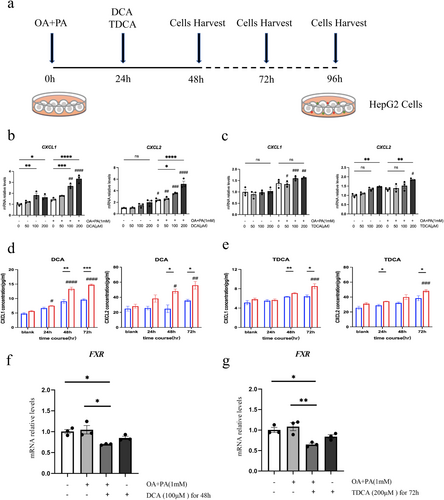
Results showed that liver cells treated with 1 mM OA and PA solutions exhibit significantly increased mRNA expression levels of the chemokines Cxcl1 and Cxcl2 when exposed to 100 μM DCA. With a further increase in DCA concentration to 200 μM, the expression levels of Cxcl1 and Cxcl2 showed a more pronounced increase. Compared to untreated liver cells exposed to the same concentration of DCA, liver cells treated with OA and PA exhibited a significantly higher level of Cxcl2 expression, while Cxcl1 expression was significantly elevated at DCA concentrations of 100 and 200 μM (Figure 6b). In contrast, for liver cells treated with 1 mM OA and PA, although there was a trend toward increased Cxcl1 expression with rising TDCA concentrations, no significant difference was observed. However, at a TDCA concentration of 200 μM, Cxcl2 expression was significantly increased. Compared to untreated liver cells exposed to the same TDCA concentration, Cxcl1 expression was significantly higher at TDCA concentrations of 50 μM and 100 μM, and both Cxcl1 and Cxcl2 expressions were markedly elevated at 200 μM TDCA (Figure 6c). Collectively, these results suggest that liver cells treated with OA and PA show a notable increase in Cxcl1 and Cxcl2 expression when exposed to 100 μM DCA or 200 μM TDCA.
To assess the concentrations of the chemokines CXCL1 and CXCL2 released into the culture supernatant, liver cells were stimulated with either 100 μM DCA or 200 μM TDCA for different durations. Stimulation with 100 μM DCA led to a time-dependent increase in CXCL1 and CXCL2 concentrations in the supernatant of liver cells treated with OA and PA, with significant differences observed. The supernatant of OA and PA-treated liver cells showed a significant increase in the levels of CXCL1 and CXCL2 after 48 h of DCA stimulation, in contrast to untreated liver cells (Figure 6d). Similarly, stimulation with 200 μM TDCA resulted in a significant increase in CXCL1 and CXCL2 concentrations in the supernatant of OA and PA-treated liver cells after 72 h, with markedly higher levels compared to untreated liver cells (Figure 6e). These results indicate that exposure of liver cells to 100 μM DCA for 48 h or 200 μM TDCA for 72 h significantly enhances the release of CXCL1 and CXCL2.
To further investigate the regulatory effects of DCA and TDCA stimulation on FXR in hepatocytes, we systematically analysed FXR expression under different experimental conditions. The results revealed that exposure to 100 μM DCA for 48 h significantly decreased FXR expression in OA/PA-treated groups compared with unstimulated controls (including normal controls and OA/PA-treated groups without DCA) (p < 0.05). Notably, while DCA stimulation reduced FXR expression in non-OA/PA-treated hepatocytes, this reduction did not reach statistical significance (p > 0.05). Similarly, 200 μM TDCA intervention for 72 h induced a significant FXR expression decrease in OA/PA-treated hepatocytes relative to unstimulated controls (p < 0.05). However, the same TDCA dosage in normal hepatocytes only exhibited a downward trend in FXR expression (p > 0.05). These findings collectively suggest that both DCA and TDCA treatments suppress FXR expression in hepatocytes, and hyperlipidemic conditions may enhance hepatocyte sensitivity to bile acids (Figure 6f,g).
3.9 Bile Acid Enhances Neutrophil Chemotaxis and Disrupts Lipid Metabolism in Liver Cells
In liver cells under high-fat conditions, the stimulation of bile acid has been found to notably enhance the release of CXCL1 and CXCL2. In order to evaluate the impact of chemokines on neutrophils, they were isolated from human peripheral blood and then tested in chemotaxis assays. The results revealed that DCA stimulation not only enhances neutrophil chemotaxis in untreated liver cells, but also significantly increases it in liver cells treated with OA and PA. Neutrophils from untreated liver cells did not show a significant chemotactic response to TDCA stimulation. However, when liver cells were treated with OA and PA, TDCA greatly enhanced their chemotaxis (Figure 7b–d). The findings of this research demonstrated that the introduction of DCA or TDCA to liver cells in a high-fat state leads to a significant amplification of neutrophil chemotaxis.
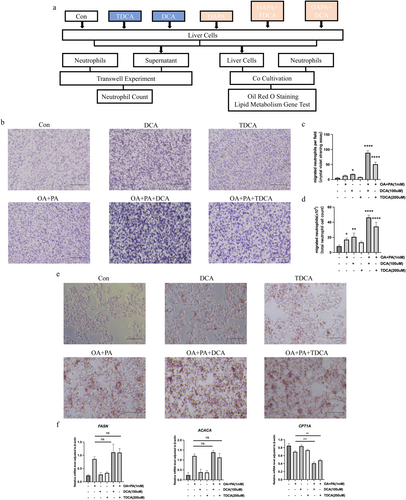
In order to delve deeper into the effects of bile acid stimulation and neutrophil chemotaxis on liver cells, we conducted co-culture experiments involving both liver cells and neutrophils. The accumulation of lipids in liver cells was evaluated, along with the expression levels of genes related to lipid metabolism. The results showed that when liver cells were treated with OA and PA and then stimulated with DCA or TDCA, they had a higher number of lipid droplets compared to cells that were not stimulated with BAs, indicating a more pronounced lipid accumulation. Some liver cells stimulated with DCA even showed morphological changes (Figure 7e). Furthermore, mRNA expression levels of lipid metabolism genes Fasn, Acaca, and Cpt1a were analysed. While Fasn and Acaca showed no significant differences in high-fat state liver cells with or without bile acid stimulation, Cpt1a expression was significantly reduced in bile acid-stimulated high-fat state liver cells compared to those without stimulation (Figure 7f). The findings indicate that, after cholecystectomy, the varying stimulation of BAs in high-fat state liver cells and the resulting neutrophil chemotaxis may worsen lipid accumulation in liver cells, potentially caused by decreased fatty acid β-oxidation.
4 Discussion
The assessment of liver phenotype, pathology, biochemical parameters, and lipid metabolism gene expression revealed that cholecystectomy significantly exacerbated hepatic lipid deposition and inflammation under high-fat dietary conditions, but not under the ND. The obtained results are in agreement with the findings reported in previous studies [30, 31]. Research indicates that the increased accumulation of lipids in the liver is driven by multiple factors, primarily including lipogenesis, lipid transport, and fatty acid β-oxidation [32-34]. Further analysis of genes involved in hepatic lipid metabolism indicated increased expression of genes related to TG synthesis, such as Acaca and Fasn, and decreased expression of genes involved in TG β-oxidation, such as Cpt1a. However, genes associated with lipid transport exhibited no significant changes. These observations suggest that post-cholecystectomy hepatic lipid metabolic dysregulation is likely driven by enhanced de novo lipogenesis and diminished β-oxidation.
Similar to previous investigations [35], this study also observed significant differences in the concentration and compositions of bile acid pools between individuals with MAFLD and healthy individuals. Previous studies have confirmed that DCA and its derivatives increase with MAFLD disease activity [36, 37]. Notably, TDCA is significantly elevated in MAFLD patients and experimental models, promoting hepatic stellate cell proliferation and fibrosis-related protein expression [38]. Spearman correlation analysis reveals a robust association between TDCA levels and the degree of hepatic steatosis and fibrosis [39]. Additionally, LCA and its derivatives demonstrate a strong correlation with MAFLD and hepatocellular ballooning [40]. These findings imply that BAs, acting as important signalling molecules, probably have a substantial impact on the disturbance of hepatic lipid metabolism and inflammation subsequent to cholecystectomy while on a high-fat diet.
Since 12-OH BAs are primarily synthesised through the classical bile acid synthesis pathway, while non-12-OH BAs are mainly produced via the alternative pathway [41], the ratio between these two groups can serve as an indicator of alterations in bile acid synthetic pathways. Our study revealed that the concentration of non-12-OH BAs was significantly lower in the PC group, resulting in a significantly higher ratio of 12-OH to non-12-OH BAs compared to the Sham group. This observation was accompanied by decreased expression of Fgf15 in ileal tissue and Fgfr4 in hepatic tissue, along with upregulated expression of the bile acid synthetic enzyme Cyp7a1 but no significant change in Cyp27a1 expression. These findings suggest that downregulation of intestinal FXR may attenuate its negative feedback inhibition on bile acid synthesis. Importantly, unlike the hepatic FXR/SHP signalling axis, the intestinal FXR/FGF15/FGFR4 axis specifically targets CYP7A1, the rate-limiting enzyme of the classical pathway, without affecting CYP27A1 in the alternative pathway [42]. Notably, an increased ratio of 12-OH to non-12-OH 12-OH BAs has been associated with more severe inflammatory responses and oxidative stress levels [41, 43]. Therefore, our results indicate that reduced bile acid synthesis through the alternative pathway following cholecystectomy under high-fat diet conditions may contribute to the exacerbation of hepatic lipid metabolism disorders.
Compared to the stable concentration of total BAs in the liver, the ratio of secondary to primary BAs and the proportion of conjugated to unconjugated BAs significantly increase in the PC group. Conjugated BAs and secondary BAs exhibit greater hydrophobicity and cytotoxicity compared to unconjugated BAs and primary BAs [44]. Consequently, these compounds can exert direct toxic effects on hepatocytes and, due to their higher affinity for various bile acid receptors, play a more significant role in diverse metabolic regulations. Furthermore, under high-fat conditions, significant increases in DCA, HDCA, and their derivatives are observed post-cholecystectomy. The elevated LCA levels in the ileum do not translate to an increase in the liver, most likely because of its high hydrophobicity and primary excretion through faeces [45]. Multi-dimensional and univariate statistical analysis reveals that secondary and hydrophobic BAs (e.g., DCA and TDCA) are upregulated, whereas primary and hydrophilic BAs (e.g., CDCA, UDCA, βMCA, and βCA) are significantly downregulated in the PC group. These differential bile acid profiles could serve as valuable markers. DCA and TDCA levels are notably increased in several population-based studies on MAFLD [35, 46], potentially inducing liver damage through mitochondrial depolarization and oxidative stress, stimulating hepatic stellate cell secretion of various inflammatory factors, and interacting with bile acid receptors to regulate metabolism [47, 48]. In this study, the increased mRNA expression levels of Ntcp and the decreased levels of Oatp and Bsep in mice following cholecystectomy under a high-fat diet may contribute to the elevated ratio of conjugated to unconjugated BAs in the liver [49, 50]. Regarding the receptors and transporters implicated in the enterohepatic circulation, a downregulation of the intestinal FXR-FGF15-FGFR4 axis was observed, alongside increased bile acid reabsorption in the gut, augmented hepatic bile acid uptake, and reduced bile acid secretion. These findings suggest that, under high-fat dietary conditions, cholecystectomy significantly affects the bile acid profile in the intestine and liver, as well as the enterohepatic circulation, potentially contributing to hepatic lipid metabolism disorders and inflammation.
Previous research has predominantly focused on monocytes/macrophages in the pathogenesis of MAFLD, but recent studies have highlighted the increasing importance of neutrophils [51-53]. Only recently have researchers begun to investigate the impact of changes in bile acid levels on MAFLD following cholecystectomy. A correlation analysis showed a strong relationship between neutrophils and differential BAs, while helper T cells showed none. Co-culture studies revealed that neutrophils from nonalcoholic steatohepatitis (NASH) patients exhibit an activated phenotype that suppresses CD4+ T cell proliferation, potentially reducing hepatic immune defence and promoting the progression from nonalcoholic fatty liver (NAFL) to NASH [54]. Additionally, under high-fat conditions, neutrophils exhibit heightened production of reactive oxygen species (ROS), release of MPO, and expression of CD11b [55]. The elevated MPO and CXCL1 levels in the livers of high-fat diet and cholecystectomy mice were consistent with the findings of this study. These findings suggest that changes in hepatic BAs following cholecystectomy may exacerbate hepatic lipid metabolism disorders and inflammation through increased neutrophil infiltration.
In our experiments, CXCL1 and CXCL2 release was significantly elevated in OA/PA-treated hepatocytes following stimulation with DCA or TDCA, and DCA and TDCA interventions suppressed FXR expression in hepatocytes. Previous studies similarly suggest that TDCA acts as an antagonistic bile acid for FXR [56]. As a nuclear receptor, FXR directly inhibits the NF-κB signalling pathway, and its downregulation relieves this suppression, triggering transcriptional activation of downstream inflammatory mediators (e.g., IL-6, TNF-α) and chemokines (CXCL1, CXCL2) [57]. Second, FXR negatively regulates pro-inflammatory genes by inducing small heterodimer partner (SHP); diminished FXR expression attenuates SHP-mediated inhibition of inflammation-associated transcription factors (e.g., AP-1, STAT3), thereby amplifying chemokine production [58]. Additionally, FXR deficiency exacerbates mitochondrial dysfunction and oxidative stress, with accumulated reactive oxygen species (ROS) promoting chemokine release (e.g., IL-1β) via activation of the NLRP3 inflammasome and MAPK/JNK pathways [23]. Collectively, FXR downregulation drives the upregulation of hepatocyte chemokine expression through multi-dimensional disinhibition of inflammatory cascades. Thus, the increased release of CXCL1 and CXCL2 from hepatocytes following DCA and TDCA intervention may be attributed to a marked reduction in hepatocyte FXR receptor levels.
Mechanically, changes in hepatic BAs are often associated with increased neutrophil recruitment [59]. Multiple studies have demonstrated that neutrophils are crucial immune cells in acute liver injury induced by cholestasis [60, 61]. Neutrophil depletion markedly alleviates hepatocellular injury [62], indicating that neutrophils are the primary effector cells in mediating the chemotactic immune response of hepatocytes under the influence of BAs. Additionally, liver biopsies indicate that excessive neutrophil infiltration is a prominent histological feature of MAFLD, and neutrophil depletion can significantly reduce liver injury in murine models of this condition [55], suggesting a potential impact of neutrophils on the induction of MAFLD. These findings underscore the bridging role of neutrophils in bile acid changes and hepatic lipid metabolic disorders, further supporting the results of this study. By analysing the expression of lipid metabolism genes, we infer that neutrophils are likely causing disruption in hepatic lipid metabolism by interfering with liver fatty acid β-oxidation. Research has revealed that with neutrophil-induced mitochondrial dysfunction and β-oxidation defects in hepatocytes, excessive accumulation of lipids and lipotoxic metabolic products occurs in hepatocytes, exacerbating metabolic disturbances [63, 64]. Due to the short in vitro half-life, neutrophils can only be co-cultured with hepatocytes for a limited time. This explains why neutrophils were observed to primarily affect hepatocyte fatty acid β-oxidation, likely representing an initial effect. As neutrophils persist in the liver, they may exert broader effects on lipid metabolism, including synthesis, oxidation, and transport.
Increasing evidence suggests that neutrophils impact hepatic lipid metabolism and lipid accumulation through multiple pathways. Neutrophil-derived MPO disrupts lipid metabolism and causes hepatocellular injury. Neutrophils can also release various granule proteins, including neutrophil serine proteases (NSPs), such as neutrophil elastase (NE) and proteinase 3 (PR3), which are elevated in MAFLD patients [65]. NE is a significant source of various key cytokines, such as IL-1β, which can stimulate TG and free cholesterol accumulation, as well as the formation of lipid droplets in hepatocytes [66]. Similarly, PR3 has been shown to promote MAFLD via IGF-1 and IGFBP-3 [67]. In HFD-fed mouse models, dual knockout of NE and PR3 or single knockout of NE markedly improved hepatic steatosis [68, 69]. Furthermore, the unique structure released by neutrophils—neutrophil extracellular traps (NETs)—contribute to hepatic lipid metabolism disorders and inflammation by influencing hepatocyte lipid metabolism and enhancing neutrophil infiltration, thus recruiting macrophages and establishing a positive feedback loop during MAFLD progression [63, 70]. These findings indicate that neutrophils play diverse roles in hepatic lipid metabolism disorders and lipid accumulation, warranting further investigation of their mechanisms.
The study has some limitations: first, the use of liver homogenates in animal studies inherently limits cell type-specific resolution, as non-parenchymal cells (e.g., Kupffer cells, stellate cells) may obscure hepatocyte-selective FXR alterations. Second, the downstream signalling pathways mediating FXR-dependent chemokine regulation remain to be further investigated. Third, further investigation into specific mechanisms underlying diet–cholecystectomy interactions in MAFLD pathogenesis is valuable. Last, while this study delineates neutrophil-driven liver damage pathways in MAFLD, it does not comprehensively address their crosstalk with other immune populations (e.g., macrophages, T-cells) that jointly orchestrate disease progression, which requires future investigation using single-cell transcriptomics or multicellular coculture models to map intercellular communication pathways.
5 Conclusion
In summary, a high-fat diet after cholecystectomy is more likely to induce hepatic lipid deposition and inflammation compared to the ND, indicating that the dietary composition following cholecystectomy may significantly influence the risk of developing MAFLD in patients. Additionally, modifications in the bile acid compositions of the gastrointestinal tract and liver have been observed subsequent to cholecystectomy, accompanied by corresponding alterations in bile acid receptors and transporters. The alterations in hepatic BAs prompt the secretion of chemokines from liver cells, thereby facilitating the recruitment of neutrophils to the liver. This subsequently exacerbates hepatic lipid metabolic disorders and inflammation. Therefore, this research supplies theoretical support in comprehending the long-term metabolic complications, such as MAFLD, that arise after cholecystectomy. This study introduces novel complexities in preoperative assessment and indications for cholecystectomy among high-risk populations. Additionally, it proposes potential therapeutic targets for the prevention and treatment of MAFLD after cholecystectomy. The findings highlight the substantial clinical implications and stress the imperative for thorough mechanistic examinations and comprehensive clinical evaluations in future research to fully harness the potential of these insights.
Author Contributions
Yulan Liu and Jun Xu designed this study. Yanting Xu, Ziliang Ke, Feng Zhang, Yiken Lin, Yang Zhang, Yun Liu, and Yifan Zhang performed the experiments. Yanting Xu, Jun Xu, and Feng Zhang conducted data analysis and visualisation. Feng Zhang and Yanting Xu wrote, and Yulan Liu and Jun Xu revised this manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgements
The authors have nothing to report.
Ethics Statement
This study was approved by the Institutional Medical Ethics Review Board of Peking University People's Hospital (Document ID: 2021PHB346-001).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.




