Randomized Open-Label Clinical Trial Comparing Prednisolone and Cyclosporine With a Nonrandomized Active Control for Treating Presumed Chronic Pancreatitis in Cats
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Current management for chronic pancreatitis in cats is largely symptomatic. Anecdotal reports suggest that immunomodulatory treatment can be helpful in some cases, but limited data is available.
Objectives
Compare the effects of symptomatic treatments alone, an immunosuppressive dosage of prednisolone, or modified cyclosporine on serum feline pancreatic lipase immunoreactivity (fPLI) concentration and clinical activity index (CAI).
Animals
Forty-eight client-owned cats with a presumptive diagnosis of chronic pancreatitis were managed on an outpatient basis.
Methods
Three-week randomized open-label trial with a nonrandomized active control. Owners elected to join either the control or the treatment group; cats enrolled in the treatment group were randomized to receive either prednisolone or cyclosporine. Serum fPLI concentration and clinical signs were recorded at baseline and on Days 10 and 21.
Results
The average decrease in serum fPLI concentration was 13.0 μg/L (95% CI, −23.9 to −0.9 μg/L) larger for the cyclosporine group (n = 17) than for the control group (n = 16) and 27.6 μg/L (95% CI, −41.2 to −11.4 μg/L) larger than for the prednisolone group (n = 15). The average decrease in CAI was 1.9 points (95% CI, −2.7 to −1.2) larger for the prednisolone group than for the control group and 1.2 points (95% CI, −2.1 to −0.4) larger than for the cyclosporine group.
Conclusions
Over a 3-week treatment period, cats with presumed chronic pancreatitis that received cyclosporine had a larger decrease in serum fPLI concentration compared with cats that were treated with an immunosuppressive dosage of prednisolone or cats that received only symptomatic treatments. However, clinical improvement was more apparent with prednisolone, but not cyclosporine.
Abbreviations
-
- BCS
-
- body condition score
-
- CAI
-
- clinical activity index
-
- CBC
-
- complete blood count
-
- CI
-
- confidence interval
-
- cPLI
-
- canine pancreatic lipase immunoreactivity
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- fPLI
-
- feline pancreatic lipase immunoreactivity
-
- fTLI
-
- feline trypsin-like immunoreactivity
-
- GI
-
- gastrointestinal
-
- PC
-
- principal component
-
- PO
-
- per os
-
- RI
-
- reference interval
-
- Spec fPL
-
- specific feline pancreatic lipase
1 Introduction
Chronic pancreatitis is widely considered common in cats. Although pancreatitis is amenable to antemortem diagnosis by integrating all available clinical and diagnostic information, a definitive diagnosis of chronic pancreatitis by current definition requires histopathologic confirmation [1]. In one study of cats presented for necropsy regardless of the cause of death, the overall histologic prevalence of pancreatitis was 66.1%, with 50.4% of cats having histologic evidence of chronic pancreatitis alone [2]. However, little research is available on the treatment of chronic pancreatitis in cats. The cause of pancreatitis in cats often remains idiopathic. Hence, the current management for chronic pancreatitis in cats is largely symptomatic (e.g., analgesics and anti-emetics). Concurrent and potentially predisposing diseases (e.g., chronic enteropathy and cholangitis) should be diagnosed and managed when present [1].
Targeted treatment for chronic pancreatitis has not been defined [1]. Anti-inflammatory or immunosuppressive treatment can be helpful in some cases, and variable dosing protocols of prednisolone or cyclosporine have been proposed [1]. A retrospective study reviewed 19 cats with presumed chronic pancreatitis that were treated with cyclosporine and found that serum feline pancreatic lipase immunoreactivity (fPLI) concentration was significantly lower after treatment, but the follow-up period was not standardized, and no other treatments were compared [3]. A case report also documented a favorable response to prednisolone in a cat with lymphoplasmacytic pancreatitis (tapering dose starting from 1 mg/kg PO q24h) [4]. However, no studies have compared the effects of symptomatic treatment alone, prednisolone, and cyclosporine for the treatment of pancreatitis in cats.
Our aims were to compare the effects of symptomatic treatment alone, an immunosuppressive dosage of prednisolone, and modified cyclosporine on serum fPLI concentration and a clinical activity index (CAI) defined by relevant clinical signs in cats with presumed chronic pancreatitis.
2 Materials and Methods
2.1 Trial Design
Three-week open-label randomized clinical trial comparing prednisolone and cyclosporine with a nonrandomized active control for the treatment of presumed chronic pancreatitis in cats. The study consisted of two screening steps and two scheduled reevaluations. The second screening step was used as pretreatment baseline for the immunosuppressive treatment groups. Reevaluations were scheduled on Days 10 and 21 of the treatment trial. Figure 1 depicts the study timeline and the main outcomes assessed.
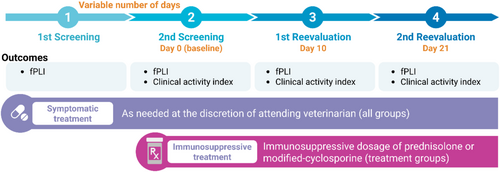
2.2 Study Population and Eligibility (First Screening Step)
Cats were recruited from veterinarians in the United States who submitted testing of serum fPLI concentration as part of their patient's diagnostic evaluation. A first screening serum fPLI concentration > 10 μg/L (reference interval [RI], ≤ 4.4 μg/L) along with serum cobalamin concentration > 400 ng/L (RI, 290–1500 ng/L) and serum folate concentration within the RI (9.7–21.6 μg/L) were used as search criteria in the laboratory database to identify potential candidates for enrollment. The rationale for setting the first screening fPLI criterion at 10 μg/L is based on our empirical observation that patients with higher serum fPLI concentrations are more likely to continue to have increased serum fPLI concentrations when compared to those cats with a serum fPLI concentration that is only mildly increased. The inclusion of normal cobalamin and folate concentrations in the search criteria was intended to help decrease the chance of enrolling cats with concurrent intestinal diseases. The cutoff for serum cobalamin concentration was chosen because previous work showed that some cats with serum cobalamin concentrations in the lower end of the RI had increased serum methylmalonic acid concentrations, suggesting cobalamin deficiency on a cellular level [5, 6].
Additional medical histories and diagnostic tests were requested from veterinarians with patients who met the search criteria to confirm eligibility. Cats of any age, breed, and sex were eligible for enrollment. Criteria for eligibility consisted of (1) diagnosed by the primary veterinarian as having pancreatitis for > 2 weeks based on clinical evaluation (e.g., medical history and physical examination); (2) no diet changes within 2 weeks, but if this was the only violation of criteria, cats were allowed to be enrolled 2 weeks after the diet change; (3) no treatment with any medications that might cause pancreatitis (e.g., phenobarbital, organophosphates, calcium); and (4) no recent (within 3 months) or current use of corticosteroids or other immunosuppressants. Concurrent disease was not an exclusion factor, but patients who were stable and had fewer concurrent diseases were prioritized for recruitment.
2.3 Treatment Assignment and the Second Screening Step
For cats that met the first screening criteria and were considered eligible, the owner and the attending veterinarian were given the choice to enroll the cat into either the control or the treatment group. A choice was given, instead of being based on randomization, mainly for ethical reasons, understanding that the balance of measured and unmeasured covariates cannot be ensured between the control group and the two treatment groups.
Cats that were enrolled in the treatment group were randomly assigned to receive prednisolone or cyclosporine (described in Section 2.5). Randomization was performed before the second screening step, and results were revealed to the care team immediately to avoid delaying potentially beneficial immunosuppressive treatment after blood collection at baseline. If the second screening serum fPLI concentration was < 5.4 μg/L, the cat was excluded from the study. The second screening fPLI criterion was set at 5.4 μg/L because the study followed the previous Spec fPL cutoff for diagnosis of pancreatitis [7] and was conducted before the cutoff was adjusted to ≥ 8.8 μg/L [8].
2.4 Sample Size Determination
Sample size was not calculated because no studies have documented the crucial variables regarding the effects of symptomatic treatment alone, prednisolone, or cyclosporine on serum fPLI concentration or the CAI in cats with pancreatitis. A desirable difference in clinically meaningful outcomes for pancreatitis in cats has not been determined. Therefore, we aimed to enroll a reasonable number of cats in hopes that doing so would lead us to the ideal sample size for future studies. Hence, the number of cats per group was empirically determined to be 15. To account for the number of cats that might be excluded at the second screening step or die or be euthanized because of their illness, five additional cats were requested for each group. Both first and second screening serum fPLI concentrations were initially set at > 20 μg/L following a previous study [9]; during this period, two cats were enrolled but later excluded after failing to meet the second screening fPLI criterion. The screening fPLI criteria were revised (detailed in Sections 2.2 and 2.3), and only 58 cats enrolled with these criteria were analyzed and discussed in the study.
2.5 Intervention Regimen
Cats in the control group only received symptomatic treatment as needed at the discretion of the attending veterinarian, and these treatments were not necessarily started at baseline. Cats in the prednisolone group received prednisolone at 2 mg/kg PO q12h for the first 5 days and were then transitioned to 1 mg/kg PO q12h for the remainder of the 16 days. Cats in the cyclosporine group received cyclosporine at 5 mg/kg PO q24h for 21 days.
For ethical reasons, all groups were allowed to receive symptomatic treatments or treatments for concurrent diseases as needed at the discretion of the attending veterinarian, understanding that doing so could confound clinical outcome assessment. Owners were required to keep the current diets unchanged throughout the trial period. No additional anti-inflammatory or immunosuppressive treatments were allowed during the study period.
2.6 Outcome Assessments and Assays
Serum fPLI concentration and CAI were used as outcomes for the evaluation of treatment effects. Outcomes were assessed at baseline, Day 10, and Day 21 of the study period. All visits were conducted at the patient's local hospital with the attending veterinarians. Serum fPLI concentrations were measured using a commercially available ELISA Kit (Spec fPL, IDEXX Laboratories, Westbrook, ME, USA) [8]. The CAI, modified from the feline chronic enteropathy activity index [10], was the sum of scores for seven clinical variables that often are related to the clinical presentation of cats with pancreatitis, which was recorded by the attending veterinarian at each visit. These consisted of activity, appetite, weight loss, vomiting, icterus, abdominal pain, and diarrhea. Each clinical variable was assessed with an ordinal value of 0, 1, 2, or 3; a higher score corresponds to a more severe clinical sign. Definition of score and corresponding severity for each clinical variable is shown in Table S1.
Complete blood counts (CBCs) were submitted to the Texas A&M Veterinary Medical Diagnostic Laboratory. Serum biochemistry profiles were automatically analyzed (AU 480 chemistry analyzer, Beckman Coulter, Indianapolis, Indiana); serum cobalamin and folate concentrations were measured by automated competitive binding chemiluminescence immunoassay (IMMULITE 2000 XPi, Siemens Healthineers, Erlangen, Germany), and serum feline trypsin-like immunoreactivity (fTLI) concentrations were measured by radioimmunoassay [11] at the Gastrointestinal Laboratory, Texas A&M University.
2.7 Statistical Analyses
Spearman correlations were used to evaluate associations between changes in serum fPLI concentration and days between the first and second screening tests. Relevant clinical signs that developed within the last 3 months before baseline and demographics at baseline were described using descriptive statistics. Binomial testing was used to determine if the proportion of male cats in total or in each group was different from 50%. Normality was evaluated using Shapiro–Wilk tests. Differences in age, body condition score (BCS), serum fPLI concentration, and CAI were compared using Kruskal–Wallis tests, followed by Dunn's multiple comparison tests.
To account for the baseline clinical presentations in the regression model, principal components derived from the baseline clinical variables were used instead of individual baseline clinical variables or the baseline CAI, because (1) baseline clinical variables showed little variability across subjects and (2) sums of ordinal data (e.g., CAI) are not meaningful statistics because of unequal intervals between categories. That is, adding ordinal values together does not accurately represent the data. The significance of the principal component analysis (PCA) was analyzed [12], and only the first principal component was significant at the 5% level, which captures only 42% of the variability in the data. Therefore, the first three principal components that together explained at least 80% of variability were extracted to perform principal component regression [13].
Treatment effects on serum fPLI concentration and CAI were estimated with linear mixed-effects models using different baseline adjustment methods; the corresponding response types were (1) fPLIreevaluation: serum fPLI concentration on Days 10 and 21, (2) ∆fPLI = fPLIreevaluation − fPLIbaseline, (3) %∆fPLI = ∆fPLI/fPLIbaseline × 100%, (4) CAIreevaluation: CAI on Days 10 and 21, and (5) ∆CAI = CAIreevaluation − CAIbaseline (Table 1; fPLIbaseline: baseline serum fPLI concentration; CAIbaseline: baseline CAI). Standard errors for regression coefficients were estimated using the bootstrap method because of the moderate sample size [14]. The point estimates and 95% confidence intervals (CIs) for treatment effects comparing cyclosporine and control (1), prednisolone and control (2), and cyclosporine and prednisolone (1 − 2), as well as for other covariates (3–7), were reported for each baseline adjustment method. Seven of the 48 cats had a CAIbaseline = 0 despite the clinical history of chronic GI signs. Therefore, the percentage change in CAI was not evaluated as a response type.
| Baseline adjustment method | Regression model |
|---|---|
| Outcome: Serum feline pancreatic lipase immunoreactivity (fPLI) concentration | |
|
(1) Reevaluation fPLI (μg/L): Use fPLIreevaluation as response and include fPLIbaseline as a covariate |
fPLIreevaluation = constant + 1Cyclosporine + 2Prednisolone + 3Time + 4PC1 + 5PC2 + 6PC3 + 7fPLIbaseline + patient as random effect |
|
(2) Change in fPLI from baseline (μg/L): Use ∆fPLI = fPLIreevaluation − fPLIbaseline as response |
∆fPLI = constant + 1Cyclosporine + 2Prednisolone + 3Time + 4PC1 + 5PC2 + 6PC3 + patient as random effect |
|
(3) Percentage change in fPLI from baseline (%): Use %∆fPLI = ∆fPLI/fPLIbaseline × 100% as response |
%∆fPLI = constant + 1Cyclosporine + 2Prednisolone + 3Time + 4PC1 + 5PC2 + 6PC3 + patient as random effect |
| Outcome: Clinical activity index (CAI) | |
|
(1) Reevaluation CAI: Use CAIreevaluation as response and include PCs as covariates |
CAIreevaluation = constant + 1Cyclosporine + 2Prednisolone + 3Time + 4PC1 + 5PC2 + 6PC3 + 7fPLIbaseline + patient as random effect |
|
(2) Change in CAI from baseline: Use ∆CAI = CAIreevaluation − CAIbaseline as response and include PCs as covariates |
∆CAI = constant + 1Cyclosporine + 2Prednisolone + 3Time + 4PC1 + 5PC2 + 6PC3 + 7fPLIbaseline + patient as random effect |
- Note: fPLIreevaluation: Serum feline pancreatic lipase immunoreactivity concentration on Days 10 and 21; fPLIbaseline: Serum feline pancreatic lipase immunoreactivity concentration at baseline (second screening); CAIreevaluation: Clinical activity index on Days 10 and 21; CAIbaseline: Clinical activity at baseline (second screening); PC: Principal component derived from clinical variables at baseline.
Repeated measures of correlation between serum fPLI concentration and the CAI of three visits (baseline, Day 10, and Day 21) were examined using the R package rmcorr [15].
Statistical analyses were performed in R (version 4.4.2, R Core Team (2024), R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (version 9.0, GraphPad Software, Boston, MA, USA). Statistical significance was set at p < 0.05.
2.8 Ethics Statement
The study was approved by the Clinical Research Review Committee at Texas A&M University (IACUC 2017-0416 CA and IACUC 2020-0317 CA). Informed owner consent forms were obtained from each owner.
3 Results
3.1 Recruitment Statistics
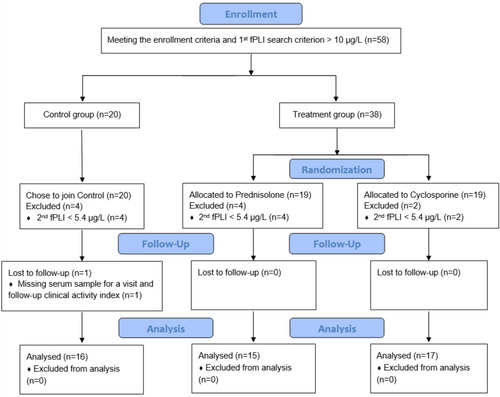
Figure 2 summarizes the recruitment statistics. Of the 58 enrolled cats, 48 (83%) cats had serum fPLI concentration ≥ 5.4 μg/L at baseline and hence continued to meet the inclusion criteria to stay in the trial, with 16 cats in the control group, 15 cats in the prednisolone group, and 17 cats in the cyclosporine group. Out of 48 cats, 1 cat in the control group missed a serum sample for one of the visits and the CAI for both reevaluation visits. Otherwise, all 48 cats finished the study without reported protocol deviations, and all were analyzed.
3.2 Changes in Serum fPLI Concentrations and Time Between First and Second Screening
For 58 enrolled cats, the median time between the first and second screening was 24 days (range, 6–52 days). The median change in serum fPLI concentration was −5.8 μg/L (range, −195.5 to 156.4 μg/L). The median percentage change in serum fPLI concentration was −31.2% (range, −97.8 to 506.3%). Time was not correlated with either type of change in serum fPLI concentration (p = 0.14 and 0.11, respectively; Figure S1).
3.3 Clinical Signs Within the Last 3 Months Before Baseline Visit (Day 0, Second Screening)
Overall, the most commonly reported signs in the 3 months before the baseline visit were weight loss, vomiting, anorexia, and lethargy (Table 2).
| Number of cats (%) | ||||
|---|---|---|---|---|
| Control (n = 16) | Prednisolone (n = 15) | Cyclosporine (n = 17) | Total (n = 48) | |
| Anorexia/decreased appetite | 10 (63%) | 6 (40%) | 8 (47%) | 24 (50%) |
| Lethargy/less active | 10 (63%) | 8 (53%) | 8 (47%) | 26 (54%) |
| Weight loss | 11 (69%) | 13 (87%) | 13 (76%) | 37 (77%) |
| Vomiting | 13 (81%) | 10 (67%) | 6 (35%) | 29 (60%) |
| Diarrhea | 3 (19%) | 5 (33%) | 8 (47%) | 16 (33%) |
| Abdominal pain | 3 (19%) | 3 (20%) | 2 (12%) | 8 (17%) |
| Icterus | 1 (6%) | 0 (0%) | 0 (0%) | 1 (2%) |
3.4 Baseline Demographics
Table 3 summarizes the baseline demographics. Most cats were domestic shorthair cats. Overall, the proportion of males was higher than females (p = 0.03); the proportion of males was higher in the cyclosporine group (p = 0.01), but the proportions of males and females were not significantly different in the control or the prednisolone group (p = 0.80 and 0.61, respectively). Cats were generally older (median, 13.25 years). Body condition score was different between groups (p = 0.004); BCS in the control group was higher than in the prednisolone (adjusted p = 0.02) or cyclosporine (adjusted p = 0.01) groups, but was not different between the prednisolone and the cyclosporine groups (adjusted p = 0.67). The fPLIbaseline was not different between groups (p = 0.18), but the CAIbaseline was different between groups (p = 0.01). The CAIbaseline in the control group was lower than in the prednisolone (p = 0.02) or the cyclosporine (p = 0.03) groups, but was not different between the prednisolone and the cyclosporine groups (p > 0.99). Seven of the 48 cats had a CAIbaseline of 0: 5 cats in the control group and 1 cat each in the prednisolone and the cyclosporine groups, respectively.
| Control (n = 16) | Prednisolone (n = 15) | Cyclosporine (n = 17) | |
|---|---|---|---|
| Breed |
DSH (11) DLH (3) Siamese mix (2) |
DSH (14) DLH (1) |
DSH (12), DLH (2) Ragdoll (2) Maine Coon (1) |
| Sex |
FS: 7 (44%) MN: 9 (56%) |
FS: 6 (40%) MN: 9 (60%) |
FS: 3 (18%) MN: 14 (82%) |
| Age (year) |
13.75 (7–20) |
13 (3–18) |
14 (5–22) |
| BCS (scale of 9) |
5a (4–7) |
4b (3–7) |
4b (1–8) |
| Baseline serum fPLI concentration (μg/L) |
13.9 (6.0–41.5) |
20.3 (6.7–158.0) |
18.9 (5.5–206.4) |
| Baseline clinical activity index |
1.5a (0–5) |
5b (0–11) |
3b (0–8) |
- Note: Median (minimum − maximum) of age, BCS, baseline serum fPLI concentration, and baseline clinical activity index were reported. Groups with different superscripts (a or b) denote statistical differences.
- Abbreviation: fPLI, feline pancreatic lipase immunoreactivity.
The CBC, biochemistry profile, serum cobalamin, folate, fPLI, and fTLI concentrations at each time point, comorbidities, and available ultrasonography reports are summarized in Tables S2–S8. The number of cats that received symptomatic and other treatments near the time of baseline is summarized in Table S9.
3.5 Serum fPLI Concentrations Over Time and Treatment Effects
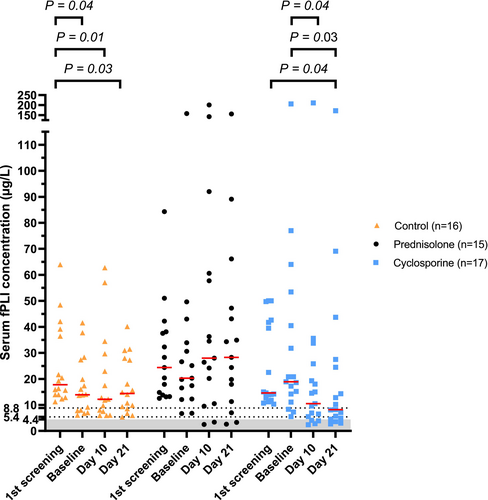
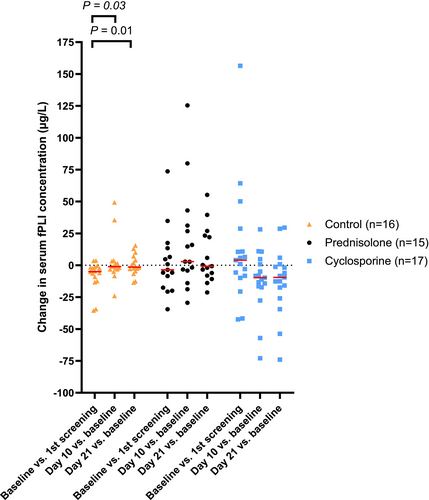
Serum fPLI concentration over time and changes in serum fPLI concentration between time points are shown in Figures 3 and 4, with corresponding descriptive statistics in Tables S10 and S11, respectively. Table 4 summarizes the number of cats in the selected ranges of serum fPLI concentration over three visits. Tables 5 and 6 summarize the point estimates and 95% CI for the treatment effect and other covariates on serum fPLI concentrations with respective baseline adjustment methods. Trends for each patient over time and percentage changes in serum fPLI concentrations between time points are shown in Figures S2 and S3, respectively.
| Number of cats (%) | ||||||
|---|---|---|---|---|---|---|
| Baseline | Day 10 | Day 21 | ||||
| Serum fPLI concentration (μg/L) | ≤ 4.4 | < 8.8 | ≤ 4.4 | < 8.8 | ≤ 4.4 | < 8.8 |
| Control (n = 16) | 0/16 | 5/16 | 0/15 | 5/15 | 0/16 | 4/16 |
| (0%) | (31%) | (0%) | (33%) | (0%) | (25%) | |
| Prednisolone (n = 15) | 0/15 | 2/15 | 2/15 | 2/15 | 2/15 | 3/15 |
| (0%) | (13%) | (13%) | (13%) | (13%) | (20%) | |
| Cyclosporine (n = 17) | 0/17 | 3/17 | 4/17 | 7/17 | 3/17 | 9/17 |
| (0%) | (18%) | (24%) | (41%) | (18%) | (53%) | |
- Note: Baseline: The time point when second (baseline) serum fPLI concentration was screened; Day 10: Day 10 of the study; Day 21: Day 21 of the study.
- Abbreviation: fPLI, feline pancreatic lipase immunoreactivity.
| Regression coefficient of treatment effect | ||||||
|---|---|---|---|---|---|---|
| Cyclosporine vs. control (1) | Prednisolone vs. control (2) | Cyclosporine vs. prednisolone (1 − 2) | ||||
| Response type | 1 (SE) | 95% CI | 2 (SE) | 95% CI | 1 − 2 (SE) | 95% CI |
| Outcome: Serum feline pancreatic lipase immunoreactivity (fPLI) concentration | ||||||
| (1) fPLIreevaluation (μg/L) | −9.4 (6.0) | (−20.9, 1.9) | 16.6 (7.1) | (2.7, 30.6) | −26.0 (7.7) | (−40.1, −10.2) |
| (2) ∆fPLI (μg/L) | −13.0 (6.3) | (−23.9, −0.9) | 14.6 (6.8) | (1.0, 28.1) | −27.6 (7.8) | (−41.2, −11.4) |
| (3) %∆fPLI (%) | −24.2 (30.0) | (−82.0, 34.8) | 60.5 (43.2) | (−25.0, 150.8) | −84.7 (38.8) | (−155.0, −0.4) |
| Outcome: Clinical activity index (CAI) | ||||||
| (1) CAIreevaluation | 0.1 (0.5) | (−0.8, 1.0) | −1.0 (0.4) | (−1.7, −0.2) | 1.0 (0.4) | (0.3, 1.8) |
| (2) ∆CAI | −0.7 (0.5) | (−1.5, 0.3) | −1.9 (0.4) | (−2.7, −1.2) | 1.2 (0.4) | (0.4, 2.1) |
- Note: fPLIreevaluation: Serum feline pancreatic lipase immunoreactivity concentration on Days 10 and 21; fPLIbaseline: Serum feline pancreatic lipase immunoreactivity concentration at baseline (second screening); ∆fPLI = fPLIreevaluation − fPLIbaseline; %∆fPLI = ∆fPLI/fPLIbaseline × 100%; CAIreevaluation: Clinical activity index on Days 10 and 21; CAIbaseline: Clinical activity index at baseline (second screening); ∆CAI = CAIreevaluation − CAIbaseline. CI in red indicates statistical significance. The corresponding regression model can be found in Table 1. The cat that was missing the CAI for both reevaluation visits was removed from evaluating treatment effect on the CAI.
- Abbreviations: CI, confidence interval; SE, standard error.
| Regression coefficient of other covariates | |||||
|---|---|---|---|---|---|
| i (95% CI) | |||||
| Response type | Time (3) | PC1 (4) | PC2 (5) | PC3 (6) | fPLIbaseline (7) |
| Outcome: Serum feline pancreatic lipase immunoreactivity (fPLI) concentration | |||||
| (1) fPLIreevaluation (μg/L) | −4.4 (−12.2, 2.6) | −0.3 (−3.3, 3.5) | −1.8 (−8.2, 4.6) | −3.7 (−9.7, 1.5) | 0.8 (0.1, 1.0) |
| (2) ∆fPLI (μg/L) | −4.4 (−12.1, 2.6) | −0.3 (−3.5, 3.0) | −2.8 (−9.2, 4.3) | −3.8 (−9.7, 1.2) | NA |
| (3) %∆fPLI (%) | −40.1 (−94.5, 8.6) | −5.1 (−22.0, 12.7) | −1.6 (−29.1, 26.2) | −12.4 (−39.5, 14.9) | NA |
| Outcome: Clinical activity index (CAI) | |||||
| (1) CAIreevaluation | 0.1 (−0.5, 0.7) | 0.5 (0.2, 0.8) | −0.6 (−0.9, −0.2) | 0.6 (0.2, 1.0) | −0.001 (−0.01, 0.01) |
| (2) ∆CAI | 0.1 (−0.5, 0.7) | −0.7 (−1.0, −0.5) | −0.7 (−1.0, −0.3) | −0.6 (−1.0, −0.3) | −0.002 (−0.02, 0.03) |
- Note: fPLIreevaluation: Serum feline pancreatic lipase immunoreactivity concentration on Days 10 and 21; fPLIbaseline: Serum feline pancreatic lipase immunoreactivity concentration at baseline (second screening); ∆fPLI = fPLIreevaluation − fPLIbaseline; %∆fPLI = ∆fPLI/fPLIbaseline × 100%; CAIreevaluation: Clinical activity index on Days 10 and 21; CAIbaseline: Clinical activity index at baseline (second screening); ∆CAI = CAIreevaluation − CAIbaseline. CI in red indicates statistical significance. The corresponding regression model can be found in Table 1.
- Abbreviations: NA, not applicable; PC, principal component derived from clinical variables at baseline.
Treatment effect on serum ∆fPLI was significant when comparing cyclosporine and control (1, Table 5), indicating that cyclosporine was associated with a larger decrease in serum fPLI concentration compared with control. When fPLIreevaluation was used as the response, the treatment effect was not significant (95% CI, −20.9 to 1.9 μg/L), but the upper limit was close to 0.
Treatment effect comparing prednisolone and control (2, Table 5) was significant when either fPLIreevaluation or ∆fPLI was evaluated. These results indicate that prednisolone treatment was associated with a higher serum fPLI concentration at the time of reevaluation and a larger increase in serum fPLI concentrations compared with control.
Treatment effect comparing cyclosporine and prednisolone (1 − 2, Table 5) was significant regardless of the baseline adjustment method used. All treatment effects indicate that cyclosporine was associated with a decrease and a larger decrease in serum fPLI concentrations compared with prednisolone.
The fPLIbaseline (7) was significantly associated with fPLIreevaluation. Regardless of the baseline adjustment methods used, time (3) and principal components derived from the baseline clinical variables (4–6) were not significant (Table 6).
3.6 CAI, Clinical Variables Over Time, and Treatment Effects
Figure 5 shows the CAI and each clinical variable over time. Figure S4 shows the changes in CAI between time points. Prednisolone was associated with a decrease and larger decrease in CAI, compared with the control (2) or the cyclosporine group (1 − 2). The treatment effect was not significant when comparing the cyclosporine and the control group (1; Table 5). Regardless of the baseline adjustment used, principal components derived from the baseline clinical variables (4–6) were significant, but time (3) and fPLIbaseline (7) were not (Table 6).
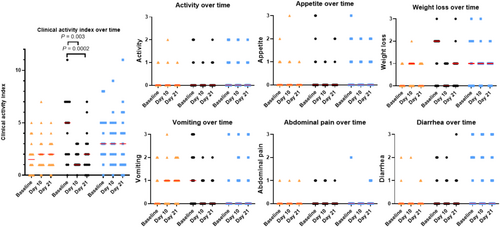
3.7 Correlation Between Serum fPLI Concentration and CAI
Serum fPLI concentrations and the CAI were not correlated, with a repeated measures correlation at 0.03 (p = 0.79; 95% CI, −0.15 to 0.17).
4 Discussion
We report the first randomized clinical trial comparing prednisolone and cyclosporine, with a nonrandomized active control for treatment in cats with a presumptive diagnosis of chronic pancreatitis that was managed on an outpatient basis. Superiority of cyclosporine compared to prednisolone or control in decreasing serum fPLI concentration was observed, given the studied period and dosage, but the clinical improvement was not apparent with cyclosporine use.
Cats enrolled in our study were presumed to have chronic pancreatitis based on clinical findings and two consecutively increased serum fPLI concentrations. Asymptomatic cats (i.e., CAI = 0 for 7/48 cats, 15%) at baseline were also enrolled, considering that cats with chronic pancreatitis can have waxing and waning clinical signs. Of 58 cats with a relevant clinical history and an increased first screening serum fPLI concentration, 48 cats (83%) continued to have increased serum fPLI concentrations at baseline, suggesting ongoing pancreatic inflammation (Figure 2). No correlation was found between changes in serum fPLI concentration and days between the first and second screening (Figure S1). These findings suggest that serum fPLI concentration is not routinely normalized with time and that many cats have ongoing pancreatic inflammation.
Pancreatitis in cats is not thought to have a sex predisposition, according to a histopathology study [1, 2]. However, there were slightly more neutered males (32/48 cats, 67%) in our study. Similar findings were observed in previous studies evaluating cats with a clinical suspicion of pancreatitis (16/19 cats, 84%) [3], or cats diagnosed with pancreatitis based on serology, ultrasonography, histopathology, or some combinations of these (94/157 cats, 60%) [16]. It is unclear if it happened by chance or if cats have a sex-related predisposition to pancreatitis, which calls for more research [17]. To account for sex, we attempted to include sex as a regressor, but encountered a convergence issue because of the small number of females in the cyclosporine group. However, when repeating the analyses with only males, the resultant treatment effects were similar (e.g., 95% CI of treatment effect on ∆fPLI comparing the cyclosporine and control group was −30.2 to −0.8 μg/L), suggesting that sex was not associated with how cats would respond to treatment in the study.
Cats in the control group had a higher and closer to ideal BCS and a milder clinical presentation than those in the treatment group, but age and serum fPLI concentration were not significantly different at baseline. This finding likely reflects that the overall clinical picture affected the choice to join the control or the treatment group in our study (cats in the treatment group potentially had a more severe clinical presentation). A comparison between the control group and either of the treatment groups therefore would need to be interpreted in light of this difference, because a higher CAI in the treatment groups could allow a chance to observe a larger decrease in CAI over the study period.
Modified cyclosporine, given at 5 mg/kg PO q24h for 21 days, was associated with a larger decrease in serum fPLI concentration compared with the control or prednisolone groups. However, the treatment effect on fPLIreevaluation or %∆fPLI comparing the cyclosporine and the control group was not significant. This finding might have been the result of insufficient sample size, or the type of baseline adjustment method might have lacked statistical efficiency [18]. Of note, although treatment with cyclosporine was associated with a larger decrease in serum fPLI concentration, it was associated with a higher CAI and a larger increase in CAI compared with the prednisolone group, but not the control group. This observed larger increase in CAI in the cyclosporine group compared with the prednisolone group, as indicated by the regression coefficient (1 − 2), could be a result of a nonsignificant change in CAI in the cyclosporine group but a significant decrease in CAI in the prednisolone group (Figure 5). The decrease in serum fPLI concentration but a non-significant change in CAI in the cyclosporine group might suggest that the improvement of clinical signs associated with pancreatitis was masked by adverse cyclosporine effects, or alternatively, a persistence of clinical signs associated with pancreatitis.
Some cats had increases in serum fPLI concentration despite cyclosporine treatment. This finding might reflect that (1) some cats did not benefit from cyclosporine treatment, or (2) cyclosporine did not reach therapeutic concentrations in some cats because of its variable bioavailability [19]. Both generic and brand name cyclosporine were used in the study, and cyclosporine concentrations or interleukin-2 effects were not assessed. Therefore, the same dose might have had different effects in different cats based on metabolism or the drug formulation administered. For cats that responded, most of the decrease in the serum fPLI concentration appeared to occur within the first 10 days because serum fPLI concentrations on Days 10 and 21 were not significantly different (Figure 3). However, some cats had an increase on Day 21. Ideal timing for intervention, treatment duration, the relationship between discontinuation and relapse, and reasons for subsequent increase despite initial decrease require further study.
It is unclear how cyclosporine could decrease pancreatic inflammation in cats. Cyclosporine exerts its effects by binding to cyclophilin, forming a complex that inhibits calcineurin, resulting in the inhibition of T cell proliferation and activation [19, 20]. Because chronic pancreatitis is common in cats, and lymphocytes are the major inflammatory component [2], lymphocytes might be a good therapeutic target, such as by modulation of T-cell alterations [21]. A mouse model of autoimmune pancreatitis showed that autoimmune pancreatitis is a T lymphocyte-driven disease responsive to cyclosporine [22]. In another mouse model of chronic pancreatitis, regulatory T cells were identified as the central regulators of the fibroinflammatory reaction [23], and it was suggested that T cells are a possible therapeutic target to prevent fibrosis and preserve functional pancreatic tissue [23]. Nonetheless, higher dosages of cyclosporine compared to the dosage used in our study were linked to adverse effects on the pancreas [24, 25]. Therefore, different dosages of cyclosporine might have different effects on the pancreas, and a carefully selected dosage is warranted to reach therapeutic goals while preventing adverse effects [26]. In addition, although not demonstrated in cats, cyclophilin and calcineurin are widely distributed among tissues, and can have different sensitivity to inhibition by cyclosporine [27, 28]. Also, different cellular sources of calcineurin were shown to exert different effects [29]. Hence, the ideal dosage of cyclosporine, the need for a specific calcineurin inhibitor, and the balance between benefits and risks, such as opportunistic infections, require further investigation before routine use of cyclosporine can be recommended in cats with pancreatitis.
Prednisolone, given at an immunosuppressive dosage, was associated with a decrease and a larger decrease in CAI compared with either the control or the cyclosporine groups, but was associated with an increase and a larger increase in serum fPLI concentration compared with either the control or the cyclosporine groups. The observed increase in serum fPLI concentration in the prednisolone group, as indicated by the regression coefficients compared with either the control (2) or the cyclosporine group (1 − 2), could be a result of failure to decrease in the prednisolone group and other groups that had a larger decrease (Figure 4). This observation suggests that the alleviation of clinical signs in the prednisolone group might not be a direct result of alleviation of pancreatic inflammation, at least for some cats. Prednisolone given at anti-inflammatory dosages was not found to increase serum pancreatic lipase immunoreactivity concentration in healthy cats [30] and healthy dogs [31]. However, prednisolone given at an immunosuppressive dosage (2–2.2 mg/kg/day) was associated with various degrees of increases in canine pancreatic lipase immunoreactivity (cPLI) concentrations in dogs with immune-mediated disease [32]. One of 6 healthy dogs that had prednisone administrated at 4 mg/kg had an increase in serum cPLI concentration ≥ 400 μg/L. [33] Prospective studies are needed to evaluate whether prednisolone exerts consistent effects on serum fPLI concentrations, or if the effect is more dosage and individual dependent.
Notably, fPLIreevaluation was associated with fPLIbaseline but not baseline clinical presentation (principal components 1–3). Also, CAI or its changes were associated with baseline clinical presentation (principal components 1–3) but not fPLIbaseline. Also, no correlation was found between the repeatedly measured serum fPLI concentrations and the CAI. Understanding that CAI can be confounded by many factors, these results, however, suggest that assessing serum fPLI concentration and clinical signs individually could allow a more comprehensive treatment assessment. Future studies that enroll a larger number of cats with variable severity of baseline clinical presentation could better assess its association with serum fPLI concentration or its changes, without the need for dimensionality reduction techniques such as PCA, and would improve interpretability without loss of information.
The outcomes to monitor during the treatment of pancreatitis in cats are debated. Serial serum fPLI concentration and its association with severity, prognosis, and other important disease progression outcomes have not been extensively documented. Serum fPLI concentration at the time of hospital admission was found to be associated with survival in 33 cats hospitalized for pancreatitis [9], of which were hospitalized for pancreatitis, but studies evaluating outcomes of outpatient cases are lacking. Additional studies are needed to evaluate whether a downward trend of serum fPLI concentration is associated with better clinical outcomes. Clinical signs as a clinical outcome can be challenging to interpret in cats with pancreatitis because clinical signs are often mild, nonspecific, and confounded by many factors, such as concurrent diseases [1], medication adverse effects, and symptomatic treatments. The CAI, a summary of relevant clinical signs formulated in our study, has not been validated as an outcome measure in pancreatitis in cats; it is also confounded by the aforementioned factors and the nature of an open-label trial. Therefore, interpretation of the treatment effects on CAI needs to be interpreted with caution. Important disease progression outcomes (e.g., development of fibrosis, exocrine pancreatic insufficiency, and diabetes mellitus) develop slowly over years and cannot be assessed in short-term studies, which also has been pointed out as a consideration in clinical trial design in human patients with pancreatitis [34]. In addition, the natural history of pancreatitis in cats is not well documented; hence, the optimal timing for intervention is unclear. A long-term prospective observational study, similar to one conducted in humans [35], along with the use of validated tools for progression outcomes such as fibrosis [36-38], and pancreatic acinar cell damage [8] would facilitate the design of future clinical trials.
Heterogeneity in pancreatitis cause, clinical signs, complications, and disease course complicates the design of clinical trials focusing on treatments [39]. Our study was a pragmatic clinical trial with less restrictive eligibility criteria and aimed to serve as an initial proof-of-concept study [34]. Because we selected patients who had not received corticosteroids or immunosuppressants within the past 3 months, we might have enrolled more cats with a relatively stable or mild clinical presentation. Ideally, the cause of pancreatitis should determine the treatment plan, but > 95% of cases of pancreatitis in cats are considered to be idiopathic [1], and the prevalence of possible immune-mediated pancreatitis is currently unclear. Therefore, our study does not necessarily support the routine use of immunosuppressants for cats with presumed chronic pancreatitis, but demonstrates a potential benefit. Each cause might require independent study to further understand the nuances of disease progression and identify differences in potential therapeutic targets. When causes, stages of disease, core outcomes, and validated tools are more defined in cats with pancreatitis, more defined populations could be targeted to conduct confirmatory clinical trials.
Our study had some limitations, including a presumptive diagnosis of chronic pancreatitis. A definitive diagnosis of chronic pancreatitis in cats currently relies on histological evidence. Because pancreatic biopsies are rarely performed, we elected to have two consecutive increased serum fPLI concentrations as an indicator of chronic pancreatitis. Ultrasonography was conducted as part of the diagnostic evaluation for some cats and also was used as an aid to assess eligibility when available, but it was not a requirement for the study. We recognize that requiring ultrasound examination would have helped strengthen the diagnosis and better define the population, but requiring a standardized ultrasound examination would have limited enrollment because cats were enrolled from veterinary first opinion practices. Also, there was heterogeneity as to the level of diagnostic evaluation and existing comorbidities, and hence, a selection bias could have been introduced when prioritizing patients during the first screening step. It is also difficult to discern whether the presumed chronic pancreatitis was a primary or secondary disease. Finally, blood cyclosporine concentrations were not measured, which would have been helpful to explain whether the failure to respond had been a result of low cyclosporine concentrations.
In conclusion, cats with presumed chronic pancreatitis that received cyclosporine and symptomatic treatments for 3 weeks had a larger decrease in serum fPLI concentration compared with those that received an immunosuppressive dosage of prednisolone and symptomatic treatments, or symptomatic treatments alone. Immunosuppressive dosages of prednisolone and symptomatic treatments appeared to decrease CAI, whereas symptomatic treatments alone or cyclosporine and symptomatic treatments failed to do so, but this observation must be interpreted in light of the confounders discussed (e.g., symptomatic treatments). Long-term studies are warranted to evaluate if serological evidence of improvement persists and if it is associated with a better clinical outcome.
Acknowledgments
The authors thank all the veterinarians, technicians, and clients for their participation. Part of the results were presented at the 2022 American College of Veterinary Internal Medicine (ACVIM) Forum, Austin, TX.
Disclosure
The authors have nothing to report.
Ethics Statement
The study was reviewed and approved by the Clinical Research Review Committee at Texas A&M University (IACUC 2017-0416 CA and IACUC 2020-0317 CA).
Consent
Informed owner consent forms were obtained from all cat owners.
Conflicts of Interest
Drs. Yu-An Wu, Jonathan A. Lidbury, and Jörg M. Steiner are affiliated with the Gastrointestinal Laboratory at Texas A&M University, which offers laboratory testing, including measurement of serum fPLI concentration, on a fee-for-service basis. Dr. Jörg M. Steiner is also a paid consultant for IDEXX Laboratories. Both Drs. Jonathan A. Lidbury and Jörg M. Steiner have acted as paid speakers for IDEXX Laboratories. Dr. Samiran Sinha declares no conflicts of interest.




