The pathobiology of platelet and megakaryocyte extracellular vesicles: A (c)lot has changed
Manuscript Handled by: Matthew T. Rondina
Final decision: Matthew T. Rondina, 02 May 2022
Abstract
Platelet-derived extracellular vesicles (PEVs) were originally studied for their potential as regulators of coagulation, a function redundant with that of their parent cells. However, as the understanding of the diverse roles of platelets in hemostasis and disease has developed, so has the understanding of PEVs. In addition, the more recent revelation of constitutively released megakaryocyte-derived extracellular vesicles (MKEVs) in circulation provides an interesting counterpoint and avenue for investigation. In this review, we highlight the historical link of PEVs to thrombosis and hemostasis and provide critical updates. We also expand our discussion to encompass the roles that distinguish PEVs and MKEVs from their parent cells. Furthermore, the role of extracellular vesicles in disease pathology, both as biomarkers and as exacerbators, has been of great interest in recent years. We highlight some of the key roles that PEVs and MKEVs play in autoimmune blood cell disorders, liver pathology, and cardiovascular disease. We then look at the future of PEVs and MKEVs as candidates for novel therapeutics.
1 THE WORLD OF EXTRACELLULAR VESICLES
In the past few decades, research into extracellular vesicles (EVs) has led to a reimagining of the dogma of secreted signals.1 The potential for membrane-bound packages of cargo including RNA, proteins, lipids, and organelles to be delivered to a recipient cell has altered how we think about communication between cells, tissues, and organs.1, 2 The more traditional outlook of cell-cell communication being mediated by individual chemical signals or cell-cell contacts has been extended to encompass the hypothesis that vesicle-mediated signals allow bundled messaging to distant sites. This exciting field continues to provide insight into cellular functions, pathogenicity of disease, and candidate therapeutics. Thus far, EVs have been identified as being produced from all tested human cell types and are found in every bodily fluid including blood, lymph, synovium, and cerebrospinal fluid.3-7 Accumulating evidence suggests that their role in communication and delivery of nucleic acids, lipids, proteins, and organelles may be essential for multicellular organism function. However, there is considerable work to be done in understanding the heterogeneity of these messages and their roles in physiology and pathology.
Of particular interest for their potent and increasingly well-characterized signaling functions are platelet-derived EVs (PEVs). In this review, we highlight the interest in PEVs for their classical role in hemostasis, as well as more recent studies that demonstrate broader functionality in disease pathogenicity and as candidate therapeutics. We will also touch upon the much less studied roles of megakaryocyte-derived EVs (MKEVs) and the limited studies that have implicated MKEVs in important biological functions.
1.1 History and nomenclature of PEVs
Initial work on PEVs relied upon ultra-centrifugation methodologies that allowed the isolation of what was originally referred to as phospholipid-rich “platelet dust.”7 This dust eventually became classified as platelet “microparticles,” a term that is now synonymous with EVs. Subpopulations of EVs are defined by their mode of production. Platelets are capable of producing both microvesicles that bud directly from the plasma membrane and exosomes that are released upon fusion of multivesicular bodies with the plasma membrane.8 Size and surface marker expression have often been used to approximate the type of vesicles; however, they do not comprehensively discriminate between EV subpopulations and there are currently no widely accepted/consensus markers to distinguish these populations.9 Therefore, per the guidance of the International Society for Extracellular Vesicles, in this review, we will refer to all populations collectively as EVs.10
Platelet activation occurs upon vascular damage to the endothelium that induces exposure of extracellular matrix proteins such as collagen and the release of Weibel-Palade bodies from endothelial cells. Platelet degranulation, the release of protein-packed granules, coincides with a conformational change in platelet shape. In conjunction with granule release, there is the accompanying formation and release of EVs; whereas most cells undergo EV release constitutively, there is little evidence that PEV release functions in this way. On the contrary, PEV production has been positively correlated with the degree of platelet activation in numerous studies. Consequently, PEVs are relatively easy to generate in vitro with platelet agonists, such as thrombin, calcium ionophores, and collagen, in addition to physical stimuli such as high shear.11-13
1.2 Megakaryocyte extracellular vesicles – hiding in plain sight
An important study from the early 2000s concluded that PEVs contribute the largest proportion of circulating EVs in plasma. By assessing annexin-V and CD61 in addition to phosphatidylserine (PS) expression, the study distinguished PEVs from endothelial EVs (Figure 1).14 Although platelets garnered much attention for their EV production and the correlation of circulating EVs with platelet activation, a 2009 study highlighted how CD41 EVs in circulation could also be derived from megakaryocytes and may contain higher levels of full-length filamin protein than PEVs.15 It was later revealed that MKEVs were marked by GPVI and CLEC-2, whereas PEVs from activated platelets only present CLEC-2, reflecting the activation-dependent cleavage of GPVI on platelets (Figure 1).16
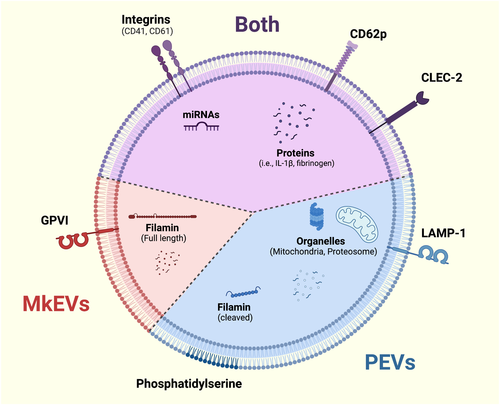
Unlike PEVs, which require platelet activation, MKEVs are constitutively released. Their mechanism of release has not been closely studied, but it is noteworthy that the microtubule inhibitor nocodazole attenuates platelet production but not MKEV production, suggesting that MKEV formation is not a microtubule-mediated event and is distinct from proplatelet extension and platelet release.15 In line with that notion, several studies have shown that EV formation from various cell types is largely driven by actin-mediated forces and signaling pathways, including members of the small GTPase families Arf, Rab, and Rho.17-19
In the aforementioned study, Flaumenhaft et al. further suggest that CD62p (P-selectin) may be a better distinguisher of PEVs than CD41 because of its distinct enrichment in the membrane of activated platelets. Building on this study, recent work from our laboratory demonstrated that both platelets and megakaryocytes produced highly heterogeneous populations of EVs when stimulated in vitro. CD62p is found on both megakaryocytes and activated platelets; however, its expression is more common in PEVs than MKEVs (Figure 1).20 Currently, there are no known surface markers that faithfully distinguish PEVs and MKEVs. Studies that have previously referred to circulating CD41+ and CD62p+ EVs as PEVs can likely now be considered as a mixed population of MKEVs and PEVs. This is an important consideration when interpreting EV studies published before 2009.
1.3 Platelet EVs and hemostasis
As early as the 1960s, the Wolf study of “platelet dust” demonstrated that platelet-derived particles accelerate clotting times of plasma more than 4-fold, indicating that PEVs harbor procoagulant capacities.7 Subsequent electron microscopy studies demonstrated the presence of vesicular structures released by platelets.21, 22 Sims et al. then positively correlated levels of activated platelet prothrombinase activity with PEV counts, suggesting that these particles could enhance thrombin generation.23 It was later demonstrated during blood draws from cardiopulmonary bypass patients that platelet numbers exiting a wound decrease and EV numbers increase, indicating that EVs are shed from activated platelets.24 The characteristic exposure of PS on the plasma membrane upon platelet activation positively correlates with vesicle production, however, through use of intracellular calcium pump inhibitors, these processes were later demonstrated to function independently.24, 25
Platelet-derived extracellular vesicles were initially thought to enhance procoagulant activity in hemostatic processes because they exhibit a 50- to 100-fold higher prothrombotic activity than platelets.26 However, it is important to note that the cumulative surface area of circulating platelets far outnumbers that of PEVs. Given the sheer number of platelets in circulation, could PEVs be playing a significant role? An important in vitro study from the Wolberg laboratory investigating purified populations of monocyte- and platelet-EVs revealed that both populations have procoagulant activity.27 However, the group made the important distinction that only monocyte-derived EVs are capable of initiating and promoting increased complexity of fibrin network formation, whereas PEVs solely act in propagating clotting reactions. Other studies have corroborated that the lack of inherent procoagulant activity in PEVs generated in vitro results from the absence of surface tissue factor.28 However, the presence of PS on the surface of some PEV populations can support the binding of coagulation factors and the propagation of the clotting cascade.29
1.4 Platelet and megakaryocyte EVs - extending the function of their parent cells
Until recently, PEVs were mostly considered in the context of their originator cells. However, more current studies have identified several nonredundant PEV functions, one of which is the role of PEV and MKEV signaling within the bone marrow microenvironment. The capacity for PEVs to modify hematopoietic stem and progenitor cell (HSPC) characteristics was first identified in a study demonstrating that hematopoiesis in lethally irradiated mice recovered more quickly when the bone marrow is reconstituted with HSPCs coated with PEVs.30 Intravenous application of human MKEVs into mice also significantly increased platelet numbers in both wild-type mice and those made thrombocytopenic through administration of anti-CD41 antibodies.31 Furthermore, our group demonstrated that PEVs can leave circulation and enter the bone marrow, where they may bind and signal to megakaryocytes and/or HSPCs, a property not shared by platelets.20 Our in vitro studies revealed that PEVs increased megakaryocyte differentiation from progenitors. This finding is corroborated by another study showing that pro-megakaryocytic effects of PEVs were due to targeted downregulation of RhoB by miR-1915-3p.32 This capacity to feed back to the bone marrow and alter its composition indicates a method through which peripheral circulating platelets may drive changes in hematopoiesis via delivery of a concentrated and protected signal in their released PEVs. The discovery of PEVs in organs and tissues external to the vasculature such as bone marrow, lymph, and synovial fluid indicates an underlying method for extravasation of circulating EVs.3, 4, 20, 33 Although bigger than individual cytokines, they lack machinery for classical extravasation performed by cells. Notably, endothelial gaps are suggested to range between 0.1 and 3 µm in diameter, with a mean of 0.4 µm. Most studies indicate that PEVs are approximately 0.2 µm in diameter, suggesting that PEV intercalation through these gaps is feasible. In addition, inflammation can result in increased endothelial permeability, which may result in increased levels of PEVs in organs such as the bone marrow.34
Unlike with platelets, communication between megakaryocytes and HSPCs has been well documented in the bone marrow, where megakaryocytes help maintain HSPC quiescence.35, 36 The potential for megakaryocyte-derived EVs signaling to HSPCs is intriguing given their shared niche in vivo, and there is evidence that exogenous delivery of MKEVs enhances megakaryopoiesis.31 Expansion of these studies to determine the effect of intramarrow delivery of MKEVs to HSPCs and other cells is necessary to better understand their role in signaling and regulation of hematopoiesis in health and disease.
2 MEGAKARYOCYTE AND PLATELET EVS IN THE PATHOGENESIS OF DISEASE
The study of PEVs and MKEVs has been advanced in recent years because of significant interest in their roles as biomarkers, causes, and exacerbators of numerous diseases, which we will aim to cover here. In this review, we have focused on benign disease and for a discussion of PEVs, MKEVs, and cancer, we direct those interested to these reviews.37, 38
2.1 Autoimmune diseases
Circulating PEVs are increasingly found to play a role in both innate and adaptive immunity. In one of the first studies to uncover this novel function of PEVs, they were identified in the synovial joint fluid of rheumatoid arthritis (RA), but not osteoarthritis, patients.33 A model of serum transfer into platelet-depleted mice uncovered that GPVI-dependent PEV production exacerbates RA by increasing pro-inflammatory cytokine release by synovial fibroblasts. Later, EVs believed to largely be of platelet origin were shown to present the known autoantigens of RA, fibrinogen, and vimentin, potentiating the formation of immune complexes.39 A subsequent study built on these findings to identify functional proteasomes in PEVs that could present antigens.40 PEVs are also identified in neutrophils from synovial fluid with uptake by neutrophils shown to occur exclusively under inflammatory conditions.41 Additional murine RA studies have identified PEVs within lymphatic fluid.4 These PEVs are smaller than blood PEVs and lack procoagulant activity and mitochondria.4 Another study of immunoglobulin-G-mediated-arthritis indicated interleukin (IL)-1 in MKEVs from transplanted megakaryocytes is responsible for mediating arthritis development.42 The role of megakaryocytes in arthritis outside of this transplant model is yet to be fully established, but raises intriguing questions around the contribution of megakaryocytes to our immune system and whether MKEVs may be effectors of this activity.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized in part by an increase in autoantigens and immune complex deposition resulting in chronic inflammation and damage to tissues and organs. Several studies report an increase in PEV levels in SLE patients, which correlates with chronic platelet activation and thrombocytopenia that often occur in SLE.43-47 Circulating PEVs can form immune complexes with immunoglobulins, which activate monocytes and increases levels of proinflammatory cytokines, including IL-1β, tumor necrosis factor-α, and interferon-α.48 This enhancement in SLE pathology was concluded to occur because of the effect of PEV-immunoglobulin-G+ immune complexes on monocyte signaling, leading to inflammation and tissue damage.48
Multiple sclerosis (MS) is an autoimmune disease that affects the brain and central nervous system. MS causes an average 1.76-fold increase in platelet activation as measured by CD62p expression, and a similar elevation in circulating platelet microaggregates.49 Patients with MS have higher levels of circulating PEVs compared with healthy individuals.49, 50 Application of EVs isolated from plasma of MS patients to endothelial cultures disrupts endothelial barrier integrity indicating a potential pathogenicity of PEVs in MS.50 Although links have been drawn between elevated PEVs and MS, further studies are needed to determine if they are drivers of disease.
Collectively, these studies provide strong evidence for the role of PEVs in the pathogenicity of various autoimmune disorders.
2.2 Blood cell disorders
Wiskott-Aldrich syndrome (WAS) is an x-linked recessive bleeding disorder that results in thrombocytopenia and dysfunctional platelets. WAS occurs as a result of a mutation within the gene encoding for the actin-nucleating protein WASP.51 PEV counts in plasma of WAS patients are commonly >5-fold of normal controls, a finding that is reproducible through activation of WAS platelets in vitro.52 The bleeding phenotype of WAS patients is not rescued by their higher number of circulating PEVs, indicating that the PEVs alone lack an inherent hemostatic function comparable to platelets. WAS platelets have dysregulated calcium storage, with up to 3-fold higher levels of intracellular calcium, leading to extreme levels of calcium-dependent vesiculation. High intraplatelet calcium concentrations identified in WAS patients has also been associated with increased prevalence of autoimmune disorder comorbidities, including RA.33, 53, 54 However, further studies will be required to determine if these comorbidities arise as a consequence of elevated PEVs.
Sickle cell disease (SCD) is caused by a hemoglobin mutation, leading to the deformation of red blood cells and chronic complications including anemia, hypercoagulation, and inflammation.55 In patients with SCD, vaso-occlusive crises can lead to pathologies such as lung injury.56 Lung injuries in a humanized mouse model can be mediated via PEV-derived IL-1β and caspase-1, which activates platelets, neutrophils, and vascular cells, thus resulting in platelet-neutrophil aggregates large enough to block pulmonary arterioles.56 Of note, SCD platelets contain an activated NLRP3-ASC caspase-1 inflammasome complex that is absent in control platelets. Platelet-neutrophil aggregation and vaso-occlusion in SCD human blood and SCD mice could be induced by PEVs and was subsequently alleviated by treatment with caspase-1 or NLRP3-inflammasome pathway inhibitors.56 Therefore, therapeutics that target and block these interactions in vivo may be viable options to treat PEV mediated lung injury in SCD patients.
2.3 Liver pathology
Platelet-derived extracellular vesicles have been implicated in pathologies of the liver where they can propagate chronic inflammatory processes. In a study of acute liver injury (ALI) and failure (ALF), PEVs, defined as CD41+, were increased nearly 19-fold in patient plasma, and EVs from patients have higher tissue factor activity.57 This phenotype is exacerbated in patients with systemic inflammatory response syndrome (SIRS), in which circulating EVs increase roughly 20% versus non-SIRS ALI patients, and increase further in cases with systemic complications and lethal outcomes.57 Each 10-fold increase in EV count correlates with a 4.9- to 11-fold increase in ALI patients' likelihood of death.57 These EVs are potentially key players in the procoagulant and thrombotic pathology of ALI/ALF given corroborating evidence revealing that EVs from ALF patients enhance clotting times.58 Further supporting this hypothesis, another study established that a decreased platelet count in ALF directly correlates with increased PEV levels following platelet hyperactivation, resulting in overabundant clearance of platelets, thrombocytopenia, and disease progression into multiorgan failure and SIRS.59
In cases of hepatic ischemia-reperfusion injury, another form of ALI, elevated PEVs in murine models are causative in hepatocyte pathogenicity, enhanced neutrophil migration, and activation of platelets.60 A subpopulation of circulating EVs, partially of platelet origin, contain hepatocyte markers, adhere to hepatocytes within 30 min of introduction in vitro, and are endocytosed within an hour.60 Hepatocyte-targeting EVs contain bioactive lipids which are capable of increasing mitochondrial membrane permeability, leading to oxidative stress and cell death.60 Together, these studies reveal that PEVs are common contributors to numerous liver pathologies and may be effective therapeutic targets to alleviate symptoms of these diseases.
2.4 Myeloproliferative disorders
Myeloproliferative neoplasms such as primary myelofibrosis and essential thrombocythemia (ET) are diseases of clonal hematopoiesis characterized by an overproduction of myeloid cells.61 Patients exhibit increased levels of PEVs and decreased MKEVs resulting from altered megakaryopoiesis and platelet hyperactivation.62, 63 Severity of myelofibrosis and splenomegaly is associated with aberrant megakaryocyte activity, and consequently, the release of pro-fibrotic cytokines in the bone marrow and into circulation via MKEVs.64 The most common mutations seen in patients with primary myelofibrosis and ET are in Janus kinase (JAK) 2 (JAK2V617F in 50%–60% of cases) as well as mutations in calreticulin and MPL.64 A study of 72 ET patients grouped by mutation revealed significantly higher CD41+ circulating EVs in the JAK2V617F subgroup, and the CD41+ EV count strongly correlates with thrombotic risk.65 Thrombotic risk in polycythemia vera patients is also associated with increased PS+ circulating EVs of platelet and erythrocyte origin.66 Interestingly, the numbers of PEVs in circulation are more pronounced upon disease progression and are restored in patients that positively responded to the JAK inhibitor ruxolitinib.62 These studies have begun to uncover the role of PEVs and MKEVs in promoting the development of myeloproliferative neoplasms, however, whether these are simply biomarkers or involved in disease pathology is yet to be determined.
2.5 Cardiovascular disease
The involvement of PEVs in cardiovascular disease is of great interest. Intracoronary and aortic CD42+CD31+ EV levels positively correlate with thrombotic burden of first-time ST-elevation myocardial infarction (STEMI) patients, and are further increased in the affected coronary artery compared to aortic blood.67 Increased levels of CD42+CD31+ PEVs in a study of STEMI patients requiring intervention supports the hypothesis of enhanced PEV generation upon disease progression.67 The observed increase in PEVs is linear as patients' thrombus score increases on a scale of 1 to 5.67 One patient with antiphospholipid antibodies, a myocardial infarction, intracardiac thrombosis, and a history of mesenteric vein thrombosis, presented with a 9- and 2-fold higher percentage of procoagulant EVs compared with healthy volunteers and STEMI patients with no history of primary antiphospholipid syndrome, respectively.68 This was further corroborated by a study of additional patients with antiphospholipid syndrome that revealed higher circulating levels of CD62p+ EVs, implying their platelet or endothelial origin.69 These studies strongly correlate PEV number with cardiovascular disease pathologies, but more work is needed to determine if PEVs play a causative role in the disease mechanism.
In atherosclerosis, CD41+ PEVs or MKEVs can also activate endothelial cells and promote production of proinflammatory cytokines within atherosclerotic plaques. EV levels in patients with both atherosclerotic plaques and coronary artery disease who required stenting are significantly increased. Monocyte-platelet aggregates are prominent sources of EVs in atherosclerosis and are associated with increased disease severity and comorbidities. Under atherosclerotic conditions ex vivo, aggregate-derived CD41+ EVs release proinflammatory cytokines, including IL-6, IL-3, and tumor necrosis factor-α.70 PEV-driven signaling was shown to contribute to pathologic progression of plaques by inducing cell proliferation, cell death, vascular remodeling and calcification, thrombus formation, and other detrimental processes.70, 71 PEVs were shown to decorate human monocytes with integrin CD42b that facilitated the adherence and rolling of the recipient cells in a murine model of atherosclerosis.72 PEVs were also identified in lymph of another atherosclerotic mouse model.3 Although the presence of PEVs in lymph may be a useful biomarker, the function of these in atherosclerosis is yet to be determined. Additionally, released EVs directly transfer cytokines to and activate endothelial cells, thus enhancing their dysfunction and stimulating production of proinflammatory cytokines resulting in increased leukocyte adhesion, accumulation, and infiltration of intima through blood vessels.70, 73
In sum, the roles of PEVs in cardiovascular disease are multifactorial, and specifically in atherosclerosis targeting their production and cellular interactions may be a promising avenue to develop therapies that can limit plaque formation and growth.
3 PLATELET AND MEGAKARYOCYTE EVS AS CANDIDATES FOR THERAPEUTICS
Through developing a greater understanding of the nuances of various diseases and their pathologies, researchers are creating novel ways to optimize drug efficacy and avoid off-target effects. Nanotechnology-based drug delivery systems such as nanoparticles and liposomes have garnered considerable attention as tools to deliver anti-inflammatory drugs or chemotherapeutics.74 EVs are efficient cargo delivery systems for long-distance communication between cells and organs, and application of therapeutic EVs may be valuable. For instance, mesenchymal stromal cell-derived EVs and PEVs can significantly enhance angiogenesis in models of ischemia.75-77 For mesenchymal stromal cell-EVs, polymer loading attenuates ischemia progression through a slow release of pro-angiogenic EVs.77 In addition, EVs may be able to access otherwise intractable tissues, based on evidence that hematopoietic cell-derived EVs are capable of crossing the blood-brain barrier and delivering mRNA.78 For example, blood-derived EVs can be loaded with dopamine and delivered to the brain to treat Parkinson disease in mice.79 Importantly, PEVs lack immunogenicity, have potential for targeted delivery, and are relatively easy to produce.80 One key benefit is their ability to maintain functionality and stability after undergoing repeated freeze-thaw cycles.81, 82 This contrasts with the short shelf-life for platelets and other cell-based therapies, where cell function diminishes over time.83
An exciting therapeutic application of PEVs is in their use to treat pathologies that can benefit from inherent PEV cargo and properties. For example, in a rat model of uncontrolled bleeding, intravenous administration of human PEVs decreases median blood-volume loss and increases mean arterial pressure compared with vehicle and platelet transfusion between a 30- and 60-min timepoint.84 These results demonstrate that PEV treatment is more effective than fresh platelets in enhancing hemodynamic stability and provide a potential use in instances of trauma.84 Moreover, PEVs augment successful hematopoietic engraftment following bone marrow transplantation, with evidence that EV-coated HSPCs exhibit improved engraftment in irradiated mice with expedited recovery of leukocyte and platelet counts.30 The recovery of platelet counts following sublethal irradiation dosages is also improved by 10 days postirradiation following application of PEV.32 This is of particular interest given that platelet recovery in allogeneic stem cell transplants or following radiation exposure is often delayed compared with other circulating cell populations.85 Collectively, these inherent properties of PEVs could prove promising in translational applications.
3.1 Potential of PEVs as proangiogenic agents
The worldwide prevalence of cardiovascular disease rose from approximately 271 million to more than 500 million globally between 1990 and 2019, indicating significant need for therapeutics to tackle this broad array of diseases.86, 87 The pro-angiogenic potential of platelets has long been recognized; however, recent efforts have begun to examine the potential of PEVs in angiogenesis.88 PEVs induce angiogenesis both in vitro and in vivo, thus offering candidate treatments for pathologies such as chronic ischemia.76 The mechanism of revascularization by PEVs has been largely attributed to delivery of protein content such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and platelet-derived growth factor.76, 89 A similar proangiogenic effect was seen in a rat aortic ring model of vascular ischemia where activation of PI3 kinase, Src kinase, and ERK increases proliferation and tube formation of human umbilical vein endothelial cells in vitro.90, 91 Further, application of PEVs encapsulated in agarose in an in vivo model of chronic myocardial ischemia in rats, where they enhance vascularization compared with VEGF/basic fibroblast growth factor application alone.76 In the same study, immediate treatment of surgically induced myocardial ischemia with PEVs results in drastic neovascularization of the myocardium. In cerebral ischemia, application of PEVs encapsulated in a polymer improves angiogenesis at the infarct site.75 Furthermore, PEVs are capable of enhancing migration of endothelial outgrowth cells and improving arterial functionality more thoroughly than cells not treated with PEVs.92
Platelet-derived extracellular vesicles also enhance the pro-angiogenic properties of adipose derived stem cells (ADSCs) in vitro. Application of PEVs to ADSCs doubles the expression of the pro-angiogenic genes VEGF, HGF, and ANGPT1.93 PEVs more than double the positive effect of ADSC conditioned medium on human umbilical vein endothelial cell viability and tube formation in vitro.93 In vivo, PEVs significantly enhance ADSC mediated recovery of hindlimb ischemia by day 7 with a higher perfusion ratio at this timepoint than saline controls reach by day 21.93 Collectively, these studies demonstrate a strong candidacy for the usage of PEVs for pro-angiogenic therapy.
3.2 Manipulation of EVs for therapy and as drug delivery systems
In addition to using natively isolated PEVs, there is significant interest in the development of therapeutics based upon manipulation of EV cargo to enhance and/or change their targeting and functionality. For example, overexpressed PPARγ in primary and megakaryoblastic cell lines is packaged into MKEVs and successfully delivered to recipient cells, where it enhances transcription of FABP4 mRNA.94 Modification of PEVs to present an antibody against IL-β on their surface results in the rapid removal of pro-inflammatory IL-β from circulation and significantly reduces infarct damage and improves cardiac function.95 Additionally, electroporation of MKEVs to load them with micro-RNAs targeting the anti-megakaryocytic protein c-myb enhances megakaryocyte differentiation in vitro.96 These successful manipulations of PEVs and MKEVs for improved therapeutic efficacy is promising for development of EV-based therapies.
Platelet-derived extracellular vesicles inherit integrins and receptors from their progenitor cells leading to the hypothesis that PEVs could also act as Trojan horses for cancer treatments because they have a stronger capacity to penetrate the tumor microenvironment while achieving passive targeting to tumor cells through an enhanced permeability and retention effect.97 At the same time, the inherited biocompatibility of drug-loaded PEVs might reduce the existing drawbacks of nanoparticles.98 The conceptual advantages of PEVs as drug delivery systems make them attractive; nevertheless, there is still a lack of standardized isolation and characterization methods as well as a low production of naturally secreted EVs, which poses challenges to their clinical use. In summary, although platelet- and megakaryocyte-derived EVs hold promise for novel therapeutic development and delivery, key aspects still need to be addressed, such as the potential for effective targeting in vivo in conjunction with an appropriate and reproducible therapeutic effect. The development of EV therapies will likely also require a fine regulation given the complexity of the “drug” and consistency in the production of a viable biological such as an EV will be key.99
4 SUMMARY
The study of PEVs and MKEVs began highly connected to the classical hemostatic function of their parent cells. However, as we have highlighted in this review, in recent years, considerable efforts have revealed alternative and nonredundant functions of these EVs. Notably, in many disease pathologies, PEVs are indeed reported to increase procoagulant activity and thrombotic risk. However, this is largely driven not by their inherent procoagulant activity but instead through their interactions with other cells such as monocytes, neutrophils, and endothelial cells that drive proinflammatory signaling. Further research will lead to not only a better understanding of how PEVs and MKEVs are involved in disease etiology, but also how they may be used and/or manipulated to improve disease diagnoses and associated morbidities and mortalities.
CONFLICT OF INTEREST
The authors declare that they have no relevant conflicts of interest.
AUTHOR CONTRIBUTIONS
A.S., E.N., D.F., and K.M. all wrote and edited the manuscript.