Increased cleavage of von Willebrand factor by ADAMTS13 may contribute strongly to acquired von Willebrand syndrome development in patients with essential thrombocythemia
Manuscript Handled by: Karen Vanhoorelbeke
Final decision: Karen Vanhoorelbeke, 07 March 2022
Abstract
Background
Patients with essential thrombocythemia (ET) often experience bleeding associated with acquired von Willebrand syndrome (AVWS) when the platelet count is markedly increased.
Objective
We investigated whether von Willebrand factor (VWF) degradation is enhanced in patients with ET.
Methods
Seventy patients with ET underwent VWF multimer (VWFM) analysis and measurement of VWF-related parameters. We calculated the VWFM index, defined as the ratio of intensities of a patient's molecular weight-categorized VWFMs, and those of a healthy subject's, using densitometric analysis. VWF degradation product (DP) was measured via ELISA using a monoclonal antibody that specifically recognizes Y1605 at the C-terminal boundary, which is exposed following ADAMTS13-mediated cleavage of the Y1605-M1606 bond of the VWF A2 domain.
Results
Patients with higher platelet counts had a significantly reduced high molecular weight (HMW)-VWFM index and an increased VWF-DP:VWF antigen (Ag) ratio compared to those with lower platelet counts. On multivariate analysis, the VWF-DP/VWF:Ag ratio was an independent predictor of the HMW-VWFM index. Patients who underwent cytoreductive therapy had a significantly higher HMW-VWFM index and lower VWF-DP/VWF:Ag ratio than those who did not. Among individual patients, there was also a significant increase in the HMW-VWFM index and a decrease in the VWF-DP/VWF:Ag ratio after cytoreductive therapy compared to pre-therapy values.
Conclusion
In patients with ET, an increased platelet count is associated with enhanced cleavage of VWF at the Y1605-M1606 bond, primarily by ADAMTS13, leading to AVWS. Cytoreductive therapy reduces the platelet count, prevents excessive VWF cleavage, and improves VWFM distributions.
ESSENTIALS
- In essential thrombocythemia (ET), bleeding often occurs with markedly increased platelets.
- We measured von Willebrand factor (VWF) degradation product to quantify the cleavage of VWF.
- Elevated platelets in ET are correlated with hyper-cleavage of VWF at the Y1605-M1606 bond.
- Cytoreductive therapy prevents excessive VWF cleavage and improves VWF multimeric distributions.
1 INTRODUCTION
Essential thrombocythemia (ET) is a BCR-ABL1-negative myeloproliferative neoplasm characterized by persistently elevated platelet counts in the peripheral blood as well as large, mature megakaryocytes in the bone marrow.1, 2 Patients with ET have an increased risk of thrombosis, which can lead to cerebral or myocardial infarction, owing to elevated platelet counts.3 The cumulative risk of thrombosis during follow-up in such patients is reportedly 14% at 10 years after diagnosis4; however, bleeding occurs when platelets are markedly increased,5 and one study showed that major bleeding occurs in 0.79% of patients with ET annually.6 Some patients have ‘acquired von Willebrand syndrome’ (AVWS) as a result of a reduction in high molecular weight (HMW) von Willebrand factor multimers (VWFMs), which have high hemostatic activity; this condition is associated with bleeding.7 In patients with ET, AVWS typically occurs when the platelet count is markedly elevated (i.e., above 1000 × 109/L), but can also occur at levels below this threshold.8, 9
Although there have been various explanations for the pathogenesis of AVWS in ET, the main causes are considered to be (i) enhanced binding of VWF to the greater number of available platelets, resulting in the consumption of HMW-VWFMs,10 and (ii) increased VWF cleavage by proteolytic enzymes.11 In terms of the former, however, the binding of VWF to platelet glycoprotein Ib in patients with ET is comparable to that in healthy subjects,12 and it is now considered unlikely that greater VWF binding to platelets in patients with ET leads to AVWS. As for the latter explanation, it has been suggested that several proteases are involved in the enhanced degradation of VWF in patients with ET, including but not limited to ADAMTS13, a member of ‘a disintegrin and metalloproteinase with thrombospondin motifs’, family.11 The proteolytic effect of ADAMTS13 is strongly exerted on platelet-VWF complexes under shear stress.13 Therefore, more contacts between circulating platelet and VWF would be expected depending on higher levels of platelet counts in patients with ET, leading to more chance of VWF cleavage by ADAMTS13. In addition, there are several proteolytic enzymes secreted by increased platelets and leukocytes thought to contribute to the degradation of VWF. However, to the best of our knowledge, no previous studies have quantitatively evaluated VWF cleavage by proteases and directly demonstrated that such cleavage is enhanced in patients with ET.
ADAMTS13 proteolyzes ultra-large VWFMs secreted into the bloodstream from vascular endothelial cells, thereby preventing thrombus formation.14 In this process, ADAMTS13 specifically cleaves VWF at the Y1605-M1606 bond in the A2 domain.15 In this study, we focused on the site of VWF cleavage by ADAMTS13 to investigate whether enhanced protease-mediated VWF cleavage is responsible for the reduction of HMW-VWFMs in patients with ET. Cleavage of the Y1605-M1606 bond in VWF’s A2 domain was quantitatively evaluated using a novel ELISA that utilizes a monoclonal antibody (N10) that specifically recognizes the Y1605 residue at the C-terminus.16
2 METHODS
2.1 Study populations and sample collection
We included 70 patients with ET in our study who had been referred to either our hospital or one of two other collaborating institutes between July 2016 and June 2021. Patients were confirmed to fulfill the World Health Organization 2016 criteria for ET at the time of inclusion,2 and comprised both untreated individuals as well as those who underwent treatments such as antiplatelet and cytoreductive therapies. None of the patients had a history of congenital von Willebrand disease. Citrated plasma samples were collected from all patients at the time of inclusion. In addition, patients who had not received any treatment at the time of inclusion underwent plasma sample collection again after the start of treatment. This study was approved by the ethics committee of Nara Medical University and the collaborating institutions; written informed consent was obtained from all patients.
2.2 VWFM analysis and its quantification
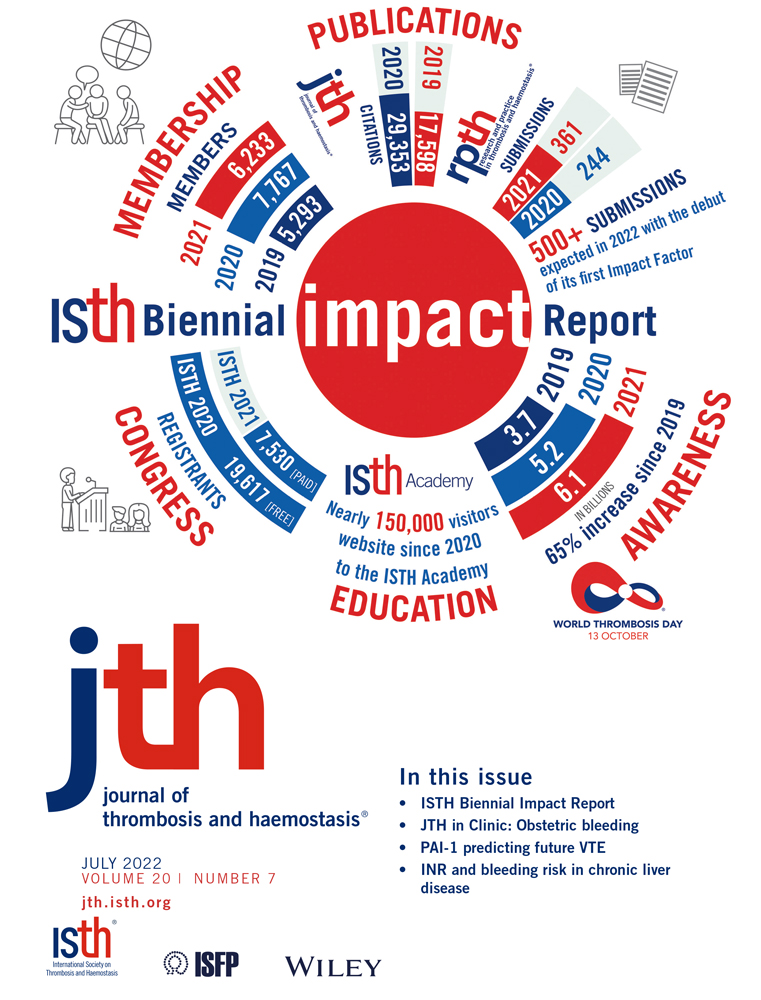
We conducted VWFM analysis in all patients using agarose gel electrophoresis as described by Ruggeri and Zimmerman17 with some modifications.18, 19 VWFM bands can be of three types: high-, medium-, and low-molecular-weight (HMW-, MMW-, and LMW-VWFMs), defined as bands ≥11, 6–10, and 1–5, respectively. To quantitatively evaluate VWFM, we used the previously described "VWFM ratio", defined as the ratio of the intensity of each molecular weight-based cluster of bands (multimers) to that of all VWFM bands combined as measured using densitometry. However, it has been pointed out that this ratio varies widely among studies20; to overcome this problem, we calculated the previously described "VWFM index", defined as the ratio of each patient's VWFM ratio to that of a control subject's that is run on the same gel.21 Pooled plasma from healthy volunteers was used as a control. We determined the HMW and LMW-VWFM indices via densitometric analysis using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Figure S1).
2.3 Measurement of VWF related parameters and ADAMTS13 activity
VWF antigen (VWF:Ag) was measured via ELISA using rabbit anti-VWF polyclonal antibodies (DakoCytomation, Copenhagen, Denmark).22 VWF ristocetin cofactor activity (VWF:RCo) was measured with an automated instrument (Sysmex, Kobe, Japan) using the ‘BC von Willebrand reagent’ (Siemens, Marburg, Germany). ADAMTS13 activity was measured using an ADAMTS13-act-ELISA (Kainos Laboratories, Tokyo, Japan).16
2.4 Quantification of VWF cleavage at the Y1605-M1606 bond in the A2 domain
To quantify VWF cleavage at the Y1605-M1606 bond in the A2 domain, we measured the amount of plasma VWF degradation product (VWF-DP) via ELISA using the two types of anti-VWF monoclonal antibodies (mAb) developed in-house.23 The first mAb N10 specifically recognizes Y1605 at the C-terminal boundary of the VWF A2 domain, which is exposed following cleavage (usually by ADAMTS13).16 Plasma samples diluted 100-, 200-, 400-, and 800-fold were applied to N10-coated plates and incubated for 2 h at room temperature. After washing, horseradish peroxidase-conjugated second anti-VWF mAb, which recognizes the N-terminal region of VWF, was added and incubated for 2 h at room temperature. After washing, the 3,3′,5,5'-tetramethylbenzidine substrate was added for 15 min, with the intensity of the color resulting from the reaction reflecting the amount of VWF-DP. The reaction was stopped with sulfuric acid and absorbance was measured at 450 nm. The amount of VWF-DP was expressed as a percentage of normal pooled human plasma.
2.5 Statistical analysis
Categorical variables are expressed as frequencies and percentages, while continuous variables are expressed as medians and interquartile ranges. Fisher's exact test was used to compare categorical variables, while the Kruskal-Wallis test followed by a multiple comparison test using the Holm method was used to compare continuous variables among three groups. The Mann–Whitney U-test was used to compare continuous variables between two groups. The Wilcoxon signed-rank sum test was used to compare continuous variables before and after treatment of individual patients. Correlations between two variables were evaluated using Spearman analysis; additionally, we performed multivariable stepwise regression analysis. Statistical significance was set at a p-value < .05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).24
3 RESULTS
3.1 Patient characteristics
The demographic characteristics, hematological parameters, and treatments of the 70 patients included in this study are listed in Table 1. The patients were divided into the following three groups according to their platelet counts: group 1 included those with normal or mildly elevated platelet counts (<500 × 109/L; n = 17), group 2 comprised those with high platelet counts (500–1000 × 109/L; n = 41), and group 3 included those with very high platelet counts (>1000 × 109/L; n = 12). Age, sex, comorbidities, and thrombotic and bleeding events prior to inclusion in the study were not significantly different between the three groups. Thrombotic events that occurred prior to the study period included cerebral infarction or transient ischemic attack (n = 7), acute coronary syndrome (n = 4), and deep vein thrombosis (n = 1). One patient had a history of both cerebral infarction and deep vein thrombosis. Bleeding events that occurred prior to the study period included purpura (n = 8), epistaxis (n = 2), and gastrointestinal bleeding (n = 2).
| Parameters | All patients (n = 70) | Patients with normal or mildly elevated platelet counts (<500 × 109/L) (n = 17, group 1) | Patients with high platelet counts (500–1000 × 109/L) (n = 41, group 2) | Patients with very high platelet counts (>1000 × 109/L) (n = 12, group 3) |
|---|---|---|---|---|
| Age in years, median (IQR) | 69 (52–75) | 71 (51–77) | 70 (59–78) | 47 (41–71) |
| Sex (female:male) | 29:41 | 8:9 | 17:24 | 4:8 |
| Comorbidities that are risk factors for cardiovascular disease | ||||
| Hypertension, n (%) | 17/69 (25%) | 5/17 (29%) | 11/40 (28%) | 1/12 (8%) |
| Diabetes mellitus, n (%) | 5/69 (7%) | 1/17 (6%) | 3/40 (8%) | 1/12 (8%) |
| Hypercholesterolemia, n (%) | 8/69 (12%) | 2/17 (12%) | 4/40 (10%) | 2/12 (17%) |
| Thrombotic events prior to inclusion n (%) | 11/69 (16%) | 4/17 (24%) | 6/40 (15%) | 1/12 (8%) |
| Bleeding events prior to inclusion, n (%) | 12/69 (17%) | 0/17 (0%) | 8/40 (20%) | 4/12 (33%) |
| Driver mutations | ||||
| JAK2 V617F mutation, n (%) | 33/63 (52%) | 8/14 (57%) | 23/38 (61%) | 2/11 (18%) |
| CALR mutation, n (%) | 18/63 (29%) | 1/14 (7%) | 10/38 (26%) | 7/11 (64%)* |
| Blood counts at inclusion | ||||
| Platelet count, ×109/L, median (IQR) | 704 (507–882) | 375 (306–384) | 711 (603–810)* | 1,234 (1,102–1,383)*,** |
| Hemoglobin, g/dl, median (IQR) | 12.8 (11.6–13.9) | 12.8 (11.5–13.9) | 12.9 (11.8–13.6) | 12.6 (12.1–14.0) |
| White blood cell count, ×109/L, median (IQR) | 7.6 (6.1–9.2) | 6.0 (4.3–7.0) | 7.9 (6.4–9.2)* | 9.1 (7.6–11.2)*,** |
| Treatment | ||||
| Antiplatelet therapy, n (%) | 28/70 (40%) | 6/17 (35%) | 19/41 (46%) | 3/12 (25%) |
| Cytoreductive therapy, n (%) | 41/70 (59%) | 17/17 (100%) | 23/41 (56%)* | 1/12 (8%)*,** |
| Hydroxyurea, n (%) | 31/70 (44%) | 11/17 (65%) | 19/41 (46%) | 1/12 (8%)*,** |
| Anagrelide, n (%) | 17/70 (24%) | 9/17 (53%) | 8/41 (20%)* | 0/12 (0%)* |
| Busulfan, n (%) | 1/70 (1%) | 1/17 (6%) | 0/41 (0%) | 0/12 (0%) |
- * p < .05 vs. group with normal or mildly elevated platelet counts (group 1)
- ** p < .05 vs. group with high platelet counts (group 2). IQR, interquartile range.
Furthermore, the proportion of JAK2 V617F mutations did not differ significantly between the three groups, but the proportion of patients with CALR mutations was significantly higher in group 3 (64%) than in group 1 (7%). In terms of hematological parameters, groups 2 and 3 had significantly higher white blood cell counts (p < .05) than group 1; on the other hand, there was no significant difference in hemoglobin levels between the three groups. In terms of treatment, 28 patients (40%) received antiplatelet therapy and 41 (59%) received cytoreductive therapy. Among the latter, 31 patients (44%) received hydroxyurea, 17 (24%) received anagrelide, and one (1%) received busulfan; nine of these patients received both hydroxyurea and anagrelide. The proportions of patients who received antiplatelet therapy were not significantly different between the three groups, but groups with higher platelet counts had smaller proportions of patients who received cytoreductive therapy.
3.2 Differences in VWF-related parameters and ADAMTS13 activity among the three groups
VWFM analysis was performed on the entire cohort; visually, group 1 showed no apparent loss of HMW-VWFM (Figure 1A). On the other hand, mild deficits in HMW-VWFM were observed in group 2 (Figure 1B), and pronounced deficits were observed in group 3 (Figure 1C). Groups with higher platelet counts appeared to have lower HMW-VWFM indices and higher VWF-DP/VWF:Ag ratios.
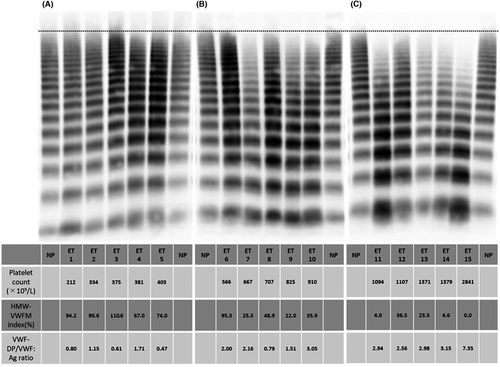
We compared VWF-related parameters and ADAMTS13 activity among the three groups (Figure 2). Groups with higher platelet counts showed a significant reduction in their HMW-VWFM indices (Figure 2A) and an increase in their LMW-VWFM indices (Figure 2B) when compared to group 1. Groups with lower platelet counts tended to have a higher VWF:Ag, but the difference was statistically significant only when comparing group 1 to group 3 (Figure 2C). The VWF:RCo/VWF:Ag ratio decreased as the platelet count increased; the differences between each of the three groups were significant (Figure 2D). Conversely, the VWF-DP/VWF:Ag ratio was significantly increased in groups 2 and 3 (Figure 2E), indicating that VWF cleavage at the Y1605-M1606 bond in the A2 domain was enhanced as the platelet count increased in patients with ET. On the other hand, ADAMTS13 enzymatic activity was not significantly different between the three groups (Figure 2F).
Additionally, 30 patients (43%) showed a VWF:RCo/VWF:Ag ratio less than 0.7, which is commonly used as the index for diagnosing AVWS.25, 26 Three (18%), 18 (44%), and nine (75%) of the patients in groups 1, 2, and 3, respectively, had VWF:RCo/VWF:Ag ratios less than 0.7. In a previous study, the HMW-VWFMs deficit was defined as an HMW-VWFM index <80%.21 Fifty patients with ET (71.4%) had a HMW-VWFM index of less than 80%. Eight (47.1%), 30 (73.2%), and 12 (100%) of the patients in groups 1, 2, and 3, respectively, had an HMW-VWFM index of less than 80%. Although the frequency was higher in group 3 than in group 1, it should be noted that the latter group included numerous number of patients with a VWF:RCo/VWF:Ag ratio <0.7 or a HMW-VWFM index <80%, even though the platelet count was normal or only mildly elevated.
3.3 Effect of enhanced VWF cleavage on VWFM structure in patients with ET
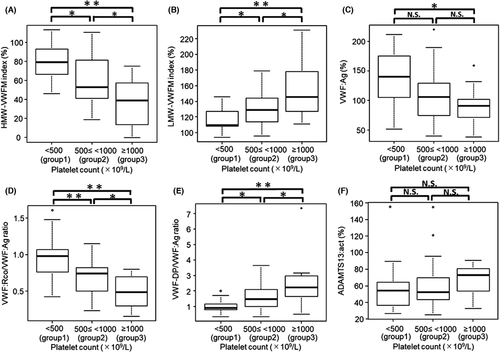
On Spearman analysis, the HMW-VWFM index was negatively correlated with the VWF-DP/VWF:Ag ratio (ρ = −0.61, p < .01) (Figure 3). Additionally, univariate analyses were also performed on other clinical and hematological parameters including age, sex, JAK2 V617F mutation, CALR mutation, platelet count, hemoglobin, and white blood cell count; in addition to its negative correlation with the VWF-DP/VWF:Ag ratio, the HMW-VWFM index as also found to be negatively correlated with platelet counts (ρ = −0.59, p < .01) and white blood cell counts (ρ = −0.46, p < .01) while being positively correlated with age (ρ = 0.25, p = .04). The four parameters found to be associated with the HMW-VWFM index on univariate analysis were then subjected to multivariate analysis, whereupon only the VWF-DP/VWF:Ag ratio was found to be an independent predictor of the HMW-VWFM index in patients with ET (regression coefficient: −16.2, p < .01).
3.4 Differences in characteristics and laboratory parameters between patients who received cytoreductive therapy and those who did not
To investigate the effect of cytoreductive therapy on VWF-related parameters, we compared the 41 patients who received cytoreductive therapy (hydroxyurea, anagrelide, and busulfan) to those who did not (Table 2). Patients who received cytoreductive therapy were significantly older than those who did not. Many elderly patients received cytoreductive therapy given their higher risk for thrombosis. There were no significant differences in sex or driver mutation status between the two groups. As for hematological parameters, platelet count, hemoglobin, and white blood cell count were all significantly lower in patients who received cytoreductive therapy than in those who did not. Those who received cytoreductive therapy also had a significantly higher HMW-VWFM index, lower LMW-VWFM index, higher VWF:Ag, higher VWF:RCo/VWF:Ag ratio, and lower VWF-DP/VWF:Ag ratio than the group that did not undergo such therapy. ADAMTS13 activity was similar in both groups.
| Cytoreductive therapy (n = 41) | No cytoreductive therapy (n = 29) | p-value | |
|---|---|---|---|
| Age in years, median (IQR) | 72 (58–78) | 67(41–71) | <.01 |
| Sex (female:male) | 15:26 | 14:15 | .46 |
| Driver mutations | |||
| JAK2 V617F mutation, n (%) | 18/35 (51%) | 15/28 (54%) | 1.0 |
| CALR mutation, n (%) | 12/35 (34%) | 6/28 (21%) | .40 |
| Antiplatelet therapy, n (%) | 20/41 (49%) | 8/21 (38%) | .09 |
| Blood counts | |||
| Platelet count, ×109/L, median (IQR) | 530 (378–707) | 910 (727–1105) | <.01 |
| Hemoglobin, g/dl, median (IQR) | 12.4 (11.4–13.6) | 13.2 (12.5–14.1) | .02 |
| White blood cell count, ×109/L, median (IQR) | 6.7 (5.0–7.9) | 9.0 (7.6–10.2) | <.01 |
| VWF-related parameters | |||
| HMW-VWFM index, %, median (IQR) | 74.0 (57.4–90.6) | 44.3 (25.8–55.1) | <.01 |
| LMW-VWFM index, %, median (IQR) | 118.9 (108.3–128.4) | 142.1 (129.2–148.5) | <.01 |
| VWF:Ag, %, median (IQR) | 117.7 (93.6–149.0) | 95.9 (70.7–111.3) | <.01 |
| VWF:RCo/VWF:Ag ratio, median (IQR) | 0.80 (0.67–0.94) | 0.56 (0.40–0.74) | <.01 |
| VWF-DP/VWF:Ag ratio, median (IQR) | 1.07 (0.79–1.34) | 2.01 (1.58–2.56) | <.01 |
| ADAMTS13 activity, %, median (IQR) | 54.0 (41.2–68.3) | 63.2 (43.3–80.4) | .17 |
Note
- Abbreviations: HMW-VWFM, high molecular weight von Willebrand factor multimer; IQR, interquartile range; LMW-VWFM, high molecular weight von Willebrand factor multimer; VWF:Ag, von Willebrand factor antigen; VWF:RCo, von Willebrand factor ristocetin cofactor activity; VWF-DP, von Willebrand factor degradation product.
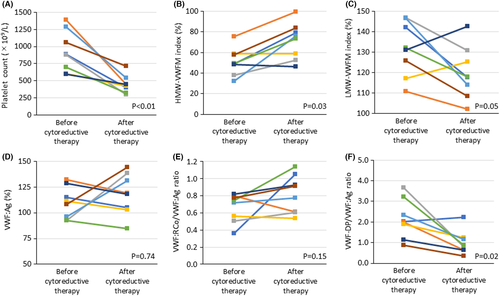
Additionally, we evaluated the longitudinal changes in platelet count and VWF-related parameters before and after cytoreductive therapy in eight patients for whom data were available (Figure 4); we observed a significant decrease in platelet count (Figure 4A), an increase in HMW-VWFM index (Figure 4B), and a decrease in the VWF-DP/VWF:Ag ratio (Figure 4F) after cytoreductive therapy compared to baseline. There were no significant differences in the LMW-VWFM index (Figure 4C), VWF:Ag (Figure 4D), and VWF:RCo/VWF:Ag ratio (Figure 4E) before and after cytoreductive therapy.
4 DISCUSSION
Our study shed light on the contribution of VWF cleavage at the Y1605-M1606 bond by ADAMTS13 to the development of AVWS in patients with ET; we achieved this by measuring VWF-DP via a novel ELISA using mAb N10. Because mAb N10 does not bind to VWF before cleavage, it specifically recognizes the Y1605 residue of the VWF A2 domain that is exposed upon cleavage16; as such, VWF-DP was ideal for quantifying the cleavage of VWF.
As previously shown by another group,7 our VWFM analysis in 70 patients with ET found that the VWFM distribution was similar to VWD type 2, where the HMW-VWFMs reduced (as reflected by lower VWF:RCo/VWF:Ag ratios) as the platelet count increased. We found that the VWF-DP/VWF:Ag ratio was significantly higher in the higher platelet count groups than in group 1. There was a negative correlation between VWF-DP/VWF:Ag ratio and HMW-VWFM index, with the former found to be an independent predictor of the latter; this suggested that VWF cleavage at the Y1605-M1606 bond is accelerated as the platelet count increases, resulting in a reduction of HMW-VWFMs and leading to AVWS. Aortic stenosis also develops AVWS due to excessive cleavage of VWF. In the previous study of patients with severe aortic stenosis, 21 of 31 patients (67.7%) had deficit of HMW-VWFMs, defined as HMW-VWFM index <80%.21 In this study, 50 patients with ET (71.4%) showed HMW-VWFM index less than 80% and deficit of HMW-VWFMs was observed as frequently in patients with ET as in those with aortic stenosis. Additionally, groups with lower platelet counts tended to have a higher VWF:Ag. In these groups, there were significantly more patients who received hydroxyurea, a cytotoxic agent, which may stimulate the secretion of VWF from vascular endothelial cells and therefore, have contributed to increased VWF:Ag levels.
We then investigated the effect of cytoreductive therapy on VWFM alterations in patients with ET. Patients who received cytoreductive therapy experienced a decrease in blood cell counts (mainly platelet counts), a decrease in the VWF-DP/VWF:Ag ratio, an increase in the HMW-VWFM index, and an increase in the VWF:RCo/VWF:Ag ratio compared to those who did not receive therapy. A similar pattern was observed in treated patients when comparing post-therapy values to baseline. In patients with ET, cytoreductive therapy suppresses the hyper-cleavage of VWF, leading to the improvement of VWFM distributions. As such, cytoreductive therapy not only prevents thrombosis by decreasing the platelet count but also contributes to reducing the risk of bleeding associated with AVWS.
The proteases that may be responsible for increased cleavage of VWF in patients with ET include ADAMTS13, proteases released from the greater number of leukocytes, and proteases released from activated platelets. In healthy individuals, the regulation of VWF is mainly dependent on ADAMTS13, and the severe deficiency of which causes thrombotic thrombocytopenic purpura.27, 28 Because of the high platelet counts in patients with ET, more contact between platelets and VWF would be expected, increasing the chance of VWF cleavage by ADAMTS13; however, the mechanism and existence of higher shear stress that promotes the stretching of VWF still requires clarification. Hence, further investigation into the enzymes contributing to the increase in VWF cleavage is needed. Normally, VWF is folded into a globular shape while in the bloodstream, rendering the ADAMTS13 cleavage site concealed.29, 30 However, when subjected to high shear stress such as in small arteries, VWF is stretched and the cleavage site is exposed. Hence, the high shear stress associated with the increased blood cell count in patients with ET would putatively enhance VWF stretching and cleavage site exposure to ADAMTS13. However, this proposition has some pitfalls, as blood viscosity is not expected to be higher in individuals with ET than in those with polycythemia vera. Moreover, ADAMTS13 activity was not significantly correlated with the platelet count in our cohort. Conversely, degradation by other proteases (as described below) may alter the morphology of VWF, making it more susceptible to further cleavage by ADAMTS13.
In terms of leukocyte-derived proteases, several have been suggested to cleave VWF. Among them, cathepsin G cleaves the Y1605-M1606 bond of the VWF A2 domain, similar to ADAMTS13.31 However, marked leukocytosis is less common in ET, and the leukocyte count was also normal or only mildly elevated in our cohort. Therefore, it is unlikely that proteases released from leukocytes are heavily involved in VWF degradation in patients with ET.
Finally, proteases derived from platelets (which are activated in patients with ET32), may play a role in the enhanced cleavage of VWF. In particular, ADAM10 and ADAM17 are both proteolytic enzymes expressed in platelets and are involved not only in the cleavage of glycoproteins VI and Ibα, respectively,33, 34 but also in VWF degradation. Lancellotti et al. showed that enhanced ADAM10 and ADAM17 activity may be involved in the reduction of HMW-VWFMs in patients with ET; they further showed that ADAM10 degraded VWF in vitro in the absence of ristocetin.11 Their findings indicate that, unlike ADAMTS13, ADAM10 may cleave VWF independently of high shear stress. However, while reversed-phase high-performance liquid chromatography has shown that ADAM10 and ADAM17 may share the VWF cleavage site with ADAMTS13,11 mass spectrometry has not been performed; hence, the exact site at which these enzymes cleave VWF remains unconfirmed. Both enzymes have also been reported to cleave the N-terminal of leucine,35 therefore, it is conceivable that these proteolytic enzymes could cleave other bonds near the VWF cleavage site by ADAMTS13. Further research is required to explore these hypotheses.
There were some limitations to our study. First, the correlation between VWF-related parameters and bleeding events could not be sufficiently investigated. Although we identified many patients with decreased HMW-VWFMs in our cohort, only a small number of them actually experienced bleeding events and there was no significant difference in bleeding events prior to inclusion among the three groups according to their platelet counts. However, bleeding events prior to inclusion tended to be more prevalent in the group with higher platelet counts compared to the group with lower platelet counts. Hence, we compared platelet counts and VWF-related parameters between the groups with and without bleeding events prior to inclusion. The group with bleeding events prior to inclusion (n = 12) had a significantly higher platelet counts (Figure S2A), lower HMW-VWFM index (Figure S2B), and higher VWF-DP/VWF:Ag ratio (Figure S2D) than the group without such events (n = 57). This result suggests that in patients with bleeding events prior to inclusion, the deficit in HMW-VWFM due to higher cleavage of VWF may be contributary to the bleeding. However, the study included patients who underwent treatments as well as those who were not receiving treatment at the time of inclusion which may skew the correlation so that it cannot be sufficiently assessed. Therefore, further investigation is needed, such as limiting the subjects to pre-treatment patients or conducting long-term observation in a larger number of patients. Second, samples from patients with ET were compared with pooled counterparts from healthy subjects, while individuals with reactive thrombocytosis were not included. Therefore, we were unable to evaluate whether VWF-related parameters share similar patterns in patients with thrombocytosis unrelated to ET. Finally, we evaluated longitudinal changes in platelet counts and VWF-related parameters before and after cytoreductive therapy in individual patients, and confirmed a similar pattern to that observed when comparing patients who received such therapy to those who did not. However, as we were only able to obtain pre- and post-treatment samples for a small number of patients, further studies with a larger sample size are planned.
In conclusion, we successfully measured VWF-DP via ELISA using a monoclonal antibody that specifically recognizes the VWF-Y1605 residue, allowing us to quantitatively evaluate VWF cleavage in patients with ET. Our results showed that VWF cleavage at the Y1605-M1606 bond in the A2 domain was enhanced as the platelet counts increased, thereby illustrating a potential mechanism whereby HMW-VWFM is reduced and AVWS develops. Although ADAMTS13 appears to be the main enzyme responsible for the cleavage of VWF at this site, further studies are warranted given that proteases released from leukocytes and platelets may also be involved.
CONFLICT OF INTERESTS
MM, the inventor of the ELISA used to assess ADAMTS13 activity, received funding from Chugai Pharmaceutical. The remaining authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
MK performed data and sample collection, experiments, statistical analysis, interpretation of the results, and writing of the manuscript. KS and MH performed the experiments. HK, HY, YS, AH, HT, IA, and YT performed data and sample collection. MM designed the research, interpreted the results, and wrote the paper.