Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: A multicenter retrospective study
Jun-Ying Li and Hong-Fei Wang: Contributed equally
Manuscript handled by: Willem Lijfering
Final decision: Willem Lijfering, 29 January 2021
Funding Information
This work was supported by Program for HUST Academic Frontier Youth Team (No. 2018QYTD14) and National Natural Science Foundation of China (No. 81973995 and No. 31620103909). The funder of the study had no role in study design, data collection, data analysis, data interpretation.
Abstract
Background
High incidence of asymptomatic venous thromboembolism (VTE) has been observed in severe COVID-19 patients, but the characteristics of symptomatic VTE in general COVID-19 patients have not been described.
Objectives
To comprehensively explore the prevalence and reliable risk prediction for VTE in COVID-19 patients.
Methods/Results
This retrospective study enrolled all COVID-19 patients with a subsequent VTE in 16 centers in China from January 1 to March 31, 2020. A total of 2779 patients were confirmed with COVID-19. In comparison to 23,434 non-COVID-19 medical inpatients, the odds ratios (ORs) for developing symptomatic VTE in severe and non-severe hospitalized COVID-19 patients were 5.94 (95% confidence interval [CI] 3.91–10.09) and 2.79 (95% CI 1.43–5.60), respectively. When 104 VTE cases and 208 non-VTE cases were compared, pulmonary embolism cases had a higher rate for in-hospital death (OR 6.74, 95% CI 2.18–20.81). VTE developed at a median of 21 days (interquartile range 13.25–31) since onset. Independent factors for VTE were advancing age, cancer, longer interval from symptom onset to admission, lower fibrinogen and higher D-dimer on admission, and D-dimer increment (DI) ≥1.5-fold; of these, DI ≥1.5-fold had the most significant association (OR 14.18, 95% CI 6.25–32.18, p = 2.23 × 10−10). A novel model consisting of three simple coagulation variables (fibrinogen and D-dimer levels on admission, and DI ≥1.5-fold) showed good prediction for symptomatic VTE (area under the curve 0.865, 95% CI 0.822–0.907, sensitivity 0.930, specificity 0.710).
Conclusions
There is an excess risk of VTE in hospitalized COVID-19 patients. This novel model can aid early identification of patients who are at high risk for VTE.
Essentials
- There is an excess risk of venous thromboembolism (VTE) in severe and non-severe hospitalized COVID-19 patients.
- Risk factors are aging, cancer, longer duration of symptoms prior to admission, lower fibrinogen, higher D-dimer, and D-dimer increment.
- D-dimer increment≥1.5-fold has the most significant association with VTE in hospitalized COVID-19 patients.
- A novel experimental model is a promising approach for symptomatic VTE prediction.
1 INTRODUCTION
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs in approximately 1 out of 1000 individuals in the general population, but is often secondary to other clinical conditions.1 During the coronavirus disease 2019 (COVID-19) pandemic, high prevalence of DVT was observed in severe COVID-19 patients, especially in the intensive care unit (ICU).2-4 High prevalence of incident thrombosis in small and mid-sized pulmonary arteries has been demonstrated in clinicopathologic case series, despite thromboprophylaxis.5, 6 Most VTE are asymptomatic and whether they are the cause of death or only concurrent events remains controversial.7 On the other hand, COVID-19 patients at high risk for VTE are also at high risk for bleeding, and sometimes catastrophic intracranial hemorrhage may occur.8 Therefore, anticoagulation may potentially be harmful, and it would be important to distinguish those who will develop thromboses in hospitalized COVID-19 patients.9
The prevalence, clinical characteristics, and risk factors for clinically relevant symptomatic VTE in hospitalized COVID-19 patients continue to be debated, and there remains a lack of satisfactory VTE risk prediction in these patients. Given this context, we performed a multicenter study to explore the prevalence, risk factors, and prediction models for symptomatic VTE in hospitalized COVID-19 patients.
2 MATERIALS AND METHODS
2.1 Study design and participants
The flowchart for the study is summarized in Figure S1 in supporting information. This multicenter, retrospective, observational study was conducted in 16 centers including Wuhan, China, the first epicenter of the COVID-19 pandemic in the world.
First, we investigated the absolute and relative risk for symptomatic VTE in hospitalized COVID-19 patients using a retrospective cohort study design, with data from 3 of the 16 centers in which a Big Data system was present. From January 1 to March 31, 2020, all laboratory-confirmed hospitalized COVID-19 patients were included, and were compared to a historic cohort of 23,434 non-COVID-19 medical inpatients from January 1 to March 31, 2018. Data on age, sex, and symptomatic VTE events were extracted by the Lex Clinical Data Application 3.2 (Shanghai Lejiu Healthcare Technology Co., Ltd), a validated big data system designed to query a clinical data warehouse and return tabular data for analysis and visualization.10 Second, we investigated the potential risk factors and predictors for symptomatic VTE in hospitalized COVID-19 patients using a case-control study design. From January 1 to March 31, 2020, those COVID-19 patients who had a subsequent symptomatic VTE event during hospitalization were included from 16 centers (“cases”) and compared to disease-severity matched COVID-19 patients without symptomatic VTE (“control group”) at an approximate 2:1 ratio (Figure S2 in supporting information).
This study was registered in the Chinese Clinical Trial Registry (ChiCTR2000033055) and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Informed consent was waived by the Ethics Committee.
2.2 Data collection and definitions
For the case-control study, data on demographic, clinical, laboratory, chest radiographs or CT scan, treatment, outcome, and VTE and bleeding events were extracted from electronic medical records of each center. Missing or uncertain records were clarified through communication with involved clinicians or patients. All data were checked by two investigators (LVT and HFW), and any difference in interpretation was adjudicated by a third clinician (WZL).
COVID-19 was confirmed by the laboratory tests of SARS-CoV-2 RNA as described previously.11, 12 Severity of COVID-19 was divided into mild, moderate, and severe categories, based on World Health Organization guidelines (https://apps.who.int/iris/handle/10665/331446). Virus clearance was defined as at least two consecutive negative RNA tests for SARS-CoV-2. The time of follow-up was defined as the duration from illness onset of COVID-19 to outcomes (symptomatic VTE, discharge, or death) of patients. No cases were lost to follow-up in this study.
Symptomatic VTE was diagnosed based on both clinical manifestations and elevated level of D-dimer (>0.5 μg/ml), and was confirmed by objective imaging: compression ultrasonography for deep vein thrombosis, computed tomography (CT) pulmonary angiography for pulmonary embolism. Clinical manifestations included swelling and pain of the lower extremities, superficial varicose veins, severe chest pain and hemoptysis under a stable disease state, worse PaO2/FiO2, hemodynamic impairment requiring fluid challenge, or dilated right ventricle. Catheter-associated thrombosis and visceral VTE were not examined. According to the ISTH criteria, major bleeding following anticoagulation was defined as clinically overt bleeding accompanied by a decrease in the hemoglobin level of at least 20 g/L or transfusion of at least 2 U of packed red blood cells, occurring at a critical site (such as intracranial), or resulting in death. In this study, D-dimer increment (DI) was defined as D-dimer level on day 4 to day 6 divided by that on day 1 to day 3 following hospitalization.
2.3 Risk prediction for VTE
Three clinical risk assessment models (the Padua model, the Improve model, and the Geneva model) for VTE in hospitalized medical patients were evaluated for each participant.13, 14 A “6-factor model” was tentatively defined as the combination of six independent variables for VTE in the final logistic regression model: age, cancer, interval from COVID-19 onset to admission, fibrinogen concentration and D-dimer level on admission, and D-dimer increment ≥1.5-fold. A simplified “3-factor model,” or “Wuhan score” was tentatively defined as the model consisting of the three coagulation variables (fibrinogen and D-dimer on admission, and DI ≥1.5-fold) that were significantly associated with symptomatic VTE in COVID-19 patients described in the analysis below.
2.4 Statistical analysis
Continuous and categorical variables were presented as median (interquartile range [IQR]) and number (%), respectively. The Mann-Whitney U test, chi-squared, or Fisher's exact test were employed to compare differences between VTE group and non-VTE group where appropriate. To explore the risk factors associated with symptomatic VTE in-hospital, univariable and multivariable logistic regression analyses were performed to estimate odds ratios (ORs) and 95% confidence intervals (CIs). There were 13 factors showing significant differences in univariable analysis (Table 1 and Table S3 in supporting information). To avoid overfitting in the model, three factors (white blood cell count, neutrophils, and lymphocytes on admission) were excluded from further analysis because we think they would have collinearity with coagulation variables. Finally, 10 factors were included in multivariable analysis, including: age, hypertension, active cancer, venous catheterization, glucocorticoid, days from COVID-19 onset to admission, C-reactive protein, D-dimer on admission, DI, and fibrinogen.
| Characteristics |
VTE group N = 104 |
Non-VTE group N = 208 |
p |
|---|---|---|---|
| Severity of COVID-19 | |||
| Moderate | 40 (39.5%) | 88 (42.3%) | .52 |
| Severe | 64 (61.5%) | 120 (57.7%) | |
| Age (year, median, IQR) | 66 (61–79) | 60.5 (49–68) | 6.77 × 10−7 |
| Male sex | 45 (43.3%) | 95 (45.7%) | .69 |
| Smoking | 8 (7.7%) | 8 (3.8%) | .18 |
| Top temperature | |||
| <37.5℃ | 29 (27.9%) | 68 (32.7%) | .69 |
| ≥37.5 and <39℃ | 48 (46.2%) | 90 (43.3%) | |
| ≥39℃ | 27 (25.9%) | 50 (24.0%) | |
| Cough | 87 (83.6%) | 155 (74.5%) | .08 |
| Dyspnea on admission | 33 (31.7%) | 61 (29.3%) | .70 |
| Pulmonary radiography on admission | |||
| Focal small patchy lesions | 48 (46.2%) | 94 (45.2%) | .87 |
| Extensive or diffuse lesions | 56 (53.8%) | 114 (54.8%) | |
| Comorbidities | |||
| Coronary heart disease | 24 (23.1%) | 35 (16.8%) | .22 |
| Hypertension | 46 (44.2%) | 58 (27.9%) | 8.99 × 10−4 |
| Diabetes | 22 (21.2%) | 38 (18.3%) | .54 |
| Atrial fibrillation | 10 (9.6%) | 8 (3.8%) | .07 |
| COPD | 15 (14.4%) | 20 (9.6%) | .25 |
| Chronic heart failure | 19 (18.3%) | 23 (11.0%) | .11 |
| Active cancer | 12 (11.5%) | 5 (2.4%) | .001 |
| Autoimmune disease | 2 (1.9%) | 1 (0.5%) | .26 |
| Therapies | |||
| Arbidol | 71 (68.3%) | 155 (74.5%) | .24 |
| Hydroxychloroquine | 12 (11.5%) | 24 (11.5%) | 1.00 |
| Lopinavir-ritonavir | 20 (19.2%) | 44 (21.1%) | .77 |
| Remdesivir | 2 (1.9%) | 1 (0.5%) | .26 |
| Convalescent plasma | 2 (1.9%) | 2 (1.0%) | .60 |
| Antibiotics | 97 (93.3%) | 189 (90.9%) | .52 |
| Antifungal agents | 24 (23.1%) | 36 (17.3%) | .23 |
| Tocilizumab | 10 (9.6%) | 11 (5.3%) | .16 |
| Glucocorticoid | 44 (42.3%) | 54 (26.0%) | .003 |
| Immunoglobin | 50 (48.1%) | 82 (39.4%) | .14 |
| Mechanical ventilation | 18 (17.3%) | 24 (11.5%) | .16 |
| Laboratory findings on admission (median, IQR) | |||
| WBC count (109/L) | 6.33 (4.70–9.67) | 4.28 (2.96–6.56) | 8.08 × 10−10 |
| Neutrophil (109/L) | 4.51 (3.15–8.16) | 3.26 (2.20–5.46) | 3.02 × 10−6 |
| Lymphocyte (109/L) | 0.82 (0.54–1.33) | 1.19 (0.79–1.56) | .001 |
| Platelet count (109/L) | 205.5 (153.25–306) | 216 (156.2–278) | .73 |
| ALT (U/L) | 35 (20.8–50) | 26.5 (19–48.2) | .11 |
| AST (U/L) | 29.5 (21.8–49.2) | 28 (20–39) | .10 |
| CRP (mg/L) | 59.4 (32.9–83.4), n = 104 | 12.3 (4.4–49.2), n = 206 | 6.16 × 10−10 |
| IL−6 (pg/ml) | 14.09 (5.34–27.09), n = 81 | 9.47 (4.78–24.19), n = 164 | .28 |
| D-dimer (μg/ml) | 2.07 (0.8–6.57), n = 96 | 0.61 (0.29–1.46), n = 187 | 1.62 × 10−10 |
| Fibrinogen (g/L) | 4.12 (2.89–4.98), n = 94 | 4.47 (3.66–5.39), n = 185 | .005 |
Notes
- Cases (VTE group) and controls (non-VTE group) were 1 to 2 matched by severity of COVID-19. Some laboratory tests were not performed on admission and comparisons were made by available data.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; IL-6, interleukin 6; IQR, interquartile range; Top temperature, the highest temperature during hospitalization; VTE, venous thromboembolism; WBC, white blood cell.
Multivariable analysis with the Cox proportional-hazard model was employed to assess the simultaneous effects of related factors on symptomatic VTE. VTE-free survivals were estimated by the Kaplan-Meier method stratified by Padua score, Improve score, Geneva score, and D-dimer increment. Any differences in VTE incidence were evaluated with a log-rank test. Receiver-operating-characteristic (ROC) analysis was performed to estimate the sensitivity, specificity, and overall accuracy of predictors for symptomatic VTE. Area under the curve (AUC) was calculated for each factor included. A two-sided P value <.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0.
3 RESULTS
From January 1 to March 31, 2020, a total of 2779 COVID-19 patients were enrolled from three centers: there were 1139 non-severe and 1640 severe COVID-19 patients. Of these, 42 developed symptomatic VTE during hospitalization.
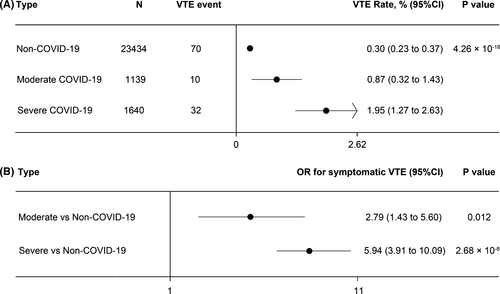
A historical cohort of 23,434 non-COVID-19 medical in-patients was retrospectively enrolled from the same centers during the same month period in 2018 (Figure S1). The historical cohort consisted of non-COVID-19 patients hospitalized for a variety of medical conditions, all of whom were de-identified. The median age was 61.5 years (IQR 46–79.5 years) and 53.3% of the patients were male; 71.6% of the patients were admitted mainly for cardiovascular diseases, hematological tumors, nervous system diseases, or respiratory diseases and 8.0% of them were transferred to the ICU. The following cases were excluded: receiving surgical procedures during hospitalization, hospital stay less than 3 days, age less than 14 years, or readmission due to transfer to another ward. Of these, 70 patients of the historical cohort developed symptomatic VTE. The crude rates of VTE were 1.95% (1.27% to 2.63%) in severe COVID-19 patients, 0.87% (0.32% to 1.43%) in non-severe COVID-19 patients, and 0.30% (0.23% to 0.37%) in non-COVID-19 medical patients (P = 4.26 × 10−16, Figure 1A). After adjustment for age and gender, the ORs for developing symptomatic VTE were 5.94 (95% CI 3.91–10.09) and 2.79 (95% CI 1.43–5.60) in severe and non-severe hospitalized COVID-19 patients, compared to non-COVID-19 patients (Figure 1B).
3.1 VTE vs Non-VTE patients
We compared 104 VTE cases and 208 disease-severity–matched controls without symptomatic VTE from 16 centers (Figure S1 and Figure S2). The 104 VTE cases consisted of 88 DVT events and 16 PE events (also combined with DVT). During hospitalization of the DVT, PE, and non-VTE groups, there were 9, 6, and 17 deaths, respectively (Figure S3 in supporting information). All other patients were discharged. The crude case-fatality rates in hospital were broadly comparable between COVID-19 patients with DVT (10.23%) and those without VTE (8.17%). Nevertheless, PE cases had a significantly higher rate for death compared to non-VTE ones (OR 6.74, 95% CI 2.18–20.81, P = .001).
There were 64 and 120 severe cases in the VTE group and non-VTE group (61.5% vs. 57.7%, P = .52), respectively (Table 1). The median age of the VTE patients was 66.0 years (IQR 61.0–79.0), significantly higher than the non-VTE group (60.5, IQR 49–68). The most frequently used antiviral drug was arbidol, and more than 90% of cases were prescribed broad-spectrum antibiotics. Systematic glucocorticoid use was significantly higher in VTE cases compared to non-VTE cases (P = .003). On admission, the VTE group had significantly higher levels of median white blood cells, neutrophil count, C-reactive protein, and D-dimer levels, and lower fibrinogen concentrations, compared to the non-VTE group (Table 1).
3.2 VTE prophylaxis and treatment
Pharmacological thromboprophylaxis was employed in 13 (12.5%) VTE cases and 35 (16.8%) non-VTE cases with COVID-19 (Table S1 in supporting information); low molecular weight heparin (LMWH) was most commonly prescribed (95.8%), usually 4000 IU per day. One gastrointestinal major bleeding occurred in the VTE group, whereas one gastrointestinal major bleeding and one intra-abdominal fatal bleeding occurred in the non-VTE group. All the major and fatal bleeding events developed in patients receiving LMWH of 4000 IU per day. Treatment for VTE was shown in Table S2 in supporting information.
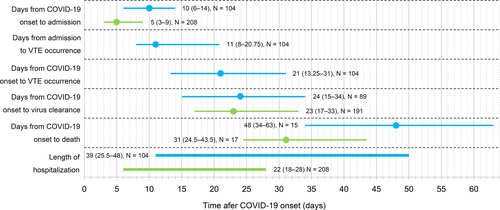
The timeline for clinical outcomes from COVID-19 onset is summarized in Figure 2. Symptomatic VTE developed at a median of 21 days (IQR 13.25–31) after illness onset and 11 days (IQR 8–20.75) after hospitalization. Median time from illness onset to admission was longer in VTE cases than in non-VTE cases (10 days, IQR 6–14, vs. 5 days, IQR 3–9, P = 7.86 × 10−10). The VTE group had a longer duration of hospitalization than that in non-VTE group (median 39 days, IQR 25.5–48, vs. 22 days, IQR 18–28, P = 6.77 × 10−15). Median times from COVID-19 onset to virus clearance were similar in the two groups (P = .53).
3.3 Risk prediction for VTE
Possible factors and prediction models related to VTE in hospitalized COVID-19 patients were assessed (Table S3). The proportion of Padua score ≥4, Improve score ≥3, Geneva score ≥3, and D-dimer increment ≥1.5-fold were higher in the VTE cases than in non-VTE cases.
On multivariable analysis (Table 2), independent factors for symptomatic VTE were advancing age, cancer, longer interval from onset to admission, lower fibrinogen and higher D-dimer on admission, and D-dimer increment ≥1.5-fold. Of these, D-dimer increment ≥1.5-fold had the most significant association (OR 14.18, 95% CI 6.25–32.18, P = 2.23 × 10−10), followed by D-dimer level on admission (OR 1.33, 95% CI 1.17–1.50, P = 7.46 × 10−6), and fibrinogen level on admission (OR 0.64, 95% CI 0.49–0.83, P = 7.56 × 10−4).
| Factors | Crude OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| Age | 1.05 (1.03–1.07) | 2.86 × 10−6 | 1.04 (1.01–1.07) | .008 |
| Days from COVID-19 onset to admission | 1.10 (1.05–1.16) | 2.07 × 10−4 | 1.12 (1.05–1.20) | .001 |
| Hypertension | 2.60 (1.60–4.21) | 8.99 × 10−4 | 1.62 (0.76–3.44) | .208 |
| Active cancer | 5.30 (1.81–15.46) | .001 | 6.14 (1.29–29.21) | .022 |
| CRP on admission | 1.008 (1.000–1.015) | .054 | 1.007 (0.996–1.014) | .067 |
| D-dimer on admission | 1.26 (1.14–1.38) | 5.10 × 10−6 | 1.33 (1.17–1.50) | 7.46 × 10−6 |
| DI ≥1.5 fold | 8.32 (4.63–14.96) | 9.54 × 10−14 | 14.18 (6.25–32.18) | 2.23 × 10−10 |
| Fibrinogen on admission | 0.72 (0.58–0.89) | .002 | 0.64 (0.49–0.83) | 7.56 × 10−4 |
| Glucocorticoid | 2.09 (1.27–3.44) | .004 | 1.95 (0.86–4.44) | .113 |
| Central venous catheterization | 4.64 (2.20–9.76) | 5.36 × 10−5 | 2.10 (0.58–7.57) | .255 |
Note
- DI: D-dimer increment, defined as D-dimer level on day 4 to day 6 divided by that on day 1 to day 3 following admission; DI values were available for 88 VTE cases and 169 Non-VTE ones; adjusted OR: odds ratio for symptomatic VTE was adjusted for age, hypertension, active cancer, venous catheterization, glucocorticoid, days from COVID-19 onset to admission, CRP, D-dimer on admission, DI, fibrinogen.
- Abbreviations: CI, confidence interval; CRP, C-reactive protein; OR, odds ratio; VTE, venous thromboembolism; WBC, white blood cell.
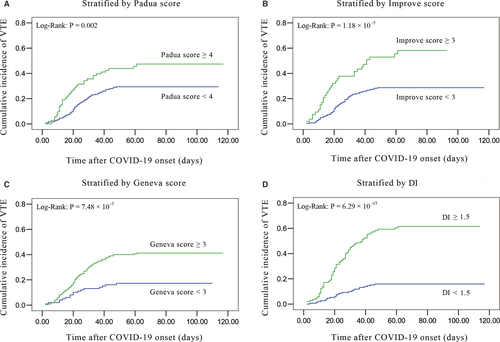
Kaplan-Meier curves showed a higher cumulative symptomatic VTE incidence in hospitalized COVID-19 patients with Padua score ≥4, Improve score ≥3, Geneva score ≥3, and D-dimer increment ≥1.5-fold (Figure 3A-D). Multivariable analysis using a Cox proportional-hazard model also indicated that D-dimer increment ≥1.5-fold had the most significant association with symptomatic VTE (Table S4 in supporting information; hazard ratio [HR] 4.66, 95% CI 2.76–7.85, P = 7.65 × 10−9).
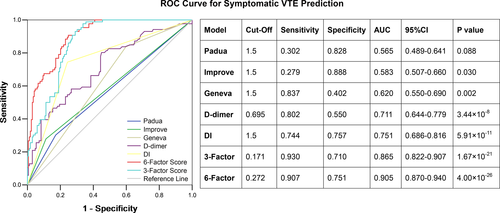
ROC curve analysis was performed for the possible predicting factors (Figure 4). We tentatively defined the “6-factor model” as the combination of six independent variables for VTE in the final logistic regression model. We also defined a simpler “3-factor score” (“Wuhan score”) as the model using the three coagulation variables significantly associated with symptomatic VTE: fibrinogen and D-dimer on admission, and DI ≥1.5-fold. The 6-factor model showed the highest prediction accuracy for symptomatic VTE (AUC 0.905, 95% CI 0.870–0.940), followed by the simple Wuhan score (AUC 0.865, 95% CI 0.82–0.907), DI ≥1.5-fold (AUC 0.751, 95% CI 0.686–0.816), and D-dimer on admission (AUC 0.711, 95% CI 0.644–0.779). The Wuhan score (3-factor model) had a sensitivity of 0.93 and a specificity of 0.71 for predicting symptomatic VTE in COVID-19 patients.
4 DISCUSSION
To our knowledge, this is the largest multicenter study that systematically investigated the risk of, and predicting factors for, symptomatic VTE in hospitalized COVID-19 patients. Our principal findings are as follows: (a) there is an excess risk of VTE in severe and non-severe hospitalized COVID-19 patients compared to non-COVID-19 medical inpatients; (b) new-onset PE increased in-hospital deaths, and six factors independently predicted symptomatic VTE in hospitalized COVID-19 patients; and (c) a simple “3-factor” model (Wuhan score) consisting of three coagulation variables (fibrinogen and D-dimer levels on admission, and DI ≥1.5-fold) showed very good prediction for symptomatic VTE.
Several other studies and case series on incidence of VTE in COVID-19 patients have been published.15-28 The reported incidence of VTE in these cohorts varied widely (4.1% to 85.4%) due to the different characteristics of study population, different diagnostic methods, and various thromboprophylaxis modalities. Most of the studies enrolled critically ill patients and employed a screening strategy with ultrasonography. Asymptomatic VTE events were also commonly included, which accounted for 65.2% to 87.8% of the total VTE events. Therefore, strikingly high rates of VTE in hospitalized COVID-19 patients were observed.
In contrast, we focused on symptomatic VTE, which was more clinically relevant and has always been used as the primary outcome in clinical trials. Indeed, asymptomatic VTE is widely prevalent in special populations, such as patients suffering from acute infectious diseases or cancer, and those who are critically ill, even before hospitalization. Some studies suggested that less than 5% of the asymptomatic VTE could progress to a clinically symptomatic event. Thus, asymptomatic DVT is associated with a low risk of recurrence as well as a low risk of post-thrombotic syndrome, and long-term mortality in asymptomatic DVT is broadly comparable to that seen in non-DVT patients.29 Moreover, anticoagulant prophylaxis confers an absolute risk reduction in asymptomatic VTE events of only 2.6%, but results in significantly increased risk in major bleeding.30 In contrast, there is also evidence that asymptomatic DVT is associated with increased short-term mortality in medical patients.31, 32 Therefore, the clinical importance and prognosis of asymptomatic VTE is still uncertain and there is no consensus on the necessity of detection and treatment.33
In this study, 12.5% of VTE cases and 16.8% of non-VTE cases with COVID-19 received pharmacological thromboprophylaxis (Table S1). Rate of thromboprophylaxis in VTE group did not significantly differ from that in the non-VTE cases and a considerable number of COVID-19 patients developed symptomatic VTE despite anticoagulation. COVID-19 patients who are at high risk for VTE may also be at high risk for bleeding.34 As shown in the current study, 3 out of the 48 COVID-19 patients (6.25%) receiving thromboprophylaxis developed major bleeding. A more precise method should be developed to recognize patents who are at high risk for thrombosis and those who are at greater risk for major bleeding where caution is needed with full-dose anticoagulation.35-39
In early studies on COVID-19, the most typical finding was a higher D-dimer concentration on admission in patients with VTE than that in those without VTE.18, 21, 26 Therefore, D-dimer on admission was considered a diagnostic marker for VTE in COVID-19.40 Nevertheless, D-dimer has a low specificity, and we have observed that many patients with a high D-dimer level would not necessarily develop a symptomatic VTE if the level kept stable or increased slowly. In contrast, in COVID-19 patients who are likely to develop a symptomatic VTE, the D-dimer will rise sharply within the first week during hospitalization.
Because the duration from admission to symptomatic VTE ranged from 8 to 20.75 days (Figure 2), we proposed using a D-dimer increment of ≥1.5-fold increase, from day 1–3 to day 4–6 following hospitalization. Indeed, such a D-dimer increment was the most significant risk factor for developing symptomatic VTE using multivariable analysis. Additionally, D-dimer increment improved the specificity significantly, and predictive accuracy for VTE prediction comparing single clinical or laboratory variables (Figure 4).
The Padua score, the IMPROVE model, and the Geneva risk score are robust risk assessment models evaluating VTE risk in hospitalized medical patients (Table S5 in supporting information).12 Consistent with previous findings, the above three models were also associated with developing symptomatic VTE in hospitalized COVID-19 patients. They are clinical assessment models that included various demographic and clinical characteristics. All these clinical variables will ultimately contribute to a hypercoagulable state, which will be reflected by coagulation variables. Therefore, we proposed a simple “3-factor score” (Wuhan score) consisting of three independent coagulation predictors for VTE identified using multivariable analysis: lower fibrinogen on admission (coagulation consumption), higher D-dimer on admission (secondary hyperfibrinolysis), and D-dimer increment ≥1.5-fold (persistent hyperfibrinolysis). The Wuhan model showed very good predictive accuracy for symptomatic VTE, which was broadly comparable to the more complex 6-factor model (sensitivity 0.930 vs. 0.907, specificity 0.710 vs. 0.751).
4.1 Strengths and limitations
This research has some strengths. This was a multicenter study covering all hospitalized COVID-19 patients with VTE, with complete follow-up. Therefore, the study population would be representative of the whole hospitalized COVID-19 population with VTE in China. In addition, we focused on symptomatic VTE, which has more clinical relevance, and investigated the associated factors comprehensively. Moreover, we proposed a simple experimental model for symptomatic VTE prediction, which could more directly reflect the underlying hypercoagulable state.
This study also has several limitations. First, it was a retrospective study, with lack of regular dynamic clinical and laboratory data. Second, the sample size was not large enough to adjust for possible differences in patients’ characteristics across centers, and interpretation of our findings might be limited by the sample size. By including all patients with symptomatic VTE in the 16 major designated COVID-19 hospitals, we believe our study population is representative of cases managed in the epicenter of China. Third, genetic factors may play an important role in VTE, but these data were not available in this study. Fourth, there is no internal or external validation, because the COVID-19 outbreak was controlled rapidly in China. Fifth, other major VTE risk factors of the historic cohort were not taken into account when predicting the OR for VTE. Fifth, in Western populations the majority of patients admitted with COVID-19 receive thromboprophylaxis. There is ethnic variation in risk of VTE and therefore the estimates of absolute and relative risk may not apply to other populations. There is also ethnic variation in D-dimer and fibrinogen, so the proposed model may not apply elsewhere. Sixth, in the Kaplan-Meier analyses, the cumulative VTE incidences have not been adjusted for deaths. Therefore, the VTE incidence may have been overestimated. Last, follow-up after discharge was not conducted, and post-discharge VTE events were not analyzed.
5 CONCLUSIONS
There is an excess risk of symptomatic VTE in severe and non-severe hospitalized COVID-19 patients. New-onset PE increased in-hospital deaths, and three coagulation variables (fibrinogen and D-dimer on admission, and DI ≥1.5-fold) predicted symptomatic VTE in hospitalized COVID-19 patients. This “3-factor” model can aid in early identification of COVID-19 patients who are at high risk for symptomatic VTE.
ACKNOWLEDGMENTS
We wish to acknowledge the dedication, commitment, and sacrifice of the staff, providers, and personnel in each center through the local COVID-19 crisis and express our profound sadness about the suffering and loss of our patients, their families, and our community. Special thanks go to the participants included in this report.
CONFLICTS OF INTEREST
GYHL: consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo; speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. The other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
LVT and YH were the overall principal investigators in this study who conceived the study and obtained financial support, were responsible for the study design, and supervised the entire study. JYL, LVT, HFW, DL, DLW, PP, WHW, LW, XWY, JYX, FZ, NX, FS, CXW, XT, HY, WJW, BDL, and WZL recruited participants and collected the data. JYL, LVT, PY, DL, QL, and WZL completed the statistical analyses. JYL, LVT, HFW, DLW, FS, CXW, WJW, BDL, QL, and YH completed data analysis. JYL, LVT, HFW, and GYHL drafted the paper. All authors participated in interpretation of data, critically revised the manuscript for important intellectual content, and gave final approval for the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.