BBX9 forms feedback loops with PIFs and BBX21 to promote photomorphogenic development
Edited by: Hongtao Liu, Center for Excellence in Molecular Plant Sciences, Institute of Plant Physiology and Ecology, CAS, China
ABSTRACT
Light is one of the most essential environmental factors that tightly and precisely control various physiological and developmental processes in plants. B-box CONTAINING PROTEINs (BBXs) play central roles in the regulation of light-dependent development. In this study, we report that BBX9 is a positive regulator of light signaling. BBX9 interacts with the red light photoreceptor PHYTOCHROME B (phyB) and transcription factors PHYTOCHROME-INTERACTING FACTORs (PIFs). phyB promotes the stabilization of BBX9 in light, while BBX9 inhibits the transcriptional activation activity of PIFs. In turn, PIFs directly bind to the promoter of BBX9 to repress its transcription. On the other hand, BBX9 associates with the positive regulator of light signaling, BBX21, and enhances its biochemical activity. BBX21 associates with the promoter regions of BBX9 and transcriptionally up-regulates its expression. Collectively, this study unveiled that BBX9 forms a negative feedback loop with PIFs and a positive one with BBX21 to ensure that plants adapt to fluctuating light conditions.
INTRODUCTION
Light is a key environmental factor that regulates a variety of physiological and developmental processes in a plant's life cycle. Plants have evolved at least five classes of photoreceptors that perceive distinct wavelength-specific light signals from the sun. Phytochromes (phys) sense red and far-red light, cryptochromes (CRYs), phototropins (PHOTs), and ZEITLUPE family members (ZTL, FKF1, and LKP2) absorb UV-A and blue light, while UV RESISTANCE LOCUS 8 (UVR8) acts as a UV-B photoreceptor (Galvao and Fankhauser, 2015; Paik and Huq, 2019; Cheng et al., 2021). Light-activated photoreceptors promptly initiate and induce a set of molecular and biochemical events, including conformational changes, nucleocytoplasmic partitioning, phosphorylation, ubiquitination, and transcriptional reprogramming in plant cells (Paik and Huq, 2019; Cheng et al., 2021; Qi et al., 2022; Jia et al., 2024; Shi et al., 2024), ultimately resulting in the adaptation of plants to changing light environments.
PHYTOCHROME B (phyB) is the predominant red light photoreceptor in Arabidopsis. Upon exposure to red light, the Pr isoform of phyB is converted into a biologically active Pfr version that rapidly translocates from the cytoplasm to the nucleus, where it forms nuclear bodies (NBs) (Bauer et al., 2004). The steady-state pattern of phyB NBs are tightly corelated with light-dependent seedling development (Chen et al., 2003, 2005; Van Buskirk et al., 2012; Kwon et al., 2024). Pfr form of phyB interacts with a subgroup of basic Helix-Loop-Helix (bHLH)-type transcription factors, namely PHYTOCHROME-INTERACTING FACTORs (PIFs: PIF1, PIF3, PIF4, and PIF5), triggering their phosphorylation and ubiquitination through distinct kinases and E3 ubiquitin ligases, and eventually leading to their degradation via the 26S proteasome system within minutes (Cheng et al., 2021). In addition, CRYs also modulate the transcription and abundance of PIF4 and PIF5 to control blue light and warm temperature-mediated development (Ma et al., 2016; Pedmale et al., 2016; Zhai et al., 2020). PIFs control the expression of hundreds of genes, especially those related to plant hormone auxin biosynthesis and signaling to promote hypocotyl growth (Leivar et al., 2009; Shin et al., 2009; Franklin et al., 2011; Sun et al., 2012, 2013; Li et al., 2024). Moreover, increasing studies revealed that PIFs integrate diverse internal (e.g., circadian clock and hormones) or external signals (e.g., light, shade, and temperature) to regulate plant growth and development (Franklin et al., 2011; Nusinow et al., 2011; Sun et al., 2012, 2013; Cheng et al., 2021; Bian et al., 2022; Legris, 2023; Martinez-Garcia and Rodriguez-Concepcion, 2023; Fu et al., 2024; Liu et al., 2024; Luo et al., 2024).
A group of B-box domain containing proteins (BBXs) acting downstream of various photoreceptors coordinately control light-dependent development in plants (Gangappa and Botto, 2014; Song et al., 2020a, 2024; Xu, 2020; Cao et al., 2023). BBXs can be classified into five subfamilies according to their domain structure. Each BBX member contains at least one or two B-box domains at the N-terminal regions. Some of them also possess Val–Pro (VP) motif(s) or a CONSTANS, CONSTANS-like, TIMING OF CAB EXPRESSION 1 (CO, CO-like, TOC1; CCT) domain at the C-terminus, while others lack these conserved domains (Khanna et al., 2009). CONSTANS (CO/BBX1), which directly activates the FLOWERING LOCUS (FT) to initiate flowering, is the first BBX protein characterized and identified in plants (Putterill et al., 1995; Samach et al., 2000). The CO-FT module is linked to various endogenous and exogenous signals, including light, temperature, and the circadian clock, in the control of floral induction (Suárez-López et al., 2001; Jang et al., 2008; Fernández et al., 2016). In addition to flowering, multiple BBXs, along with ELONGATED HYPOCOTYL 5 (HY5), form a molecular regulatory network, controlling the expression of a large number of genes for photomorphogenesis. Previous studies have shown that BBX11, BBX20, BBX21, BBX22, and BBX23 interact with HY5 to enhance its action (Datta et al., 2007, 2008; Bursch et al., 2020, 2021; Zhao et al., 2020; Job et al., 2022; Podolec et al., 2022). BBX11, BBX21, and HY5 transcriptionally form a positive feedback loop (Xu et al., 2016, 2018; Zhao et al., 2020; Job and Datta, 2021), and HY5 up-regulates the transcription of BBX22 (Chang et al., 2008). BBX24, BBX25, BBX28, and BBX29 function as negative regulators of light signaling by directly associating with HY5, and inhibiting its transcriptional activation activity (Gangappa et al., 2013; Lin et al., 2018; Song et al., 2020b). These facts suggested that these BBXs act as rate-limiting cofactors of HY5 in the regulation of photomorphogenic development. In addition, BBX4, BBX11, BBX16 and BBX23 participate in the signaling of PIFs (Zhang et al., 2017a; Heng et al., 2019; Job and Datta, 2021; Song et al., 2021; Veciana et al., 2022), indicating that BBXs also regulate light-mediated developmental processes via other transcription factors or signaling pathways.
In this study, we identify BBX9 as a positive regulator of light signaling. The Pfr form of phyB interacts with BBX9 and promotes its accumulation in light. Loss of BBX9 function mutant seedlings show significantly elongated hypocotyls, whereas transgenic plants overexpressing BBX9 exhibit markedly shortened hypocotyl phenotypes. BBX9 interacts with PIFs and inhibits their transcriptional activation activity toward target genes. PIFs associate with the promoter regions of BBX9 to repress its expression. In addition, BBX9 interacts with BBX21 to enhance its biochemical activity at the protein level, and in turn, BBX9 is positively controlled by BBX21 at the transcriptional level. Together, our results suggest that BBX9 participates in the PIFs- as well as BBX21-mediated signaling to promote photomorphogenesis in light.
RESULTS
Phytochrome B interacts with BBX9
Phytochrome B is the predominant photoreceptor responsible for perceiving red light signals in Arabidopsis (Sharrock and Quail, 1989; Li et al., 2011). We performed a yeast two-hybrid screen to identify the novel components of light signaling using BD-phyB-C possessing both PRD and HKRD domains as bait. This screen identified that BBX9 could interact with phyB-C, but not phyB-C1 containing only an HKRD domain, suggesting that BBX9 is a phyB-interacting protein and that the intact C-terminal of phyB is required for its interaction with BBX9 in yeast cells (Figure 1A, B). In line with these results, the firefly luciferase (LUC) complementation imaging (LCI) assays showed that LUC signals were markedly detectable when phyB-LUCN and LUCC-BBX9 were transiently co-expressed in Nicotiana benthamiana leaves. Various negative controls, as indicated, when transiently co-expressed in the same leaves produced barely detectable LUC signals (Figure 1C). Next, we incubated recombinant glutathione S-transferase (GST) or GST-BBX9 with protein extracts from dark-grown phyB-myc transgenic seedlings irradiated with far-red or red light for 15 min respectively. GST-BBX9 was able to pull-down phyB-myc proteins from extracts treated with red light, but not from those treated with far-red light, while GST could not pull-down any detectable phyB-myc from the same protein extracts (Figure 1D). These data indicate that BBX9 preferentially associates with the Pfr form of phyB.
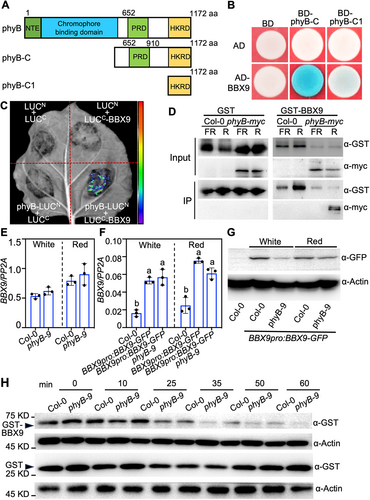
Phytochrome B (phyB) interacts with and stabilizes B-box CONTAINING PROTEIN 9 (BBX9)
(A) Schematic diagram of various constructs used in the yeast two-hybrid assays. Numbers indicate the amino acid positions in phi. (B) Yeast two-hybrid interactions between the various truncated phyB and BBX9 proteins. (C) Luciferase (LUC) complementation imaging (LCI) assays showing the interaction of phyB with BBX9. Full-length phyB and BBX9 were fused to the split N- or C-terminal (LUCN or LUCC) fragments of LUC. LUCN and LUCC were used as negative controls. (D) Recombinant BBX9 interacted with the Pfr form of phyB; 0.38 pmol glutathione S-transferase (GST) or 0.16 pmol GST-BBX9 were incubated with plant extracts from myc-phyB transgenic seedlings irradiated with far-red (FR) (13.03 μmol/m2/s) or red (R) (60 μmol/m2/s) light for 15 min, respectively. The immunoprecipitates were detected using anti-myc and anti-GST antibodies. (E) Reverse transcription – quantitative polymerase chain reaction (RT-qPCR) analysis of BBX9 transcript levels in Col-0 and phyB-9 seedlings grown in white (36.1 μmol/m2/s) or red (60 μmol/m2/s) light for 5 d. Three biological replicates, each with three technical repeats, were performed. The data represent means ± SD of three biological repeats. (F) RT-qPCR analysis of BBX9 transcript levels in Col-0, BBX9pro:BBX9-GFP #2 and BBX9pro:BBX9-GFP phyb-9 #2 seedlings grown in white (36.1 μmol/m2/s) or red (60 μmol/m2/s) light for 5 d. Three biological replicates, each with three technical repeats, were performed. The data represent means ± SD of three biological repeats. Letters above the bars indicate significant differences (P < 0.05), as determined by one-way analysis of variance with Tukey's post hoc analysis. (G) Immunoblot assays showing the BBX9-GFP (green fluorescent protein) protein levels in BBX9pro:BBX9-GFP #2 and BBX9pro:BBX9-GFP phyB-9 #2 seeding grown in white (36.1 μmol/m2/s) or red (60 μmol/m2/s) light for 5 d. Col-0 was used as the negative control. Actin was used as the loading control. (H) Degradation of recombinant GST-BBX9 in the cell-free system. Protein extracts were prepared from 5-d-old Col-0 and phyB-9 seedlings and then incubated with GST-BBX9 or GST (negative control) over the indicated time course. Actin was used as a nondegraded loading control.
Phytochrome B positively regulates BBX9 protein levels
Photoexcited phyB regulates the transcription and/or protein abundance of its multiple interacting factors (Pham et al., 2018; Cheng et al., 2021). We thus compared the transcript levels of BBX9 in Col-0 and phyB-9 seedlings grown in white or red light. The transcript levels of BBX9 in Col-0 were comparable with those in phyB-9 seedlings (Figure 1E), indicating that phyB likely does not affect the expression of BBX9 in light-grown Arabidopsis. We next transformed a construct encoding BBX9 fused with green fluorescent protein (GFP) at the C-terminus driven by its own promoter (BBX9pro:BBX9-GFP) into Col-0 and generated BBX9pro:BBX9-GFP #2 transgenic plants, in which BBX9 was overexpressed and BBX9-GFP proteins were clearly detectable using immunoblot assays (Figure S1A, B). The phyB-9 mutation was introgressed into this transgenic line (BBX9pro:BBX9-GFP phyB-9 #2) through genetic crosses. The transcript levels of BBX9 in BBX9pro:BBX9-GFP phyB-9 #2 were comparable to that in BBX9pro:BBX9-GFP #2, which was significantly increased compared to those in Col-0 seedlings grown in white or red light (Figure 1F). These results were consistent with the conclusion that phyB does not regulate BBX9 at the transcriptional level. We next investigated whether phyB controls BBX9 abundance in Arabidopsis. BBX9pro:BBX9-GFP phyB-9 #2 accumulated significantly fewer BBX9-GFP proteins compared to BBX9pro:BBX9-GFP #2 when grown in constant white or red light for 4 d (Figure 1G). In addition, we performed cell-free degradation assays to validate the effects of phyB on BBX9. The recombination of GST-BBX9 proteins degraded obviously faster when incubated with extracts from phyB-9 than Col-0. However, GST remained stable when incubated with extracts from Col-0 or phyB-9 seedlings (Figure 1H). Together, these results suggest that phyB positively controls BBX9 abundance in Arabidopsis.
Red light can trigger phyB to form NBs which is essential for its molecular and biological roles in the regulation of seedling development (Chen et al., 2003, 2005). We thus tested whether BBX9 affects the formation of NBs. and created bbx9 mutants using the clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease 9 (Cas9) technique (Wang et al., 2015) (Figure S1C, D). bbx9-1 mutation was introduced into PBC (phyB-CFP) transgenic plants through genetic crosses. Consistent with previous studies (Chen et al., 2005), NBs were observed in the hypocotyl cells of PBC grown in red light. The NBs in PBC bbx9-1 seedlings were not significantly changed compared to those in PBC (Figure S2A). PBC and PBC bbx9-1 transgenic seedlings grown in red light accumulated comparable phyB-CFP protein levels as detected by immunoblot assays (Figure S2B). These results suggest that BBX9 does not affect the formation of phyB NBs and their abundance in the red light-grown seedlings. To examine the genetic links between phyB and BBX9, Col-0 (wild-type), phyB-9, PBC, bbx9-1, phyb-9 bbx9-1, and PBC bbx9-1 were grown in red light for 5 d. phyB-9 displayed drastically elongated hypocotyls, whereas PBC showed shortened hypocotyls. The hypocotyl length of bbx9-1 was significantly longer compared to Col-0 and PBC, but shorter than phyB-9. phyB-9 bbx9-1 and PBC bbx9-1 resembled phyB-9 or PBC, respectively, for hypocotyl phenotypes (Figure S2C–F), suggesting that BBX9 is involved in phyB signaling.
B-box CONTAINING PROTEIN 9 is a positive regulator of light signaling
We next searched the public database (Arabidopsis eFP Browser) and performed reverse transcription – quantitative polymerase chain reaction (RT-qPCR) assays to analyze the transcription of BBX9 in Arabidopsis seedlings. BBX9 was ubiquitously expressed in cotyledons, hypocotyls and roots (Figure S3A, B). To investigate the roles of BBX9 in light signaling, we examined the hypocotyl phenotypes and length of bbx9-1 and bbx9-2 single mutant seedlings grown under various light conditions for 5 d. The expression of BBX9 in bbx9-1 and bbx9-2 was comparable to that in Col-0 (Figure S3C).The hypocotyl length of bbx9-1 and bbx9-2 was similar to that of Col-0 when grown in darkness (Figure S4A, B). Both of these independent mutants displayed elongated hypocotyls when grown under white, red, blue, and far-red light conditions (Figure 2A–H), suggesting that BBX9 promotes photomorphogenesis in light. To validate the roles of BBX9 in light signaling, the hypocotyl phenotypes of two independent BBX9pro:BBX9-GFP transgenic lines were analyzed. The transgenic plants overexpressing BBX9 were indistinguishable from Col-0 grown in darkness (Figure S4A, B), while they were significantly shorter compared to Col-0 grown in all the light conditions tested (Figure 2A–H). These results verify that BBX9 is a positive regulator of light signaling.
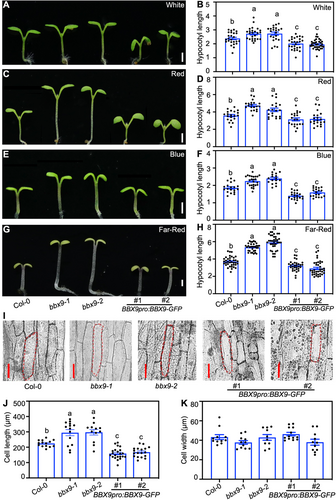
B-box CONTAINING PROTEIN 9 (BBX9) is a positive regulator of light signaling
(A–J) Hypocotyl phenotypes and length of 5-d old Col-0, bbx9-1, bbx9-2, BBX9pro:BBX9-GFP #1 and BBX9pro:BBX9-GFP #2 grown in white (36.1 μmol/m2/s) (A, B), red (102 μmol/m2/s) (C,D), blue (3.88 μmol/m2/s) (E, F), and far-red (2.05 μmol/m2/s) (G, H) light conditions. Hypocotyl length is expressed in millimeters (mm). Scale bars = 1 mm. Data are means ± SE; n ≥ 20. Letters above the bars indicate significant differences (P < 0.05), as determined by one-way analysis of variance (ANOVA) with Tukey's post hoc analysis. The experiments were performed three times with similar results. The graphs depict the results of one of three experiments. (I) Hypocotyl cell length observed by electron microscope. Scale bars = 100 µm. (J, K) Cell length (J) and cell width (K) quantification of indicated seedlings shown in (A). Data are means ± SD. n = 30. Letters above the bars indicate significant differences (P < 0.05), as determined by one-way ANOVA with Tukey's post hoc analysis.
We next measured and quantified the epidermal cell size in hypocotyls. The length of hypocotyl epidermal cells in bbx9-1 and bbx9-2 was significantly longer compared to those in Col-0, whereas the hypocotyl epidermal cells in BBX9pro:BBX9-GFP #1 and BBX9pro:BBX9-GFP #2 were shorter compared to those in Col-0 (Figure 2I, J). The width of these cells was comparable in Col-0, bbx9-1, bbx9-2, BBX9pro:BBX9-GFP #1, and BBX9pro:BBX9-GFP #2 (Figure 2K). Thus these data indicate that BBX9 inhibits cell elongation in the hypocotyls of plants under light conditions.
B-box CONTAINING PROTEIN 9 interacts with PIFs
Phytochrome-interacting factor proteins, acting directly downstream of phyB signaling, promote both cell elongation and hypocotyl growth (Cheng et al., 2021). We thus performed yeast two-hybrid assays to examine whether BBX9 interacts with PIFs. As shown in Figure 3A, BBX9 interacted with PIF1, PIF3, PIF4, and PIF5 in yeast cells. Consistently, LCI assays showed that LUC signals were produced when PIF1-LUCN and LUCC-BBX9, PIF3-LUCN and LUCC-BBX9, PIF4-LUCN and LUCC-BBX9, and PIF5-LUCN and LUCC-BBX9 were transiently co-expressed in Nicotiana benthamiana leaves. Various negative control pairs were unable to produce any detectable LUC activity in the same experimental setting (Figure 3B). Co-immunoprecipitation (Co-IP) assays were then performed to verify these results. It was revealed that BBX9-GFP proteins could pull-down PIF4-HA (hemagglutinin) or PIF5-myc proteins in Arabidopsis seedlings, as detected by immunoblot assays (Figure 3C, D). Together, the data suggest that BBX9 physically interacts with PIF proteins in plant cells.
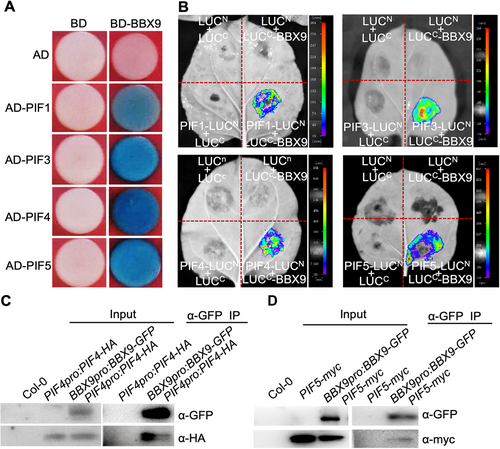
B-box CONTAINING PROTEIN 9 (BBX9) interacts with PHYTOCHROME-INTERACTING FACTORs (PIFs)
(A) Yeast two-hybrid assays showing that BBX9 interacts with PIF1, PIF3, PIF4 and PIF5 proteins. (B) Luciferase (LUC) complementation imaging (LCI) assays showing that BBX9 interacts with PIF1, PIF3, PIF4 and PIF5 in Nicotiana benthamiana leaves. Full-length PIFs and BBX9 were fused to the split N- or C-terminal (LUCN or LUCC) fragments of LUC. LUCN and LUCC were used as negative controls. (C, D) Co-immunoprecipitation (Co-IP) analysis shows that BBX9-GFP (green fluorescent protein) interacts with PIF4-HA (hemagglutinin) and PIF5-myc in Arabidopsis. Total protein was extracted from Col-0, PIF4pro:PIF4-HA, BBX9pro:BBX9-GFP PIF4pro:PIF4-HA, PIF5-myc, and BBX9pro:BBX9-GFP PIF5-myc grown in white light for 5 d. The immunoprecipitates were detected using anti-GFP, anti-HA or anti-Myc antibodies.
B-box CONTAINING PROTEIN 9 represses the transcriptional activation activity of PIF4 and PIF5 toward target genes
To explore the biological significance of BBX9-PIF interactions, we studied whether BBX9 affects the transcriptional activation activity of PIFs. Previous studies have revealed that PIF4 and/or PIF5 positively regulate the transcription of SMALL AUXIN UP RNA 24 (SAUR24), SAUR29 and INDOLE-3-ACETIC ACID 29 (IAA29) (Franklin et al., 2011; Oh et al., 2012; Sun et al., 2013; Wang et al., 2019). Either PIF4 or PIF5 could activate SAUR24pro:LUC, SAUR29pro:LUC, and IAA29pro:LUC reporters. BBX9 alone did not have any effect on these reporters. However, the activation of PIF4 or PIF5 on these reporters significantly decreased in the presence of BBX9 (Figure 4A–C). We next performed chromatin immunoprecipitation (ChIP)-qPCR experiments using PIF4pro:PIF4-HA and PIF4pro:PIF4-HA bbx9-1 seedlings, both of which accumulated comparable PIF4-HA proteins when grown in white or red light conditions (Figure S5), suggesting that BBX9 may not affect the PIF4 abundance. PIF4-HA were able to associate with the promoter regions of SAUR24, SAUR29, and IAA29, while the enrichment of PIF4-HA on these binding sites was significantly increased in Arabidopsis seedlings lacking BBX9 grown in white or red light conditions (Figure 4D, E). In agreement, the transcription of the three PIF4- and PIF5-controlled genes, SAUR24, SAUR29, and IAA29, were significantly increased in bbx9-1 and bbx9-2, but decreased in transgenic plants overexpressing BBX9 when grown in white or red light conditions (Figure 4F, G). These results suggest that BBX9 negatively regulates the DNA binding affinity of PIF4 and PIF5 and their transcriptional activation activity toward target genes.
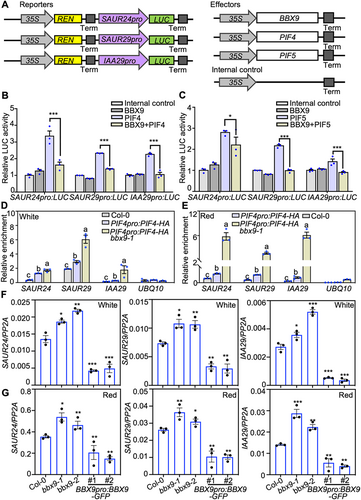
B-box CONTAINING PROTEIN 9 (BBX9) represses the DNA binding affinity and transcriptional activation activity of PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) and PIF5
(A) Schematic representation of various constructs used in the transient transfection assay in Nicotiana benthamiana leaves. (B, C) Transient dual-luciferase (dual-LUC) assay showing that BBX9 represses the transcriptional activation activity of PIF4 and PIF5 on SAUR24pro:LUC, SAUR29pro:LUC and IAA29pro:LUC reporters. Error bars represent SD (n = 3). Asterisks indicate significant differences (*P < 0.05, ***P < 0.001), as determined by two-tailed Student's t-test. (D, E) Chromatin immunoprecipitation – quantitative polymerase chain reaction (ChIP-qPCR) assays show that BBX9 inhibits the PIF4 binding to the SAUR24, SAUR29 and IAA29 promoter regions in Arabidopsis grown in white (D) or red (E) light conditions. ChIP-qPCR assays were performed using 5-d-old Col-0, PIF4pro:PIF4-HA, and PIF4pro:PIF4-HA bbx9-1 seedlings with anti-HA (hemagglutinin) antibodies. Plants were grown in white (36.1 μmol/m2/s) (D) or red (60 μmol/m2/s) (E) light for 5 d. The data represent the means ± SD of three biological repeats. The letters above the bars indicate significant differences (P < 0.05), as determined by one-way analysis of variance (ANOVA) with Tukey's post hoc analysis. (F, G) Reverse transcription (RT)-qPCR analysis showing that BBX9 negatively regulates the transcription of SAUR24, SAUR29 and IAA29. Col-0, bbx9-1, bbx9-2, BBX9pro:BBX9-GFP #1 and BBX9pro:BBX9-GFP #2 grown in white (36.1 μmol/m2/s) (F) or red (60 μmol/m2/s) light (G) for 5 d, then harvested for total RNA extraction. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001), as determined by two-tailed Student's t-test.
Phytochrome-interacting factors inhibit the transcription of BBX9
Phytochrome-interacting factors are a subfamily of bHLH-type transcription factors that regulate the expression of numerous genes (Leivar et al., 2009; Shin et al., 2009). We examined whether PIFs modulate the transcription of BBX9. The transcript levels of BBX9 were significantly elevated in pif1-2, pif3-1, pif4-2, pif5-3, and pifq mutant seedlings, but markedly reduced in PIF4-myc and PIF5-myc transgenic plants grown in white light or darkness (Figures 5A, B, S6A, B), suggesting that PIF transcription factors inhibit BBX9 at the transcriptional level. Consistently, PIF1, PIF3, PIF4, and PIF5 inhibited the activity of the BBX9pro:LUC reporter when transiently co-expressed in Nicotiana benthamiana leaves (Figure 5C, D). In addition, AD-PIF4 or AD-PIF5 activated BBX9pro:LacZ in yeast cells (Figure 5E), implying that PIF4 and PIF5 could bind to the promoter regions of BBX9. The BBX9 promoter possesses one G-box, one ACE, and one T/G-box (Figure S7), which are potential binding sites for PIFs (Zhang et al., 2013; Pfeiffer et al., 2014; Kim et al., 2016). The activation of PIF4 and PIF5 on these reporters was completely absent in yeast cells when each of the DNA-cis-elements was mutated (Figures 5E, S7), suggesting that intact G-box, ACE, and T/G-box within the BBX9 promoter were required and necessary for PIF4 and PIF5 binding. ChIP-qPCR experiments were performed to validate these results. As shown in Figure 5F–H, PIF4 and PIF5 associated with BBX9 promoter regions in vivo. Together, these results suggest that PIFs can directly bind to the BBX9 promoter and inhibit its transcription.
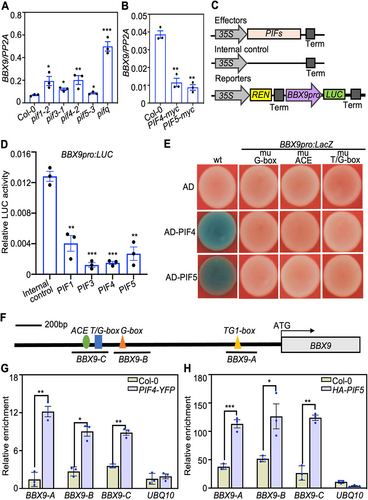
PHYTOCHROME-INTERACTING FACTORs (PIFs) inhibit the transcription of B-box CONTAINING PROTEIN 9 (BBX9)
(A, B) Reverse transcription – polymerase chain reaction (RT-qPCR) analysis of BBX9 transcript levels in Col-0, pif1-2, pif3-1, pif4-2, pif5-3, pifq, PIF5-myc and PIF4-myc seedlings grown in white light (36.1 μmol/m2/s) for 5 d. Three biological replicates, each with three technical repeats, were performed. The data represent the means ± SD of three biological repeats. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001), as determined by two-tailed Student's t-test. (C) Schematic representation of various constructs used in the transient transfection assay in Nicotiana benthamiana leaves. (D) Transient transcriptional activation assays showing that PIF1, PIF3, PIF4 and PIF5 inhibit the activation of the BBX9pro:LUC. Error bars represent SD of three independent transient transfections in Nicotiana benthamiana leaves. Asterisks represent statistically significant differences (**P < 0.01,***P < 0.001), as determined by Student's t-test. (E) Yeast one-hybrid assays showing that PIF4 and PIF5 activate the BBX9pro:LacZ reporter. (F) Schematic representation of the BBX9 promoter with the location of the G-box, ACE and T/G-box motifs. (G, H) Chromatin immunoprecipitation (ChIP)-qPCR assays showing that PIF4 and PIF5 associate with the BBX9 promoters in vivo. The 7-d-old Col-0, PIF4-YFP or HA-PIF5 seedlings were used for ChIP assays. Chromatin fragments were immunoprecipitated using green fluorescent protein (GFP)-Trap or anti-HA (hemagglutinin) antibodies. The UBQ10 gene was used as the negative control. Error bars represent SD (n = 3). Asterisks indicate significant differences (*P < 0.05, **P < 0.01,***P < 0.001), as determined by two-tailed Student's t-test.
B-box CONTAINING PROTEIN 9 interacts with BBX21 and enhances its transcriptional activation activity
To further elucidate the molecular roles of BBX9 in promoting light signal transduction, we carried out a yeast two-hybrid screen using BD-BBX9 as bait. BBX21, a positive regulator of light signaling, was recovered as a BBX9-interacting factor (Figure 6A). Next, we employed LCI assays to verify these results. LUC signals were observed when BBX21-LUCN and LUCC-BBX9 were transiently co-expressed in Nicotiana benthamiana leaves, but not the indicated pairs serving as controls (Figure 6B). Co-IP assays showed that BBX9-GFP was able to co-immunoprecipitate with BBX21-myc in Arabidopsis (Figure 6C). These results suggest that BBX9 interacts with BBX21 in plants.
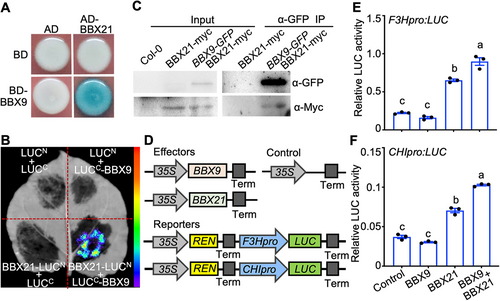
B-box CONTAINING PROTEIN 9 (BBX9) interacts with BBX21 and enhances its biochemical activity
(A) Yeast two-hybrid showing that BBX9 interacts with BBX21. (B) Luciferase (LUC) complementation imaging (LCI) assays showing that BBX9 interacts with BBX21 in Nicotiana benthamiana leaves. Full-length BBX21 and BBX9 were fused to the split N- or C-terminal (LUCN or LUCC) fragments of LUC. LUCN and LUCC were used as negative controls. (C) Co-immunoprecipitation (Co-IP) analysis showing that BBX21-myc interacts with BBX9-Flag. Total protein was extracted from wild tobacco leaves transiently expressing 35S:BBX21-myc alone or together with 35S:BBX9-Flag. The immunoprecipitates were detected using anti-myc and anti-Flag antibodies. (D) Schematic representation of various constructs used in the transient transfection assay in Nicotiana benthamiana leaves. (E, F) Transient transfection assays showing BBX9 enhances the activation of BBX21 on F3Hpro:LUC and CHIpro:LUC reporters in Nicotiana benthamiana leaves. Error bars represent SD (n = 3). The letters above the bars indicate significant differences (P < 0.05), as determined by one-way analysis of variance (ANOVA) with Tukey's post hoc analysis.
Next, we examined whether BBX9 affects the transcriptional activation activity of BBX21. BBX21 activated F3Hpro:LUC and CHIpro:LUC reporters, which was consistent with a previous study (Datta et al., 2007), whereas BBX9 did not affect these reporters. The activation of these reporters significantly increased when BBX9 and BBX21 were present in the same experimental setting (Figure 6D–F), suggesting that BBX9 enhances the biochemical activity of BBX21, likely through the formation of BBX9-BBX21 heterodimers.
B-box CONTAINING PROTEIN 21 positively regulates BBX9 expression
B-box CONTAINING PROTEIN 21 controls the transcription of a number of genes (Crocco et al., 2010; Gómez-Ocampo et al., 2021). To this end, we sought to examine whether BBX21 regulates the expression of BBX9. The transcript level of BBX9 significantly decreased in bbx21-1, but increased in YFP-BBX21 bbx21-1 #1 and BBX21-myc bbx21-1 #1 transgenic plants (Figure 7A), indicating that BBX21 up-regulates BBX9 at the transcriptional level. Consistently, BBX21 was able to activate the BBX9pro:LUC reporter when transiently co-expressed in Nicotiana benthamiana leaves (Figure 7B). Yeast one-hybrid assays showed that BBX21 activated the BBX9pro:LacZ reporter, but could not do so when each of the three cis-elements (G-box, ACE, and T/G-box) was mutated (Figures 7C, S7). These data indicate that these intact cis-elements were required for BBX21 binding. Further, we carried out ChIP-qPCR assays and found that BBX21 associated with the BBX9 promoter regions in vivo (Figure 7D, E). Consistently, the transcription of three BBX9-controlled genes (SAUR24, SAUR29, and IAA29) were elevated in bbx21-1, but decreased in BBX21-myc bbx21-1 #1 seedlings (Figure 7F). Together, these findings suggest that BBX21 associates with the promoter of BBX9 to up-regulate its transcription in plants.
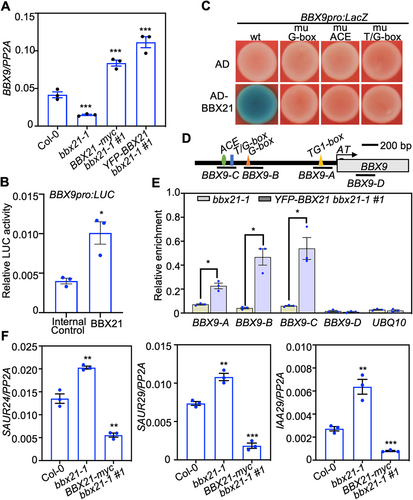
B-box CONTAINING PROTEIN 21 (BBX21) positively regulates the expression of BBX9
(A) Reverse transcription – polymerase chain reaction (RT-qPCR) analysis showing BBX21 up-regulates the expression of BBX9. Col-0, bbx21-1, BBX21-myc bbx21-1 #1 and YFP-BBX21 bbx21-1 #1 grown in white light (36.1 μmol/m2/s) for 5 d, then harvested for RNA extraction. (B) Transient transfection assays showing that BBX21 activates the BBX9pro:LUC. Error bars represent SD of three independent transient transfections in Nicotiana benthamiana leaves. The asterisk represents statistically significant differences (*P < 0.05), as determined by Student's t-test. (C) Yeast one-hybrid assays showing that BBX21 activates the BBX9pro:LacZ. (D) Schematic representation of the BBX9 promoter with the location of the G-box, ACE and T/G-box motifs. (E) Chromatin immunoprecipitation (ChIP)-qPCR assays showing that BBX21 associates with the BBX9 promoters in vivo. The 7-d-old bbx21-1 and YFP-BBX21 bbx21-1 #1 seedlings were used for ChIP assays. Chromatin fragments were immunoprecipitated using green fluorescent protein (GFP)-Trap. The UBQ10 gene was used as the negative control. Error bars represent SD (n = 3). Asterisks indicate significant differences (*P < 0.05), as determined by two-tailed Student's t-test. (F) RT-qPCR analysis showing that BBX21 negatively regulates the transcription of SAUR24, SAUR29 and IAA29. Col-0, bbx21-1, and myc-BBX21 bbx21-1 #1 grown in white light (36.1 μmol/m2/s) for 5 d, then harvested for total RNA extraction. Asterisks indicate significant differences (**P < 0.01, ***P < 0.001), as determined by two-tailed Student's t-test.
Genetic interactions between BBX9, PIF4, PIF5, and BBX21
To investigate the genetic links between BBX9, PIF4, PIF5, and BBX21, we generated various double or triple mutants. Consistent with previous studies (Huq and Quail, 2002; Leivar et al., 2012), pif4-2 and pif4-2 pif5-3 showed shortened hypocotyls, while bbx9-1 mutants displayed elongated hypocotyls in white and red light (Figure 2). bbx9-1 pif4-2 and bbx9-1 pif4-2 pif5-3 were slightly longer compared to pif4-2 or pif4-2 pif5-3, respectively, but significantly shorter compared to Col-0 and bbx9-1 grown in white light (Figure 8A, B). bbx9-1 pif4-2 displayed intermediated hypocotyl phenotypes, while bbx9-1 pif4-2 pif5-3 were indistinguishable from pif4-2 pif5-3 in red light (Figure 8C, D). These results suggest that BBX9-mediated hypocotyl growth is likely partially dependent on PIF4 and PIF5 in white light, but dependent on PIF4 and PIF5 in red light. bbx21-1 developed significantly elongated hypocotyls in white and red light (Datta et al., 2007). The hypocotyl length of bbx9-1 bbx21-1 was similar to that of bbx9-1 in white light, and was intermediate between that of bbx9-1 and bbx21-1 (Figure 8E–H). These results suggested that BBX9 likely acts downstream of BBX21 with respect to hypocotyl phenotypes in white light, and may be partially dependent on BBX21 in red light. BBX21 promotes anthocyanin accumulation in the light (Datta et al., 2007). The content of anthocyanin in bbx9-1 was less than that in Col-0, but increased compared to that in bbx21-1. The anthocyanin levels in bbx9-1 bbx21-1 were similar with that in bbx21-1, suggesting that the anthocyanin phenotype of bbx9-1 is dependent on BBX21 (Figure S8). bbx21-1 pif4-2 were similar to pif4-2 in white light, but slightly longer than pif4-2 in red light. bbx21-1 bbx9-1 pif4-2 seedlings exhibited significantly longer hypocotyls than bbx9-1 pif4-2 and bbx21-1 pif4-2 in white and red light. These triple mutants were slightly shorter than bbx21-1 in white light, while they were comparable to bbx21-1 in red light (Figure 8E–H). These genetic results suggest that BBX9 and BBX21 function both independently and dependently of PIF4 in the control of light-inhibited hypocotyl growth.
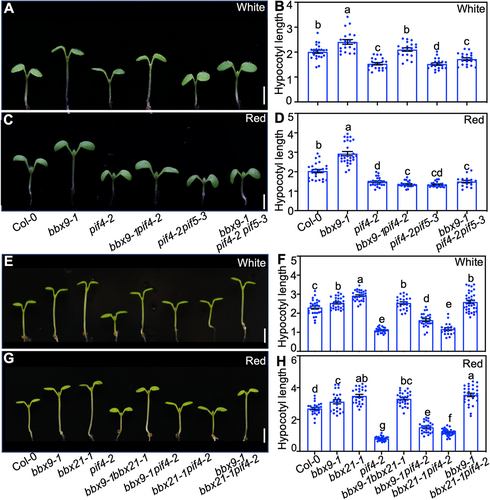
Genetic interactions between B-box CONTAINING PROTEIN 9 (BBX9), PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) and BBX21
(A–D) Hypocotyl phenotypes and length of Col-0, bbx9-1, pif4-2, bbx9-1 pif4-2, pi4-2 pif5-3, bbx9-1 pif4-2 pif5-3 mutant seedlings grown in continuous white (36.1 μmol/m2/s) (A, B) and red (223 μmol/m2/s) (C, D) light for 5 d. Scale bars = 1 mm. (E–H) Hypocotyl phenotypes and length of Col-0, bbx21-1, pif4-2, bbx9-1 bbx21-1, bbx21-1 pif4-2, bbx9-1 pi4-2, bbx9-1 bbx21-1 pif4-2 mutant seedlings grown in continuous white (36.1 μmol/m2/s) (E, F) and red (223 μmol/m2/s) (G, H) light for 5 d. Scale bars = 1 mm. In panel (B, D, F and H), letters above the bars indicate significant differences (P < 0.05), as determined by one-way analysis of variance (ANOVA) with Tukey's post hoc analysis.
Global analysis of genes regulated by BBX9
To identify the genes regulated by BBX9 at the genome-wide scale, we performed a paired-end RNA sequencing (RNA-seq) assay and compared the gene expression profile between the Col-0 and bbx9-1 seedlings. The expression of approximately 387 genes was significantly changed in bbx9-1 compared with that in Col-0 (Figure S9A; Tables S1, S2). Of these genes, 156 were down-regulated in bbx9-1 (Table S1), and 231 were up-regulated in bbx9-1 compared to Col-0 (Table S2). The expression of SAUR24, and SAUR29 was significantly reduced in bbx9-1 (Table S1), which was consistent with our real-time qPCR results (Figure 4F, G). Gene Ontology enrichment analysis revealed that BBX9-regulated genes are involved in the response to multiple stimuli, including various hormones (Figure S9B).
B-box CONTAINING PROTEIN 9 inhibits hypocotyl elongation dependent on auxin
Considering that BBX9 negatively controls SAUR24, SAUR29, and IAA29 involved in auxin biosynthesis and signaling, we investigated whether BBX9 inhibits hypocotyl growth through auxin. The hypocotyl length in Col-0, bbx9-1, bbx9-2, BBX9pro:BBX9-GFP #1 and BBX9pro:BBX9-GFP #2 seedlings was elongated, and each of two independent bbx9 mutants and two independent BBX9 overexpressors were comparable to that of Col-0 seedlings in the presence of 0.1 μmol/L synthetic auxin, picloram (PIC, 4-amino-3, 5, 6-trichloropicolinic acid) (Figure S10A, B). In addition, the hypocotyl elongation of Col-0, bbx9 mutant and BBX9 transgenic seedlings was significantly inhibited by the application of 0.1 μmol/L naphthylphthalamic acid (NPA), an inhibitor of auxin transport. These mutants and transgenic seedlings were indistinguishable from Col-0 (Figure S10B, C). These results suggested that BBX9 inhibits hypocotyl elongation, likely in an auxin-dependent manner.
DISCUSSION
Light sensed by photoreceptors promptly triggers transcriptional reprogramming to initiate photomorphogenic development in plants (Pham et al., 2018; Paik and Huq, 2019). Here, we report that red light photoreceptor phyB interacts with and stabilizes BBX9 in light. BBX9 forms a negative feedback loop with PIFs and a positive feedback regulation with BBX21, ultimately suppressing the action of PIFs but enhancing the same in BBX21. These molecular events consequently lead to the promotion of photomorphogenesis.
Photoexcited phyB is promptly converted to Pfr isoform and shifts into the nucleus, where it forms NBs with its interacting factors, including PIFs. phyB promotes the degradation of PIFs upon red light irradiation (Pham et al., 2018; Cheng et al., 2021). Considering that BBX9 interacted with both phyB and PIFs and that phyB stabilized BBX9 (Figures 1, 3), we cannot rule out the possibility that BBX9 may affect the phyB-imposed degradation of PIFs in the nucleus of plant cells. BBX9 inhibited the DNA binding affinity of PIF4 and PIF5 toward target sites and their transcriptional activation activity (Figure 4), suggesting that the association between BBX9 and PIFs serves as an essential inhibitory molecular mechanism for the repression of PIFs' action and that BBX9 is a repressor of PIFs. In turn, PIFs negatively controlled BBX9 at the transcriptional level. PIF4 and PIF5 associated with BBX9 promoter regions and the PIF4- and PIF5-activated genes (SAUR24, SAUR29, and IAA29) were negatively controlled by BBX9 (Figures 4, 5). These findings suggest that BBX9 and PIFs form a negative feedback loop that likely fine-tunes the action of PIFs, and in turn, orchestrates the PIFs-mediated transcriptional cascade during light-mediated seedling development. PIFs are a subgroup of key regulators that promote hypocotyl growth. Among these, PIF4 and PIF5 play central roles in the shade- and warm temperature-induced hypocotyl elongation predominantly by promoting auxin biosynthesis and signaling (Sun et al., 2012, 2013; Cheng et al., 2021). BBX9 inhibited hypocotyl elongation through an auxin-mediated pathway (Figure S10). It is of interest to investigate whether BBX9 functions in response to shade or elevated temperature cues.
BBX21 is a positive regulator of light signaling (Datta et al., 2007; Xu et al., 2016). BBX9 formed heterodimers with BBX21 and enhanced its transcriptional activation activity toward target genes (Figure 6). On the other hand, BBX21 positively regulated the transcription of BBX9 (Figure 7A–C). As BBX21 accumulates in light (Xu et al., 2016), it is speculated that the BBX21-promoted BBX9 expression might at least in part contribute to the phyB-mediated stabilization of BBX9 during light irradiation in plants. BBX21 associated with the promoters of BBX9 and activated its expression (Figure 7), indicating that BBX21 is an activator of BBX9. BBX21 promotes photomorphogenesis by directly activating HY5 at the transcriptional level and enhancing HY5 biochemical activity at the protein level (Datta et al., 2007; Xu et al., 2016; Bursch et al., 2020). BBX21 repressed the BBX9-controlled genes involved in auxin signaling (Figure 7F), thus implying that BBX9 and BBX21 work jointly to inhibit hypocotyl growth at least partially in an auxin-dependent manner.
BBX9 possesses two B-box domains in the N-terminus, and a CCT motif in the C-terminal region (Khanna et al., 2009). Both B-box and CCT domains have been shown to be able to bind to specific DNA cis-element(s) to activate target genes' expression (Tiwari et al., 2010; Xu et al., 2018). BBX9 directly or indirectly controlled the expression of approximately 387 genes, as revealed by RNA-seq analysis (Figure S9; Tables S1, S2). These findings suggest that BBX9 might directly associate with the promoter regions of its target genes to regulate their transcription.
Phytochrome-interacting factors and BBX21 play central roles in the regulation of photomorphogenesis, where PIFs repress photomorphogenic development and BBX21 positively regulates light signaling (Datta et al., 2007; Leivar et al., 2009). However, the exact molecular and genetic links between PIFs and BBX21 in the regulation of photomorphogenesis require further characterization. BBX9 was observed to act as a critical component of PIFs- and BBX21-mediated seedling development. BBX9 was controlled by PIFs and BBX21 at the transcriptional level, while BBX9 modulated the biochemical activity of PIFs and BBX21 at the protein level (Figures 4–7). BBX9 likely links PIFs and BBX21 signaling as a part of a fine-tuning regulatory mechanism to regulate photomorphogenesis. Either PIF4/5 or BBX21 directly bound to the BBX9 promoter regions containing an ACE, a T/G-box and a G-box, and these three intact DNA cis-elements were essential and necessary for PIF4/5 and BBX21 binding (Figures 5E, 7C). These specific DNA cis-elements locating in a 161 bp region (−665 to −504) may form a specific conformational structure that is required for PIF4/5 and BBX21 binding. PIF4/5 and BBX21 antagonistically regulated the expression of BBX9 (Figures 5A, B, 7A). These facts raise a hypothesis that PIF4/5 and BBX21 might compete with each other to bind to the BBX9 promoter to modulate its expression. Thus detailed investigations are required to elucidate the exact molecular relationship between PIF4/5 and BBX21 in the control of BBX9 expression during seedling development.
Collectively, BBX9 promotes photomorphogenesis via PIFs- and BBX21-mediated signaling. BBX9 interacts with PIFs and represses their DNA binding affinity and transcriptional activation activity toward target genes. PIFs associate with the promoter regions of BBX9 and inhibit its transcription. Meanwhile, BBX9 interacts with BBX21, a photomorphogenesis-promoting factor, and enhances its biochemical action. BBX21 binds to the BBX9 promoters and up-regulates its transcript levels (Figure 9). Thus, BBX9-PIFs and BBX9-BBX21 feedback loops may enable plants to respond quickly and appropriately to their surrounding changing light environments.
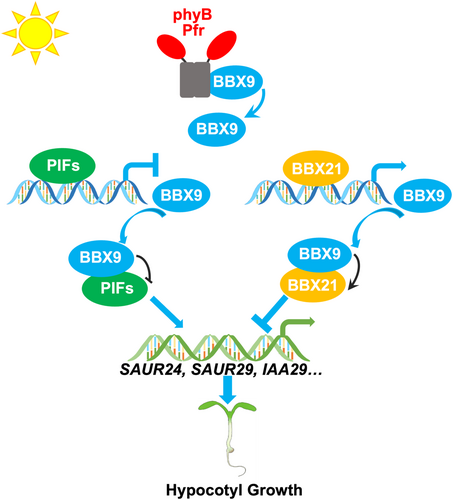
A proposed working model depicting that B-box CONTAINING PROTEIN 9 (BBX9) works coordinately and synergistically with PHYTOCHROME-INTERACTING FACTORs (PIFs) and BBX21 to promote photomorphognesis
Upon light exposure, the biologically active Pfr form of phytochrome b (phyB) associates with PIFs and BBX9 to promote the degradation of PIFs, but stabilize BBX9. BBX9 interacts with remaining PIFs and inhibits their DNA binding affinity and transcriptional activation activity, while PIFs repress the transcription of BBX9, thereby forming a negative feedback loop. On the other hand, BBX9 associates with BBX21 and enhance its biochemical action. BBX21 directly activates the expression of BBX9. Thus the BBX9-PIFs and BBX9-BBX21 regulatory loop consequently promotes photomorphognenesis.
METHODS
Plant materials and growth conditions
The phyB-9 (Neff and Chory, 1998), pif1-2 (Oh et al., 2006), pif3-1 (Ni et al., 2013), pif4-2 (Leivar et al., 2008), pif5-3 (Khanna et al., 2007), pif4-2 pif5-3 (Leivar et al., 2012), pifq (Leivar et al., 2008), bbx21-1 (Datta et al., 2007), bbx9-1 and bbx9-2 (this study) mutants, phyB-CFP (Chen et al., 2005), phyB-myc (Xu et al., 2019) PIF4pro:PIF4-HA pif4-101 (Zhang et al., 2017b), PIF4-YFP (Oh et al., 2012), PIF5-myc (Sakuraba et al., 2014), HA-PIF5 (Shen et al., 2007), BBX21-myc bbx21-1 #1 (Xu et al., 2016), BBX9pro:BBX9 #1 and BBX9pro:BBX9 #2 (this study) transgenic lines are in the Columbia-0 (Col-0) ecotype. Double-mutant/transgenic plants were generated by genetic crossing, and homozygous lines were verified by PCR genotyping or antibiotic screen. Seeds were surface-sterilized with 20% NaClO and sown on 1× Murashige and Skoog (MS) medium containing 1% sucrose and 0.8% agar. After stratification in darkness at 4°C for 3 d, seeds were transferred to light chambers (PERCIVAL) maintained at 22°C.
Generation of bbx9 mutant using CRISPR/Cas9 technique
Gene editing using the CRISPR/Cas9 technique was performed as previously described (Wang et al., 2015). The 23-bp target sites (5′-N20NGG-3′) within exons of genomic DNA sequences of BBX9 were manually searched and identified, and then each of these sites was evaluated by target specificities on the website of potential off-target finder (http://www.rgenome.net/cas-offinder/). Two independent sgRNA target sites of BBX9 were sub-cloned into the pHEE401E vector. These vectors were transformed into Agrobacterium strain GV3101 by the freeze-thaw method respectively, and then introduced into Col-0 plants via the floral dip method (Clough and Bent, 1998). T1 transgenic plants were selected on MS medium containing 25 mg/L hygromycin. The specific mutations in BBX9 were analyzed using gene-specific primers by PCR amplification and sequencing. The pHEE401 T-DNA insertion region including CRISPR/Cas9 in bbx9-1 and bbx9-2 single mutant at T1 generation were removed by genetic crossing with Col-0.
Construction of plasmids
To generate BBX9pro:BBX9-GFP construct, the BBX9 promoter and coding sequence, were ligated into the pCAMBIA1300 vector at the SalI/BamHI. For yeast two-hybrid assays, we amplified the full-length BBX9, PIF1, PIF3, PIF4 and PIF5 coding sequence (CDS) with respective pairs of primers and then cloned the PCR products at the EcoRI/BamHI sites of the pB42AD vector to generate the prey constructs pB42AD-BBX9, pB42AD-PIF1, pB42AD-PIF3, pB42AD-PIF4 and pB42AD-PIF5. The full-length BBX9 CDS was cloned at the EcoRI/BamHI sites of the pLexA vector to generate bait constructs of pLexA-BBX9. pB42AD-BBX21 (Xu et al., 2016), pLexA-phyB-C, pLexA-phyB-C1 (Song et al., 2021) were previously described. For yeast one-hybrid assays, 2,000 bp BBX9 promoter upstream of ATG were amplified and cloned into the KpnI/XhoI sites of the pLacZ-2u vector (Lin et al., 2007). For LCI assay, the coding sequences of PIF1, PIF3, PIF4, PIF5 and BBX21 were cloned into the KpnI/SalI sites of pCAMBIA1300-nLUC vector. The coding sequence of BBX9 was cloned into the KpnI/SalI sites of pCAMBIA1300-cLUC vector to generate the corresponding constructs. For the transient transfection assays, the promoters of SAUR24, SAUR29, IAA29, HY5, F3H1, CHI and BBX9 were amplified and cloned into the KpnI/NcoI sites of pGreen0800 Ⅱ-LUC vector to generate pGreen0800Ⅱ-proSAUR24:LUC, pGreen0800Ⅱ-proSAUR29:LUC, pGreen0800Ⅱ-proIAA29:LUC, pGreen0800-proHY5:LUC constructs, pGreen0800 Ⅱ-proF3H1:LUC and pGreen0800Ⅱ-proBBX9:LUC. The coding sequence of BBX9 was cloned into the XbaI/KpnI sites of pCAMBIA1307-3×Flag. The coding sequences of PIF4 and PIF5 were cloned into the XbaI/KpnI sites of pCAMBIA1305-4×myc vector. pEarleyGate 203-BBX21-myc was previously described (Xu et al., 2016). Primers used for plasmid constructions are listed in Table S3.
Transgenic plants
The pCAMBIA1300-BBX9pro:BBX9-GFP construct was transformed into Agrobacterium tumefaciens GV3101 by the freeze-thaw method, and then introduced into Col-0 plants via the floral dip method (Clough and Bent, 1998). Transgenic plants were selected on MS medium containing 20 mg/L hygromycin.
Measurement of hypocotyl length
To measure the hypocotyl length of seedlings, seeds were sown on plates and stratified at 4°C in darkness for 3 d, and then kept in continuous white light for 8 h in order to induce uniform germination. The seeds were then transferred to dark, white, blue, red, and far-red light conditions, and incubated at 22°C for 5 d (Xu et al., 2016). The hypocotyl length of seedlings was measured using ImageJ software.
Firefly LCI assays
The firefly LCI transient expression assay was performed as previously described (Chen et al., 2008). Constructs of pCAMBIA1300-PIF1-LUCN, pCAMBIA1300-PIF3-LUCN, pCAMBIA1300-PIF4-LUCN, pCAMBIA1300-PIF5-LUCN, pCAMBIA1300-BBX21-LUCN and pCAMBIA1300-LUCC-BBX9 were generated and then transformed into Agrobacterium strain GV3101 respectively. The Agrobacterium strain GV3101 containing the indicated transformant pairs was infiltrated into tobacco leaves. The corresponding empty vector pCAMBIA1300-LUCN or pCAMBIA1300-LUCC was infiltrated as a negative control. After a 24 h incubation in darkness at 22°C and an additional 24–36 h incubation under a 16-h light/8-h dark photoperiod, the leaves were harvested, and 0.33 mmol/L D-luciferin (Yesaen, China) solution was sprayed onto the tobacco leaves. LUC activity was measured with the LB 985 NightSHADE Spectrum imaging system (Berthold, Germany).
Co-immunoprecipitation assays
Co-immunoprecipitation assays were performed as previously described (Lin et al., 2018). Agrobacterium strain GV3101 cells carrying the 35S:PIF4-4×myc, 35S:PIF5-4×myc, 35S:BBX9-3×Flag or 35S:BBX21-myc constructs were transiently infiltrated into Nicotiana benthamiana leaves. Two grams of plant tissues were collected after 24–48 h infiltration and then lysed to extract total proteins. Extracts were incubated with 4 μL of anti-myc antibodies (1:250 v/v) (Sigma-Aldrich, USA) coupled with 20 μL of Protein-A Sepharose (GE Healthcare, USA) for 3 h at 4°C. The Sepharose was washed three times with protein extraction buffer. After centrifugation (500 g for 5 min at 4°C) and washing three times with protein extraction buffer, the precipitates were mixed with 5× sodium dodecyl sulfate (SDS) protein loading buffer and then boiled for 10 min before SDS-PAGE (polyacrylamide gel electrophoresis) and immunoblot analysis using anti-Flag (Medical & Biological Laboratories, Japan) antibodies.
Semi-in vivo pull-down assays with Arabidopsis protein extracts
Semi-in vivo pull-down assays were performed as previously described with minor modification (Yan et al., 2020). Proteins were extracted from 5-d-old dark-grown phyB-myc seedlings with an extraction buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 10 mmol/L MgCl2, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.2% NP-40, 10% glycerol, 1 mmol/L phenylmethylsulfoxide (PMSF), 80 μmol/L MG132, and 1× EDTA-free protease inhibitor cocktail (Roche, Swiss). Protein extracts were irradiated with far-red (13.03 μmol/m2/s) or red (60 μmol/m2/s) light for 15 min respectively. After that, 0.38 pmol GST or 0.16 pmol GST-BBX9, and GST beads were incubated with equivalent amounts of phyB-myc protein extract treated with far-red or red light in 1 mL of 1× phosphate-buffered saline (PBS) buffer (pH 7.5) containing 137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4. The reaction mixtures were incubated at 4°C for 4 h, then the beads were washed six times with 1 mL of 1× PBS buffer containing 0.1% NP-40 and 10% glycerol. The pulled down proteins were eluted in 5× SDS loading buffer at 100°C for 10 min, separated on 10% SDS-PAGE gels, and detected by immunoblotting using anti-myc (Sigma-Aldrich) and anti-GST (Abmart, China) antibodies.
Immunoblot analysis
For immunoblots, Arabidopsis Col-0 and various transgenic seedlings were homogenized in a protein extraction buffer containing 100 mmol/L NaH2PO4, 10 mmol/L Tris·HCl, 200 mmol/L NaCl, 8 mol/L urea pH 8.0, 1 mmol/L PMSF and 1× complete protease inhibitor cocktail (Roche). Primary antibodies used in this study were anti-GFP (Abmart) and anti-Actin (Sigma-Aldrich).
Yeast one-hybrid assays
For the yeast one-hybrid assay, the respective combinations of AD-fusion effectors and LacZ reporters were co-transformed into yeast strain EGY48 and transformants were selected and grown on synthetic defined (SD)/-Trp-Ura dropout media. Yeast transformation and liquid assay were conducted as described in the Yeast Protocols Handbook (BD Clontech, USA).
Yeast two-hybrid assays
Yeast two-hybrid assays were performed using the Matchmaker LexA Two-Hybrid System as described in the Yeast Protocols Handbook (BD Clontech). The respective combinations of pLexA and pB42AD fusion plasmids were co-transformed into yeast strain EGY48 containing p8op-LacZ plasmid. The empty pLexA and pB42AD vectors were co-transformed in parallel as negative controls. Transformants were selected and grown on SD/-His-Trp-Ura dropout plates at 30°C. The transformants were grown on SD/-His-Trp-Ura dropout plates containing 80 mg/L X-gal for blue color development.
Dual-luciferase reporter system
Nicotiana benthamiana plants grown in long day conditions (16 h light/8 h dark) were used for transient transactivation assay. Agrobacterium strain GV3101 cells carrying the 35S: BBX9-Flag, 35S:PIF4-myc, 35S:PIF5-myc, 35S:BBX21-myc or SAUR24pro:LUC, SAUR29pro:LUC, IAA29pro:LUC, F3H1pro:LUC and CHIpro:LUC constructs were transiently infiltrated into Nicotiana benthamiana leaves at indicated combinations. Firefly LUC and Renilla LUC (Ren) were detected using the Dual-LUC Reporter Assay System (Vazyme, China), according to the manufacturer's instructions. Ren driven by a cauliflower mosaic virus 35S promoter was used as an internal control. The relative activity was expressed as the LUC/Ren ratios.
Chromatin immunoprecipitation
The ChIP assays were performed as described by Xu et al. (2016). Chromatin isolation was performed using Col-0, PIF4-YFP and HA-PIF5 transgenic seedlings grown under constant white light for 7 d. The resuspended chromatin was sonicated at 4°C to 250- to 500-bp fragments. The sheared chromatin was immunoprecipitated, washed, reverse cross-linked, and finally amplified. Approximately 2% of sonicated but non-immunoprecipitated chromatin was reverse cross-linked and used as an input DNA control. Both immunoprecipitated DNA and input DNA were analyzed by real-time PCR (Roche LightCycler 480 II). Monoclonal anti-GFP antibody (Abmart) and anti-HA (MBL) were used for the assays. All primers used for this assay are listed in Table S3.
RNA sequencing
The 5-d-old Col-0 and bbx9-1 mutant seedlings grown in continuous white light (36.1 μmol/m2/s) were harvested for total RNA extraction. RNA-seq libraries were constructed using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) and sequenced on the Illumina NovaSeq. 6000 platform at Personalbio company. The RNA-seq data reads were aligned to The Arabidopsis Information Resource 10 genome. All RNA-seq data analyses were carried out with TBtools (Chen et al., 2020). Genes with log2 fold changes (log2FC) > 0.58 or <−0.58, and the P-value <0.05, are considered as differentially expressed genes.
Quantitative real-time PCR
Total RNA was extracted from 5-d-old Arabidopsis seedlings grown under white light using the FastPure® Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, RC112). Complementary DNAs (cDNAs) were synthesized from 1 μg of total RNA using the HiScript® III first Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme) according to the manufacturer's instructions. Then, cDNA were subjected to real-time qPCR assays. Quantitative real-time qPCR was performed using the Light Cycler 480 II detection system (Roche) and ChamQ Universal SYBR qPCR Master Mix (Vazyme). PCR was performed in triplicate for each sample, and the expression levels were normalized to that of a PP2A gene. The primers used in this study are listed in Table S3.
Cell morphology observation
For the morphological observation on epidermal cells of Arabidopsis hypocotyl, the seedlings were grown in continuous white light at 22°C for 5 d, and then epidermal cells were observed using an electron microscope. For the phyB NBs observation, the seedlings were grown in continuous red light at 22°C for 5 d. The cyan fluorescent protein (CFP) fluorescence signals were observed and imaged using a Carl Zeiss LSM510 Meta confocal laser scanning microscope. CFP fluorescence was excited by a 405-nm laser and detected between 360 and 460 nm.
Auxin sensitivity assay
Col-0, bbx9-1, bbx9-2, BBX9pro:BBX9-GFP #1 and BBX9pro:BBX9-GFP #2 seeds were surface-sterilized with 20% NaClO and sown on 1× MS medium containing 0, 0.1 μmol synthetic auxin PIC (4-amino-3, 5, 6-trichloropicolinic acid) (Tokyo Chemical Industry, Japan) or 0.1 μmol NPA (Tokyo Chemical Industry). After stratification in darkness at 4°C for 3 d, seeds were transferred to light chambers (PERCIVAL) maintained at 22°C in white (36.1 μmol/m2/s) light for 6 d. The seedlings were then photographed and the hypocotyl lengths measured using ImageJ software.
Statistical analysis
Statistical analyses were performed in Microsoft Excel, GraphPad Prism version 5.0 or through an online website (http://astatsa.com/OneWay_Anova_with_TukeyHSD/).
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database or the GenBank/EMBL libraries under the following accession numbers: phyB (At2g18790); PIF1 (At2g20180); PIF3 (At1g09530); PIF4 (At2g43010); PIF5 (At3g59060); BBX9 (At4g15250); BBX21 (At1g75540); SAUR24 (At5g18080); SAUR29 (At3g03820); IAA29 (At4g32280). The raw RNA-seq data from this study have been deposited at the China National Center for Bioinformation under accession number CRA017469.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of Jiangsu for Distinguished Young Scholars (BK20211525), the Fundamental Research Funds for the Central Universities (YDZX2024045), the National Natural Science Foundation of China (32270256), the Postdoctoral Fellowship Program of CPSF (GZB20240319), the Core Technology Development for Breeding Program of Jiangsu Province (JBGS-2021-014) and the Jiangsu Collaborative Innovation Center for Modern Crop Production (to D.X.).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Z.S., W.Y., Q.J., H.L., Q.H., Y.X.,Y.B., and Z.F. conducted the experiments. Z.S., and D.X. designed the experiments. Z.S., J.D., and D.X. analyzed the data. D.X. wrote the article.






