The actin motor protein OsMYA1 associates with OsExo70H1 and contributes to rice secretory defense by modulating OsSyp121 distribution
Edited by: Liwen Jiang, Chinese University of Hong Kong, China
ABSTRACT
Magnaporthe oryzae (M. oryzae) is a devastating hemibiotrophic pathogen. Its biotrophic invasive hyphae (IH) are enclosed in the extrainvasive hyphal membrane produced by plant cells, thus generating a front line of the battlefield between the pathogen and the host plants. In plants, defense-related complexes such as proteins, callose-rich materials and vesicles, are directionally secreted to this interface to confer defense responses, but the underlying molecular mechanism is poorly understood. In this study, we found that a Myosin gene, Myosin A1 (OsMYA1), contributed to rice defense. The OsMYA1 knockout mutant exhibited decreased resistance to M. oryzae infection. OsMYA1 localizes to the actin cytoskeleton and surrounds the IH of M. oryzae. OsMYA1 interacts with an exocyst subunit, OsExo70H1, and regulates its accumulation at the plasma membrane (PM) and pathogen–plant interface. Furthermore, OsExo70H1 interacted with the rice syntaxin of the plants121 protein (OsSyp121), and the distribution of OsSyp121 to the PM or the pathogen–plant interface was disrupted in both the OsMYA1 and OsExo70H1 mutants. Overall, these results not only reveal a new function of OsMYA1 in rice blast resistance, but also uncover a molecular mechanism by which plants regulate defense against M. oryzae by OsMYA1-initiated vesicle secretory pathway, which originates from the actin cytoskeleton to the PM.
INTRODUCTION
Rice blast disease is one of the greatest threats to cultivated rice worldwide. The causal agent of this disease is Magnaporthe oryzae (M. oryzae), a hemibiotroph filamentous fungus propagated wherever rice is grown (Pennisi, 2010). Currently, we still lack effective methods, such as traditional breeding and chemical approaches, to control this disease due to the rapid adaptation and mutation of the fungus (Wilson and Talbot, 2009). After landing on the plant leaf cuticle, the M. oryzae conidia form a germ tube to develop a dome-shaped infection structure called the appressorium (Ryder and Talbot, 2015). The appressorium forms a penetration peg to rupture the plant cell cuticle and the invasive hyphae (IH) grow inside the live rice cell. At this stage of infection, M. oryzae and rice cells establish an intimate interaction in which intracellular IH is surrounded by a plant-derived extrainvasive hyphal membrane (EIHM) (Kankanala et al., 2007). The external space of the EIHM is a critical battlefield for both the fungus and the host plants to compete. Currently, the cellular processes and crucial functions of this plant-derived EIHM during rice defense against M. oryzae infection are not well understood.
Myosin is a motor protein that can utilize the chemical energy generated by ATP hydrolysis to move on the actin cytoskeleton. Among all eukaryotes, there are 79 Myosin classes. Plants contain only two classes of plant-specific Myosins (classes VIII and XI) (Kollmar and Muhlhausen, 2017). The typical plant Myosin consists of N-terminal motor domains conserved in all Myosins, a neck domain with several isoleucine-glutamine (IQ) calmodulin-binding motifs and a C-terminal cargo-binding domain (CBD) (Tominaga and Ito, 2015). Inadequate information about the roles of plant Myosin has been reported. In Arabidopsis thaliana, there are 13 members of the Myosin XI family, of which inactivation of 11 Myosin genes resulted in no obvious phenotypes under normal growth conditions. However, the knockout of the remaining two Myosin genes, Myosin XI2 and Myosin XIK, leads to defects in driving intracellular motility and rapid cell growth expansion (Peremyslov et al., 2008; Ueda et al., 2010). In addition to plant development, Myosin XI reportedly participates in plant defense. For example, the quadruple Myosin knockout mutants XI1, XI2, XII and XIK display impaired trafficking pathways and deposition of lignin-like compounds to the penetration site of the Arabidopsis nonadapted barley powdery mildew fungus Blumeria graminis f. sp. hordei (Bgh) (Yang et al., 2014). Although these critical roles of Myosins have been revealed in Arabidopsis, we have not characterized a rice Myosin protein in terms of both development and pathogen defense.
Plants employ sophisticated immune responses, including pattern-triggered immunity (PTI) and effector-triggered immunity (ETI), to defend against pathogen microbe infection (Jones and Dangl, 2006; Dangl et al., 2013). The secretory pathway is an integral component of the plant immune system. In PTI and ETI, surface-localized immune receptor components, antimicrobial compounds, and defense proteins actively undergo endocytosis or are delivered to pathogen invasion sites through polarized secretion (Wang et al., 2016; Gu et al., 2017). The exocyst-positive organelle (EXPO) is uniquely labeled by subunits of the exocyst complex and plays a major role in exocytosis (Žárský et al., 2009; Gu et al., 2017; Saeed et al., 2019). The exocyst complex is evolutionarily conserved and is composed of eight subunits (Heider and Munson, 2012). In recent years, increasing evidence has indicated that both exocyst complex members and SNARE proteins are involved in plant immunity. For example, mutants of the Arabidopsis exocyst subunits Exo70B2 and Exo70H1 are more susceptible to Pseudomonas syringae pv. maculicola infection. Loss of Exo70B1 function causes ectopic hypersensitive responses, thus enhancing resistance to both bacterial and fungal pathogens (Stegmann et al., 2012; Zhao et al., 2015). Intriguingly, Exo70B1 was recently found to be guarded by the NLR receptor TN2 (Liu et al., 2017). EXO70B1 plays a critical role in FLAGELLIN SENSING 2 (FLS2) homeostasis at the plasma membrane (PM), which is independent of TN2 (Wang et al., 2020). In addition to the exocyst subunits, SNARE proteins also play a role in the defense response. For instance, the Arabidopsis Syp1 (Syntaxin of Plants1) subfamily is a plant-specific syntaxin family, and its members Syp121 and Syp132 reportedly function in plant fungal pathogen defense (Kalde et al., 2007; Cao et al., 2019). Together with SNAP33 and VAMP721/VAMP722, Syp121 forms PM-localized ternary SNARE complexes that are required for plant penetration resistance (Collins et al., 2003). Significantly, Syp121 was shown to be packaged into exosomes and present in the outer space of the PM (Nielsen et al., 2012; Rutter and Innes, 2017). These findings demonstrated that vesicle secretion is clearly required for plant defense, but the mechanism by which vesicles achieve targeted secretion during plant defense is not clearly understood.
The cellular mechanism of directional vesicle transport mediated by the actin cytoskeleton system in plant defense is not clear. There are few reports about how secretary vesicles are delivered to invasion sites, the EIHM, the PM or the apoplast, especially in the context of rice defense against M. oryzae infection. In this study, we characterized a rice Myosin protein, OsMYA1. We found that OsMYA1 localizes to the actin cytoskeleton and accumulates around IH. OsMYA1 interacts with the exocyst subunit OsExo70H1 through its CBD domain. OsMYA1 facilitates the transport of OsExo70H1 to the PM and the site of the pathogen–plant interface. Moreover, OsExo70H1 could interact with OsSyp121, and loss of OsExo70H1 impaired the translocation of OsSyp121 to the PM and the site of the pathogen–plant interaction. Knockout of OsMYA1 and OsExo70H1 decreased rice defense, indicating that OsMYA1-mediated transport of OsExo70H1 and OsSyp121 is crucial for rice defense against M. oryzae infection. Overall, our results provide new evidence that rice employs a Myosin protein for the directional transport of defense-related proteins to the PM and EIHM during defense against M. oryzae.
RESULTS
Characterization of Myosin genes in ZH11
Previously, we conducted a comparative transcriptome analysis to identify a core set of genes involved in the rice response to M. oryzae infection. A set of rice Myosin genes was shown to be significantly induced in response to M. oryzae inoculation (Yang et al., 2021), indicating that Myosin genes may participate in rice defense against M. oryzae infection. Next, we searched for and blasted the motor domain of Myosin, which is conserved in all eukaryotes in the ZH11 rice genome database (https://ricerc.sicau.edu.cn/RiceRC/riceInfo/riceSpecies/ZH11). In total, six intact Myosin genes were found in the ZH11 genome, each of which encoded a protein containing a typical N-terminal motor, IQ calmodulin-binding motif and a dilute (DIL) CBD. We performed a real-time polymerase chain reaction (PCR) assay to investigate whether these genes responded to M. oryzae infection. The results showed that the expression of OsZH11G0101106000, OsZH11G0612947800 and OsZH11G0305452000 increased in response to M. oryzae infection (Figure 1A). Then, we generated these three gene knockout mutants through CRISPR-Cas9 (Figure S1). After screening, we generated homozygous mutants of the three genes (T3 generation) and found that the OsZH11G0305452000 mutant increased the tiller number, while the OsZH11G0101106000 and OsZH11G0612947800 mutants decreased the tiller number compared with the wild-type (WT) plants (Figure 1B, C). Plant height did not change significantly for any of the mutants (Figure 1B, E). The fertility of the OsZH11G0101106000 and OsZH11G0612947800 mutants decreased significantly (Figure 1D, F, G).
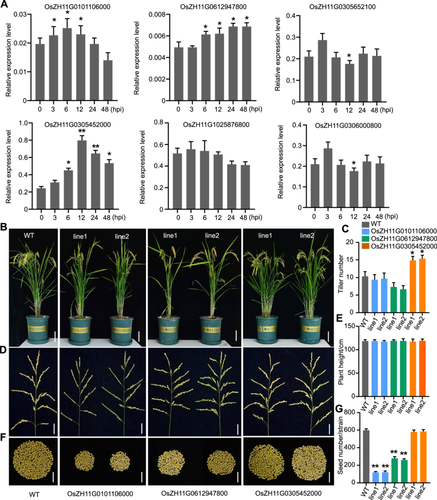
Characterization of Myosin genes in ZH11
(A) qRT-PCR analysis of the expression of Myosin genes in response to Magnaporthe oryzae infection. Total RNA was extracted from ZH11 rice infected with Guy11 conidia (1 × 105 spores/mL) for 0, 3, 6, 12, 24 or 48 h. 0 h indicates rice plants not infected with M. oryzae. The error bars represent the SDs of three independent experiments (n = 3). Asterisks indicate statistically significant differences, as determined by Student's t-test (*P < 0.05, **P < 0.01). (B) Photographs showing the rice plants for the wild-type (WT) and the mutants (T3 generation for each mutant) cultured in the field for approximately 100 d. Bars = 20 cm. (C, E) Statistical analysis of the plant tiller number (C) and plant height (E) for the WT and the mutants. Each bar represents the mean of 20 plants. (D, F) Photographs showing the rice spikes and seeds from a single strain for the WT and the mutants, respectively. Bars = 5 cm. (G) Statistical analysis of the number of seeds from the WT and the mutants. Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). Each bar represents the mean of 20 plants. PCR, polymerase chain reaction.
OsMYA1 functions in the defense against M. oryzae infection
Next, we applied a punch-inoculated assay to investigate whether these rice Myosin genes function in the rice defense against M. oryzae infection. The same amount of M. oryzae spores (Guy11, 1 × 105 spores/mL) was used to infect the leaves of the WT plants and these mutants. The results showed that the lesion area of the mutant lines of OsZH11G0305452000 was greater than that of the WT and the other gene mutants (Figure 2A, B). Then, sprayed inoculation assays were conducted, and the results also showed that more lesions appeared in the OsZH11G0305452000 mutant lines (Figure 2C, D). The investigation of fungal biomass further indicated that more M. oryzae propagated in the OsZH11G0305452000 mutants (Figure 2E). Next, we examined the expression of disease-responsive genes in the mutants. OsPR1b and OsWRKY45 are immune indicators used to evaluate disease resistance in rice (Cao et al., 2016; Meng et al., 2022). The results showed that these defense-related genes were expressed at lower levels in the OsZH11G0305452000 mutants than in the WT (Figure S2). The above results indicated that the knockout of OsZH11G0305452000 decreased rice defense and indicated that the function of this gene was associated with rice defense. We then aligned this Myosin protein with all the Arabidopsis Myosin proteins and found that OsZH11G0305452000 was most closely related to Arabidopsis Myosin XI 1 (AtMYA1) (Figure S3); thus, we designated this gene OsMYA1. The two homozygous lines of the OsMYA1 mutant were termed osmya1-1 and osmya1-2.
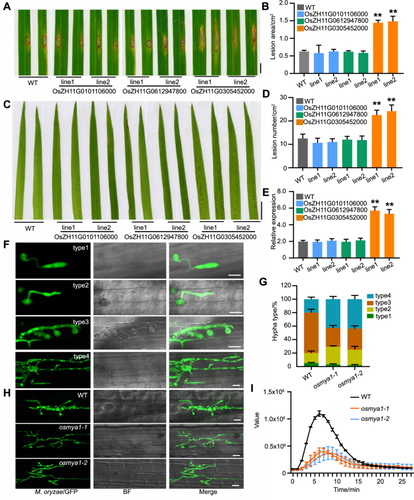
Magnaporthe oryzae resistance analysis of rice Myosin gene mutants
(A−D) Pathogenesis analysis of the mutants of the three indicated rice Myosin genes via the punched (A) or spray-inoculated (C) methods. Guy11 conidia (1 × 105 spores/mL) were used in the infection assays. Images were taken after 4 d of infection for both the punch and the spray inoculation methods. Bars = 1 cm. (B, D) Statistical analysis of the lesion area in (A) and lesion number in (C), respectively. Each bar represents the mean of 20 plant leaves. Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). (E) Relative fungal abundances of the rice Ubiquitin gene determined by qRT-PCR following the expression of the M. oryzae Pot2 gene. The data are presented as the means and SDs of three biological replicates. Asterisks indicate significant differences (**P < 0.01), Student's t-test. (F) Infection assay using GFP-labeled Guy11 to infect the rice sheath. Images showing four types of invasive hyphae (IH) (type 1, no hyphal penetration; type 2, IH with fewer than two branches; type 3, IH with more than two branches; type 4, IH penetrating neighboring cells). (G) Growth of the IH in the wild-type (WT) and the two mutant lines of OsMYA1 was quantified and statistically analyzed at 36 h after infection. Error bars represent SDs, n > 50. (H) Typical images showing IH development in the WT and the OsMYA1 mutants at 36 h after infection. Bars = 20 μm. (I) ROS burst analysis of the WT and OsMYA1 mutants triggered by chitin. The error bars represent the means of ten replicate samples for each time point. The experiments were repeated three times, and similar results were obtained. GFP, Green fluorescence protein; PCR, polymerase chain reaction; ROS, reactive oxygen species.
We then observed the infection processes of M. oryzae in the OsMYA1 mutants. We injected Guy11 spores labeled with green fluorescence protein (GFP) into the sheaths of both the WT and the two OsMYA1 mutants. We can clearly see the infection stages by monitoring the GFP. We found that the infection process in the OsMYA1 mutants occurred much faster than that in the WT plants (Figure 2F, G). For example, at 36 h after infection, M. oryzae usually infected two layers of rice sheath cells in the WT. However, more layers were processed in the mutant plants (Figure 2H). Furthermore, upon chitin triggering, the OsMYA1 mutant lines developed a much weaker reactive oxygen species (ROS) burst than the WT plants (Figure 2I). Overall, these results showed that OsMYA1 may play a crucial role in rice defense against M. oryzae infection.
OsMYA1 localized to the actin cytoskeleton and pathogen–plant interaction interface
Next, we investigated the subcellular localization of OsMYA1. We expressed OsMYA1- red fluorescence protein (RFP) driven by the 35S promoter in both rice protoplasts and tobacco cells. In both types of plant cells, OsMYA1-RFP colocalized with the actin-binding domain 2 (ABD2)-GFP-labeled actin cytoskeleton (Figure 3A). Moreover, we further found that the motor domain, but not the IQ-CBD domain, contributed to actin binding (Figure 3B−D). We further generated 35S-OsMYA1-RFP transgenic rice plants, and 35S-RFP plants were also generated as controls. The disease resistance test assay showed that overexpression of OsMYA1 increased rice defense against M. oryzae infection (Figure S4). Compared with that of 35S-RFP (Figure 3E), similar to that in rice protoplast and tobacco cells, the 35S-OsMYA1-RFP fluorescence signal exhibited fine filaments distributed in the sheath cell (Figure 3F). We infected these cells with M. oryzae, which was labeled with GFP. After 16 h of infection, M. oryzae successfully infected the rice cells. Through fluorescence observation, we found that compared with that in the control plants expressing 35S-RFP (Figure 3G), in the 35S-OsMYA1-RFP plants, much of the fluorescence accumulated around the infected hyphae (Figure 3H, I). Overall, these results indicated that OsMYA1 localized to the actin cytoskeleton and could accumulate around the IH of M. oryzae.
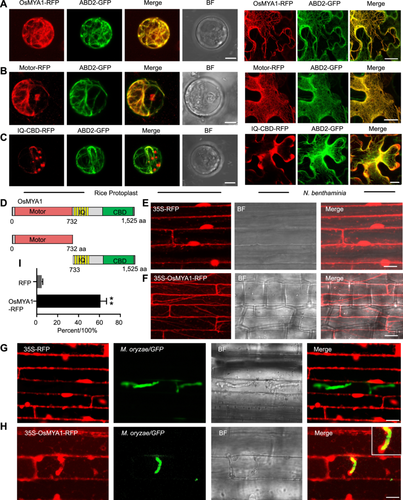
Subcellular analysis of OsMYA1
(A−C) The indicated 35S-OsMYA1-RFP, 35S-Motor-RFP and 35S-IQ-CBD-RFP constructs were coexpressed with an actin cytoskeleton marker (ABD2-GFP) in both rice protoplasts (left) and Nicotiana benthamiana (right) cells. The fluorescence signal was obtained through confocal microscopy (Zeiss 880). Bars = 20 μm. (D) A schematic diagram showing the constructed domains of OsMYA1 in the subcellular analysis. (E, F) Fluorescence images showing transgenic rice plants stably expressing 35S-RFP (E) or 35S-OsMYA1-RFP (F). Bars = 20 μm. (G, H) Images showing the RFP (G) and OsMYA1-RFP (H) distribution in the sheath cells of the transgenic rice plants. These sheath cells were infected with GFP-labeled Guy11 16 h after infection. The enlarged images of the section outlined by white lines in the right upper corner indicate that OsMYA1-RFP surrounded the invasive hyphae (IH). Bars = 20 μm. (I) Statistical analysis showing the portion of RFP and OsMYA1-RFP surrounding an IH in the wild-type (WT) rice plants. The error bars represent the SDs of three independent experiments (n = 30). Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). GFP, Green fluorescence protein; RFP, red fluorescence protein.
OsMYA1 interacts with the Exo70 exocyst complex subunit
To explore the functional basis of OsMYA1 in the defense response, we screened for OsMYA1-interacting proteins through the yeast-two-hybrid (Y2H) system. The screening of a cDNA library from rice leaves (ZH11) infected with M. oryzae revealed an exo70 exocyst complex subunit protein (OsZH11G1127022600) that strongly interacted with OsMYA1. We aligned this protein with 23 Arabidopsis Exo70 proteins and found that this protein clustered with H-type Exo70 proteins; thus, this protein was designated OsExo70H1 (Figure S5). Direct Y2H assays confirmed the interaction between the two proteins (Figure 4A). In the Y2H assay, we further found that the CBD but not the motor or IQ domain of OsMYA1 could interact with OsExo70H1 (Figure 4B, C). We performed a luciferase complementation imaging (LCI) assay to assess the interaction of OsMYA1, the CBD domain and OsExo70H1 in plant cells. As shown in Figure 4D, luciferase (Luc) activity was detected in Nicotiana benthamiana leaf cells, but no Luc activity was detected in the negative controls. We performed a coimmunoprecipitation (co-IP) assay to verify the interaction between CBD and OsExo70H1. We coexpressed CBD-GFP and OsExo70H1-RFP in ZH11 protoplast and expressed GFP as a parallel negative control. CBD-GFP and GFP were immunoprecipitated by anti-GFP agarose beads. An anti-RFP antibody detected OsExo70H1-RFP only in the precipitate from cells that expressed CBD-GFP, not from the GFP negative control (Figure 4E). The subcellular localization of OsExo70H1 was next investigated in rice protoplasts. OsExo70H1-GFP and OsMYA1-RFP were coexpressed in the cell, OsExo70H1-GFP appeared as dot-like structures, and some dots were associated with OsMYA1-RFP-formed filaments (Figure 4F). Overall, these results showed that OsMYA1 could interact with OsExo70H1 and that the CBD domain accounts for these interactions.
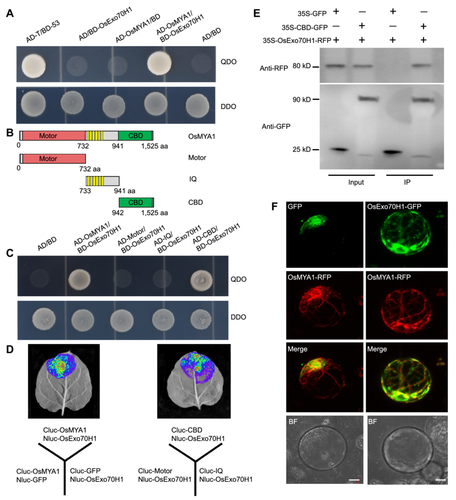
OsMYA1 interacts with OsExo70H1
(A) Yeast-two-hybrid (Y2H) assay to detect the interaction between OsMYA1 and OsExo70H1. Yeast cells containing the indicated plasmids were grown on SD/−Leu/−Trp (DDO) plates and SD/−Leu/−Trp/−Ade/−His (QDO) plates. AD/BD, AD/BD-OsExo70H1, AD-OsMYA1/BD and AD-T/BD-53 were used as negative and positive controls, respectively. (B) A schematic diagram showing the constructed domains of OsMYA1 used for Y2H. (C) Y2H assays were performed to investigate the interactions between the domains of OsMYA1 and OsExo70H1. The indicated pairs of plasmids were expressed in yeast cells, which were subsequently cultured on DDO and QDO plates. (D) Luciferase (Luc) complementation imaging (LCI) analysis of the interaction between OsMYA1, the CBD domain and OsExo70H1. (E) Coimmunoprecipitation (co-IP) assays to detect the interaction between the OsMYA1 CBD domain and OsExo70H1. The 35S-GFP and 35S-CBD-GFP plasmids were respectively coexpressed with 35S-Exo70H1-RFP in rice protoplast cells. Then, the total proteins were extracted and incubated with GFP-binding agarose. Then, western blot analysis was conducted by using anti-GFP or anti-RFP antibodies. (F) Subcellular analysis of OsExo70H1 and OsMYA1. The indicated plasmids, 35S-GFP and 35S-OsExo70H1-GFP were respectively coexpressed with 35S-OsMYA1-RFP in rice protoplast cells. Fluorescence images were obtained 24 h after plasmid expression. Bars = 10 μm. GFP, Green fluorescence protein; RFP, red fluorescence protein.
OsExo70H1 is critical for rice defense against M. oryzae infection
We found the expression of OsExo70H1 was upregulated upon M. oryzae infection (Figure S6A). A glucuronidase (GUS) reporter assay using the native promoter of OsExo70H1 showed that OsExo70H1 was expressed in the roots, sheaths and leaves (Figure S6B). We then generated OsExo70H1 mutants in ZH11 through CRISPR-Cas9 (Figure S7). The tiller number and plant height decreased in the two lines of the OsExo70H1 mutant plants when they were grown in the field (Figure S7A−C), indicating that this protein also plays a role in rice development. Next, we investigated whether OsExo70H1 plays a role in rice defense. The sprayed inoculation assay showed that more lesions appeared on the mutant leaves (Figure 5A, B). The examination of the fungal biomass showed that more pathogens developed in the mutants (Figure 5C). The punch-inoculated assay showed that the mutants formed larger lesion areas (Figure 5D, E). We also examined the expression of disease-responsive genes in the mutants. The results showed that the expression of defense-related genes was repressed in the knockout plants, indicating that OsExo70H1 functions in disease resistance signaling in rice (Figure 5F, G). A sheath infection assay using GFP-labeled Guy11 further demonstrated that invasive M. oryzae hyphae were processed faster in the cells of the rice OsExo70H1 mutants than in those of the WT (Figure 5H, I). The ROS burst assay triggered by chitin showed that mutants of OsExo70H1 generated lower ROS levels than did the WT (Figure 5J). Overall, these results indicated that OsExo70H1 is critical for rice defense against M. oryzae infection.
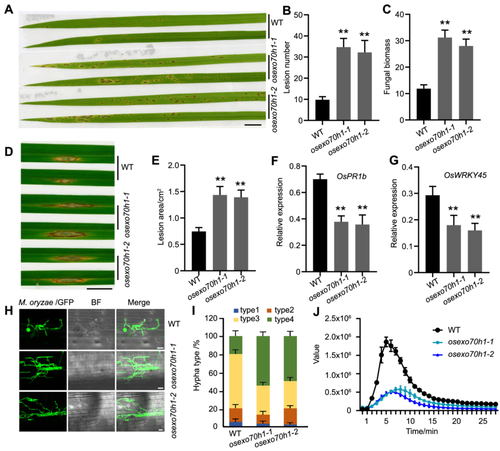
Magnaporthe oryzae resistance analysis of OsExo70H1 mutants
(A) Photograph showing that osexo70h1-1 and osexo70h1-2 produced more diseased lesions than did the wild-type (WT) after inoculation with Guy11 according to the spraying method. Bar = 1 cm. (B) Statistical analysis of the lesion number on the rice leaves of the WT plants and the OsExo70H1 mutants. Each bar represents the mean of 20 plant leaves. Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). (C) Relative fungal growth as determined by qRT‒PCR. The data are presented as the means and SDs of three biological replicates. Asterisks indicate significant differences (**P < 0.01), Student's t-test. (D) The punch-inoculated assay used for investigating the resistance of the OsExo70H1 mutants. Bar = 1 cm. (E) Statistical analysis of the lesion area in (D). Each bar represents the mean of 20 plant leaves. Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). (F, G) Expression analysis of OsPR1b and OsWRKY45 in OsExo70H1 mutants. Ten-d-old rice leaves were used for qRT‒PCR assays. Significant differences from the WT were determined by Student's t-test (**P < 0.01; the data are presented as the means ± SDs; n = 3 biological replicates). (H) Images showing the growth of GFP-labeled M. oryzae (Guy11) in the rice sheath cells of the WT and the two OsExo70H1 mutants at 36 h after infection. (I) Growth types of the invasive hyphae (IHs) in the WT and the two mutant lines of OsExo70H1 were quantified and statistically analyzed at 36 h after infection. Error bars represent SDs, n > 50. (J) ROS burst analysis of the WT and the OsExo70H1 mutants triggered by chitin. The error bars represent the means of ten replicate samples for each time point. The experiments were repeated three times, and similar results were obtained. GFP, Green fluorescence protein; PCR, polymerase chain reaction; ROS, reactive oxygen species.
OsExo70H1 responds to M. oryzae infection and accumulates around invasive hyphae
As OsExo70H1-GFP appeared as dot-like structures in the rice protoplast (Figure 4F), we further investigated its localization using the endosome cellular markers. In both tobacco and rice protoplast cells, the OsExo70H1 dots colocalized with the trans-Golgi network (TGN) marker protein Syn61-GFP but not with the late endosome marker protein ARA7-GFP (Choi et al., 2013) (Figure 6A−D). Next, we generated rice plants stably expressing pOsExo70H1::OsExo70H1-RFP. Western blot was conducted to verify the expression of RFP or OsExo70H1-RFP in the transgenic plants (Figure 6G). Compared with the 35S-RFP control plants (Figure 6E), the pOsExo70H1::OsExo70H1-RFP plants exhibited dot-like structures in the rice sheath cells, as did the rice protoplast and N. benthamiana cells (Figure 6F). We infected these sheath cells with M. oryzae/GFP and found that compared with the RFP control, the OsExo70H1-RFP signal clearly surrounded the infected M. oryzae hyphae (Figure 6I), indicating the presence of an EIHM distribution of this protein.
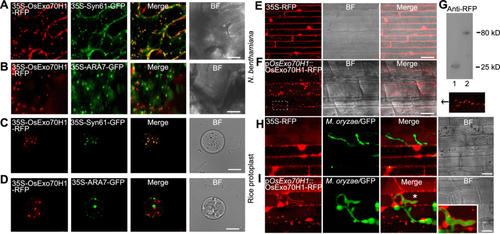
Subcellular analysis of OsExo70H1
(A−D) 35S-OsExo70H1-RFP was respectively coexpressed with a trans-Golgi network (TGN) marker (35S-Syn61-GFP) and an early/late endosome marker (35S-ARA7-GFP) in both Nicotiana benthamiana (upper) and rice protoplast (lower) cells. (E, F) Images showing the fluorescence signal distributions in the sheath cells of transgenic rice plants stably expressing 35S-RFP (E) or pOsExo70H1::OsExo70H1-RFP (F). The arrow indicates an enlarged region of the figure enclosed by a white dashed line to show the dot-like distribution of OsExo70H1-RFP. Bars = 20 μm. (G) Western blot analysis of RFP (lane 1) and OsExo70H1-RFP (lane 2) protein expression using an anti-RFP antibody. (H, I) Fluorescence images showing the RFP (H) and OsExo70H1-RFP (I) distribution in the sheath cells of the transgenic rice plants. These sheath cells were infected with GFP-labeled Guy11 16 h after infection. The white asterisk region is enlarged and outlined by white lines in the lower right corner, which shows that the OsEx70H1-RFP signal surrounded the invasive hyphae (IH). Bars = 20 μm. GFP, Green fluorescence protein; RFP, red fluorescence protein.
OsMYA1 is required for OsExo70H1 accumulation in the cell membrane during M. oryzae infection
To assess how OsMYA1 regulates OsExo70H1, we crossed OsExo70H1-RFP with OsMYA1 knockout mutants. We obtained OsExo70H1-RFP/osmya1 plants through screening. Rice sheath cells were subsequently infected with M. oryzae/GFP. In the WT, as described above, OsExo70H1-RFP accumulated around the IH (Figure 7A). However, in the OsExo70H1-RFP/osmya1-1 cells, the amount of OsExo70H1-RFP surrounding the infected M. oryzae hyphae decreased, indicating that much less OsExo70H1-RFP moved to the site of the pathogen–plant interface, the EIHM, in the mutants (Figure 7B, C). Additionally, we extracted the total proteins and the membrane proteins of the WT and the OsMYA1 knockout mutants expressing OsExo70H1-RFP at 24 h after M. oryzae infection. Through western blot analysis, we found that the protein level of OsExo70H1-RFP in the OsMYA1 knockout mutants was comparable to that in the WT. However, for the membrane proteins, the protein level of OsExo70H1-RFP in the OsMYA1 knockout mutants was much lower than that in the WT (Figure 7D). Then, the membrane proteins of the WT and the mutants were extracted at 12, 24 and 36 h after infection. In the WT, more OsExo70H1-RFP protein accumulated in the cell membrane at 24 h and 36 h than at 12 h after infection. However, in the OsMYA1 mutants, fewer OsExo70H1-RFP proteins were enriched in the cell membrane portion (Figure 7E). The results above indicated that OsMYA1 functions in OsExo70H1 accumulation at the cell membrane during M. oryzae infection.
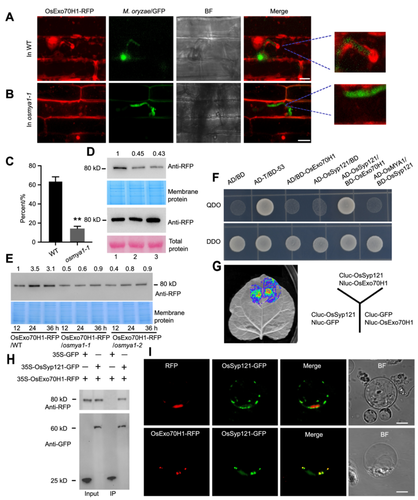
OsMYA1 plays a role in OsEx70H1 accumulation at the cell membrane
(A) Distribution of OsEx70H1-RFP upon Magnaporthe oryzae/GFP infection in rice sheath cells. OsEx70H1-RFP/osmya1-1 plants were obtained by crossing OsEx70H1-RFP plants with osmya1-1 plants. Then, Guy11/GFP conidia were used to infect the sheath cells of OsEx70H1-RFP/WT (A) and OsEx70H1-RFP/osmya1-1 (B) plants. Images were collected 16 h after infection. The invasive hyphae (IH) region was enlarged to show the distribution of OsEx70H1-RFP surrounding the IH. Bars in (A) and (B) represent 20 μm. (C) Statistical analysis showing the portion of OsEx70H1-RFP surrounding an IH in the wild-type (WT) and osmya1-1 plants. The error bars represent the SDs of three independent experiments (n = 50). Asterisks indicate statistically significant differences according to Student's t-test (**P < 0.01). (D) OsEx70H1-RFP accumulation in the total protein (lower panels) and membrane protein (upper panels) of the WT and osmya1-1 plants. Total protein and membrane protein were extracted 24 h after infection from the OsEx70H1-RFP/WT (lane 1), OsEx70H1-RFP/osmya1-1 (lane 2) and OsEx70H1-RFP/osmya1-2 (lane 3) plants. Western blot analysis was conducted using an anti-RFP antibody. The numbers above the bands indicate the fold changes in the band intensity. (E) OsExo70H1-RFP in the membrane proteins from the OsEx70H1-RFP/WT and the OsEx70H1-RFP/osmya1 plants at 12, 24 and 36 h after infection. The numbers above the bands indicate the fold changes in the band intensity. (F, G) Y2H assay (F) and LCI assay (G) to detect the interaction between OxExo70H1 and OsSyp121. (H) Co-IP assays to detect the interaction between OsExo70H1 and OsSyp121. The 35S-GFP and 35S-OsSyp121-GFP plasmids were respectively coexpressed with 35S-Exo70H1-RFP in rice protoplast cells. Then, the total proteins were extracted and incubated with GFP-binding agarose. Then, western blot analysis was conducted by using anti-GFP or anti-RFP antibodies. (I) The 35S-OsExo70H1-RFP and 35S-Syp121-GFP plasmids were coexpressed in rice protoplasts to determine the subcellular localization of the two proteins. 35S-RFP was used as the control. Bars = 10 μm. GFP, Green fluorescence protein; RFP, red fluorescence protein.
As the function of OsExo70H1 in rice defense has not been elucidated, we then performed an IP-MS experiment using OsExo70H1-RFP-expressing plants to explore how OsExo70H1 and OsMYA1 function in defense. Several rice proteins, including Syp121, CERK1, Exo70B1, and vacuolar sorting protein, were identified via IP-MS (Figure S8). The OsSyp121 protein was the most abundant, and previous reports have shown that the Syp121 protein in Arabidopsis and rice can accumulate at fungal–plant interaction sites (Collins et al., 2003; Cao et al., 2019). We then tested the interaction between OsExo70H1 and OsSyp121. The Y2H assay showed that OsSyp121 could directly interact with OsExo70H1 but not with OsMYA1 (Figure 7F). LCI and co-IP experiments further verified the interaction between OsExo70H1 and OsSyp121 (Figure 7G, H). The coexpression of OsExo70H1-RFP and OsSyp121-GFP in rice protoplasts showed that the two proteins could colocalize (Figure 7I). These results indicated that the function of OsExo70H1 may be associated with OsSyp121.
OsMYA1 and OsExo70H1 play a role in the accumulation of OsSyp121 in the cell membrane to defend against M. oryzae
We generated 35S-OsSyp121-GFP rice plants through Agrobacterium-mediated transformation. Fluorescence observation revealed dot-like structures in the sheath cells of the WT rice plants (Figure 8A). To investigate how OsMYA1 and OsExo70H1 regulate OsSyp121, we crossed the OsSyp121-GFP signal to both the OsMYA1 and OsExo70H1 mutants. After confirming the fluorescence signal by microscopy and the gene mutation background by sequencing, we observed the OsSyp121-GFP signal in these mutant lines. There were no obvious differences in the distribution of OsSyp121-GFP between the mutants and the WT (Figure 8A). We then infected these plant sheath cells with M. oryzae/RFP. After 16 h of infection, M. oryzae penetrated the plant cells. In the infected WT cells, the OsSyp121-GFP signal accumulated at the periphery of the plant cells, and the OsSyp121-GFP signal surrounded the IH (approximately 15% of the hyphae). Compared with that in the WT, in the OsMYA1 and OsExo70H1 mutants, the fluorescence intensity at the cell periphery decreased, and rare IH were surrounded by the OsSyp121-GFP signal (Figures 8B, S9A). Western blot analysis revealed that the total protein level of OsSyp121-GFP was comparable between the WT and these mutants. However, for the membrane proteins, the protein level of OsSyp121-GFP decreased in the OsMYA1 and OsExo70H1 mutants (Figure 8C). Western blot analysis further showed that after 24 h of M. oryzae infection, the OsSyp121-GFP level increased 3.6-fold in the WT membrane protein compared with that at 0 h. However, in the OsMYA1 and OsExo70H1 mutants, this increasing tendency attenuated (Figure 8D). As knockout of OsSyp121 leads to decreased deposition of callose, which is a process that depends on the exocyst complex (Du et al., 2018; Cao et al., 2019), we subsequently examined callose formation in the WT and rice mutants. The results indicated that upon chitin treatment, callose deposition in the OsMYA1 and OsExo70H1 mutants greatly decreased compared with that in the WT (Figure 8E, F). In addition, in our study, the knockout of OsMYA1 or OsExo70H1 led to a dramatic reduction in the chitin-induced ROS burst, a very early immune response. We further performed chitin-induced mitogen-activated protein kinase (MAPK) activation. The results showed that the OsMYA1 and OsExo70H1 mutants exhibited lower MAPK activation (Figure S9B). These findings support the notion that the secretion of exosomes contributes to modulating the levels of ROS, the plant's very early immune response (Hossain et al., 2013).
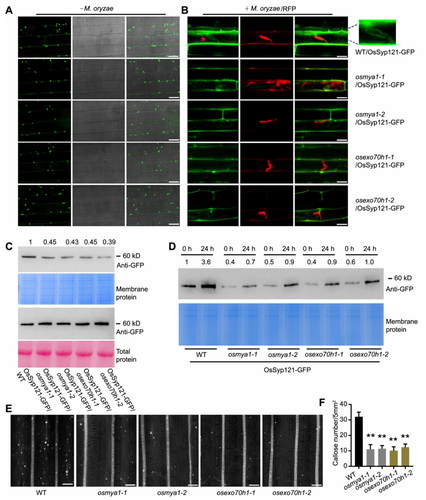
OsMYA1 and OsEx70H1 are associated with OsSyp121 accumulation in the cell membrane
(A) Distribution of OsSyp121-GFP in rice sheath cells. 35S-OsSyp121-GFP was transformed into wild-type (WT) rice plants (ZH11). The OsSyp121-GFP signal was then crossed to the mutant lines of OsMYA1 and OsExo70H1. These sheath cells all appeared as dot-like structures. Bars = 20 μm. (B) Distribution of OsSyp121-GFP in rice sheath cells upon Magnaporthe oryzae/RFP infection at 24 h after infection. OsSyp121-GFP surrounding the IH in the WT background is enlarged in the right top corner. Bars = 20 μm. (C) OsSyp121-GFP accumulation in the total protein (lower panels) and membrane protein (upper panels) of the WT, the osmya1 and osexo70h1 plants. Total and membrane proteins were extracted from these plants 24 h after infection. Western blot analysis was conducted using an anti-GFP antibody. The numbers above the bands indicate the fold changes in the band intensity. (D) OsSyp121-GFP in the membrane proteins from the WT and the osmya1 and osexo70h1 plants at 0 and 24 h after infection. The numbers above the bands indicate the fold changes in the band intensity. (E) Aniline blue staining of callose in the WT, osmya1 and osexo70h1 plants. Leaves of 2-week-old rice plants were treated with chitin (1 µM) for 12 h. These leaves were then stained with aniline blue and observed under a confocal microscope. Bars = 50 μm. (F) Quantification of the stained callose in (E). Error bars represent the standard error of the mean, as determined by Student's t-test (**P < 0.01). GFP, Green fluorescence protein; RFP, red fluorescence protein.
OsExo70H1 specifically interacts with OsMYA1, and the OsMYA1–OsExo70H1–OsSyp121 cascades may regulate SNARE complex secretion
We also investigated the interaction specificity between OsExo70H1 and OsMYA1. There are 41 OsExo70 proteins encoded in the rice genome (Chong et al., 2010). It is interesting to determine whether OsExo70H1 uniquely interacts with OsMYA1 or is shared by other OsExo70 paralogs. We subsequently cloned 41 rice Exo70 proteins, and yeast-two-hybrid assays revealed that only OsExo70H1 strongly interacts with the CBD of OsMYA1. In addition, yeast cells cotransformed with OsExo70FX7/CBD grew much slower than the positive control and OsExo70H1/CBD cotransformed yeast strains, indicating a much weaker interaction between OsExo70FX7 and CBD (Figure S10).
To test whether the OsMYA1–OsExo70H1–OsSyp121 cascades regulate the SNARE complex or only the OsSyp121 protein, we analyzed the protein accumulation of VAMP721, a protein that interacts with Syp121 in Arabidopsis to form a SNARE complex, in both the total protein and the membrane portion of the WT, osmya1 and osexo70h1 protoplasts. The results showed that the accumulation of the VAMP721 protein in the membrane decreased in osmya1 and osexo70h1 (Figure S9C). As a control, the accumulation of the subunit of the exocyst complex OsSec6 protein, in which there is only one member in rice exocyst (Chong et al., 2010), did not change significantly in the membrane portion in the osmya1 and osexo70h1 compared with that in the WT plants (Figure S9D).
In addition, we also found that the interaction between OsMYA1 and OsExo70H1 was enhanced upon chitin treatment in split luciferase assays. However, the interaction between OsExo70H1 and OsSyp121 did not significantly change upon chitin treatment (Figure S9E−H).
Overall, these results indicated that OsExo70H1 could specifically interact with OsMYA1 and that chitin could enhance this interaction. OsMYA1–OsExo70H1–OsSyp121 cascades regulate the secretion of the SNARE complex through interactions.
DISCUSSION
The actin cytoskeleton is involved in numerous fundamental cellular processes, including vesicle trafficking, the spatial distribution of organelles, protein delivery and signal transduction, during plant development and in response to stimuli (Day et al., 2011; Higaki et al., 2011). Thus, the actin cytoskeleton has been assumed to play a role in plant innate immunity against fungi and oomycetes (Li and Day, 2019). However, to date, there has been little evidence showing how the host-cell actin cytoskeleton participates in fungal disease defense. Myosin is a motor protein that can slide on actin filaments dependent on its motor domain, thus generating a driving force for the transport of cellular components (Ueda et al., 2015). There are 13 genes encoding class XI Myosins in Arabidopsis, and none of the single knockout lines exhibited obvious developmental defects under optimal growth conditions (Peremyslov et al., 2008). Multiple Myosin XI mutants need to be generated to investigate their functions. In our study, we generated three single Myosin gene mutants in rice. The plants exhibited developmental changes, such as changes in tiller number and seed generation (Figure 1B−G), indicating that the monocotyledonous rice Myosins may be functionally diverse and specific compared with those in dicotyledonous Arabidopsis.
Through multiple mutations of Arabidopsis Myosin genes, class XI Myosins were revealed to be responsible for the movement of organelles, such as the endoplasmic reticulum (ER), Golgi apparatus, peroxisomes, mitochondria, and nucleus, during plant development, including the gravity response, pollen and root hair elongation and plant nonadapted immunity (Ueda et al., 2010; Ojangu et al., 2012; Yang et al., 2014). Despite the diverse functions of class XI Myosin proteins in Arabidopsis, descriptions of the functions of this family of proteins in rice, especially in plant‒pathogen interactions, are lacking. In our work, we identified a functional rice class XI Myosin gene, OsMYA1, involved in the defense against M. oryzae infection. Knockout of OsMYA1 led to decreased resistance to pathogen infection (Figure 2A−H) and a weak ROS burst (Figure 2I), indicating that OsMYA1 is a positive regulatory factor in rice defense against this fungal disease.
Members of the Arabidopsis Class XI Myosins have different subcellular locations related to their diverse functions in endocytosis (Sattarzadeh et al., 2008), cytoplasmic streaming (van der Honing et al., 2010) and organelle movement (Peremyslov et al., 2008). Fluorescence observation revealed that Myosin XI-A1 (AtMYA1) and XI-A2 (AtMYA2) are localized to the peroxisome and Golgi, respectively. XI-F and XI-I exhibit chloroplast and nucleolus localization, respectively (Sparkes, 2011). XI-K-GFP appeared as vesicle-like bodies associated with F-actin (Peremyslov et al., 2012). In addition, the class VIII Myosin ATM1 was shown to localize to endosomes and function in vesicle trafficking (Golomb et al., 2008). In our work, although OsMYA1 is more closely related to Arabidopsis Myosin XI-A1, it did not localize to organelles. It localized to the actin cytoskeleton under optimal conditions (Figure 3A−F). Moreover, upon M. oryzae infection, OsMYA1 changed its localization to surround IH (Figure 3H). These findings may represent a new regulatory mechanism of plant Myosin proteins that can accumulate at fungus‒plant interaction sites to confer defense responses.
Myosin proteins can mediate the transport of multiple proteins, organelles and other protein complexes. How these proteins recognize and select diverse cargoes needs to be clarified. Assuming that the amino terminal motor is responsible for actin binding, it is proposed that the carboxy terminal region accounts for the specificity and diversity of cargo-binding (Ueda et al., 2015). In accordance with these findings, in our experiments, the N-terminal motor domain was bound to the actin cytoskeleton (Figure 3), and the C-terminal CBD domain was bound to the cargo OsExo70H1 through direct protein interactions (Figure 4). Thus, these results show that the actin cytoskeleton and cargo are linked through interactions with OsMYA1 during plant defense.
In plants, vesicle trafficking plays important roles in defense against pathogens and is implicated in immune receptor activation, defense signaling, and the targeting of antimicrobial compounds to infection sites to restrain pathogen colonization (Collins et al., 2003). The EXPO is a typical type of trafficking vesicle that is involved in carrying defense cargo and contributes to secretory defense (Wang et al., 2010). The exocyst consists of eight subunits, namely, Sec. 3, Sec. 5, Sec. 6, Sec. 8, Sec. 10, Sec. 15, Exo70, and Exo84. Emerging evidence indicates that the Exo70 subunit is a key player in this protein complex involved in plant‒pathogen interactions. Exo70s mediate the targeted transport of EXPO through direct binding of Exo70A1, Exo70B1 and Exo70E2 to EXPO (Wang et al., 2010). Arabidopsis has 23 Exo70 paralogs, of which Exo70B1 and Exo70B2 have been reported to be involved in both PTI and ETI (Zhao et al., 2015; Liu et al., 2017; Wang et al., 2020). There are 41 Exo70 genes in the rice genome. Although rice Exo70B1 and Exo70F have been reported to function in rice defense (Fujisaki et al., 2015; Hou et al., 2020), it is unclear whether other members of the rice Exo70 protein family also function in plant defense and how they are regulated and transferred. In this study, our results revealed that the rice Exo70 protein OsExo70H1 is critical for rice blast defense. Knockout of OsExo70H1 leads to decreased resistance to M. oryzae infection and a decreased defense response (Figure 5). OsExo70H1 was localized to the TGN, vesicles that serve as a key sorting station at the intersection of secretory proteins (Figure 6A−D). This protein could move from the inner cytoplasm to the cell membrane (Figure 7D, E) and from the pathogen–plant interaction surface to the EIHM (Figure 6I, 7A) during M. oryzae infection. These results indicated that OsExo70H1 is involved in the secretion of post-Golgi-derived vesicles toward the PM and is involved in plant secretory defense and fungal penetration site resistance. These findings broaden our understanding of the molecular mechanism of OxExo70 proteins in plant immunity.
Syp121 (also known as PEN1) has been verified to contribute to penetration resistance in Arabidopsis (Kwon et al., 2008). It has also been proven that Arabidopsis Syp121 accumulates in apoplastic vesicles and is thus enriched in the extracellular compartment upon bacterial pathogen infection (Rutter and Innes, 2017). In rice, Syp121 accumulates at the site where an M. oryzae appressorium is inoculated (Cao et al., 2019). Similarly, we further found that rice Syp121 could be present at the PM and EIHM upon pathogen infection (Figure 8A). However, the molecular mechanism underlying the variable localization of OsSyp121 during M. oryzae infection is unclear. Our results revealed that in both the OsMYA1 and OsExo70H1 mutants, the accumulation of OsSyp121 in the cell membrane and at the site of the pathogen–plant interaction surface decreased (Figure 8A−D). Given that the localization of OsExo70H1 to the cell membrane and pathogen–plant interface was associated with OsMYA1, and that OsSyp121 directly interacted with OsExo70H1 but not with OsMYA1, we speculated that OsMYA1 drove OsExo70H1 to the cell membrane and the site of the pathogen–plant interface. OsSyp121 and the other defense-related proteins identified via IP-MS, such as OsCERK1 and OsExo70B1, may act as cargoes of the exocysts to which OsExo70H1 is located (Figure S8). Considering that OsSyp121 knockout rice plants exhibit decreased rice blast resistance (Cao et al., 2019) and that exosomes function to modulate the levels of ROS and contribute to ROS signaling (Hossain et al., 2013), we hypothesized that the decreased M. oryzae defense in both the OsMYA1 and OsExo70H1 mutants was attributed to the compromised delivery of OsSyp121 and other defense-related proteins to the cell membrane and the site of the pathogen–plant interface (Figure S11). Overall, these data revealed that OsMYA1 mediated directional vesicle transport during rice defense against M. oryzae infection.
MATERIALS AND METHODS
Plant materials and growth conditions
Rice (Zhonghua11, ZH11) was used as the WT plant. Rice mutants of OsMYA1 and OsExo70H1 were constructed with the CRISPR/Cas9 system (Weimi Biotechnology, Sanya, Hainan province, China). The rice plants used in this study were grown in fields in Fujian Province or in a greenhouse at 28°C with a 16 h:8 h light:dark cycle. N. benthamiana plants were grown in a greenhouse at 22°C with a 12 h:12 h light:dark cycle.
Plasmid construction and plant transformation
For transient protein expression in rice protoplasts and N. benthamiana cells, the open reading frames of OsMYA1 and its domains OsExo70H1 and OsSyp121 were inserted into the binary expression vector pCAMBIA1300-GFP or pCAMBIA1300-RFP to generate plasmids harboring 35S-OsMYA1-RFP, 35S-Motor-RFP, 35S-IQ-CBD-RFP, 35S-OsExo70H1-GFP, and 35S-OsSyp121-GFP via homologous recombination cloning (ClonExpress MultiS One Step Cloning Kit, Vazyme Biotech, C112, Nanjing, China). The expression of the plasmids in rice protoplasts was according to the method reported previously (Meng et al., 2020).
To generate transgenic rice plants, DNA fragments harboring the native promoter of OsExo70H1 (~1500 bp) were inserted into pCAMBIA1301-RFP to generate the pOsExo70H1::OsExo70H1-RFP plasmid. The native promoter of OsExo70H1 was cloned and inserted into pCAMBIA1301-GUS to generate the pOsExo70H1::GUS plasmid. All these plasmids were introduced into rice plants through Agrobacterium tumefaciens-mediated transformation (Xiao et al., 2023). All primers used are listed in Table S1.
Gene expression analyses
Total RNA was isolated from rice plants infected with M. oryzae (Guy11) using a Plant Total RNA Purification Kit (TransGen, EC521-11, Beijing, China) according to the manufacturer's protocol. cDNA was generated using HiScript III RT SuperMix. Real-time PCR assays were performed using SYBR qPCR Master Mix (Vazyme Biotech) with rice Ubiquitin as the internal control (Zhou et al., 2020). All reactions were conducted in triplicate, and the primers used in this study are listed in Table S1.
Oxidative burst assay
The ROS measurement assay was performed as described previously (Yang et al., 2019). The same area of rice leaf segments from the WT and the OsMYA1 and OsExo70H1 mutants were collected and mixed in ddH2O for 12 h. Then, the medium was replaced with 200 μL of fresh medium containing 20 μM luminol, 10 mg ml−1 horseradish peroxidase (Sigma) and 20 µg/mL chitin (Sigma, St. Louis, MO, USA). The luminescence was recorded by a Centro XS3 LB 960 Luminometer (Berthold Technologies, Germany).
Rice blast fungus infection assay
Blast fungus inoculation and a rice sheath penetration assay were performed in this study (Wang et al., 2018; Yang et al., 2021). For the spraying assay, a spore suspension (1 × 105 spores/mL) of the M. oryzae isolate Guy11 was sprayed evenly on 15-d-old rice plants. For the punch inoculation assay, the same amount of spore suspension was added to the injured rice leaves of 30-d-old plants. The inoculated plants were placed in dark and humid containers for 24 h. The plants were then transferred to a humid growth chamber and grown under a 12 h:12 h light:dark photoperiod. The diseased lesions were examined and measured at 4 d after inoculation.
The fungal biomass assay was performed as described previously (Park et al., 2012; Zhao et al., 2020). Total DNA was extracted from diseased rice leaves. The fungal biomass was examined by comparing the total DNA of M. oryzae Pot2 to the Ubiquitin DNA of rice (Li et al., 2017).
For rice sheath inoculation assays, spores of Guy11 tagged with GFP or RFP were diluted to a concentration of 1 × 105 spores/mL and injected into the inner leaf sheath of 2-week-old rice plants (You et al., 2023). Then, the inoculated leaf sheaths were incubated in a dark and humid chamber at 28°C. At 12, 24, 36 and 48 h after infection, fungal growth in rice leaf sheath cells was observed via confocal microscopy.
Protein interaction assays
The Y2H assay was performed as described in the manuscript (Clontech, 630494, Japan). The screening of OsMYA1-interacting proteins was performed using a previously reported cDNA library of ZH11 rice seedlings infected with M. oryzae (Hou et al., 2020). To examine the interaction between OsMYA1, OsExo70H1 and OsSyp121, the indicated AD and BD fusion constructs and the respective control vector plasmids were cotransformed into yeast cells, which were subsequently cultured on SD/−Leu/−Trp (DDO) medium. After growth at 30°C for 72 h, independent colonies harboring the target constructs were verified by PCR amplification of both cotransformed genes. Verified colonies were selected on SD/−Leu/−Trp/−Ade/−His (QDO) medium.
For the firefly luciferase complementation (LUC) imaging assay, the cDNAs of OsMYA1, OsExo70H1 and OsSyp121 were amplified and ligated to the C-terminal or N-terminal ends of split Luc. Then, the plasmids were introduced into Agrobacterium strain GV3101. Equal amounts of Agrobacterium cultures containing the indicated CLuc and NLuc construct pairs were cotransformed into N. benthamiana. The plants were grown in the dark for 48 h, and the infiltrated leaves were sprayed with 1 mM luciferin (Promega, P1042, USA) and visualized with a camera (Night Owl LB985, Berthold Technologies).
Coimmunoprecipitation assays
The indicated OsExo70H1-RFP and GFP, CBD-GFP, or OsSyp121-GFP were coexpressed in rice protoplasts. Total protein was subsequently extracted using extraction/wash buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.1% Triton X-100; 0.2% Nonidet P-40; 1 mM DTT; and 1× complete protease inhibitor [Roche, 04906845001, Germany]). Anti-GFP antibody-conjugated agarose beads (Sigma) were added to the supernatant of the protein samples. After incubation, the pellet beads were washed at least five times. The bound proteins were diluted and separated by SDS‒PAGE and then subjected to immunoblot analysis using an anti-GFP antibody (Abmart, M20004L, 1:10,000 dilution) or anti-RFP antibody (Abmart, MA9041, 1:5,000 dilution).
PM protein extraction and immunoblotting analysis
The plant PM protein was extracted using a Minute Plasma Membrane Protein Isolation Kit (Invent Biotechnologies, SM-005, Beijing, China) as previously reported (Shen et al., 2017; Chen et al., 2022). Rice plants were infected with M. oryzae for 12, 24 or 36 h, after which PM proteins were extracted according to the manufacturer's instructions. GFP or RFP antibodies (Abmart, M20004L for GFP and Abmart, MA9041 for RFP) were used for the evaluation of the protein accumulation of OsSyp121 and OsExo70H1 in the PM portion, respectively.
Callose deposition and confocal microscopy
Leaves from 2-week-old rice plants (both the WT and the OsMYA1 and OsExo70H1 mutants) were incubated with 1 μM chitin in 10 mM MgCl2 and exposed to a −70 kPa vacuum for 2 h. The callose was stained with 0.1% aniline blue. The aniline blue fluorochrome was excited with a 405 nm laser, and the callose was imaged at 440–490 nm via confocal microscopy. For in vivo localization of fluorescently tagged proteins in protoplasts, plant sheath cells or tagged M. oryzae, GFP was excited at 488 nm and collected at 515–525 nm; RFP was excited at 561 nm and collected at 600–640 nm via confocal microscopy (Zeiss 880, Germany).
Phylogenetic analysis
The neighbor-joining method was used to construct a phylogenetic tree of OsMYA1, OsExo70H1 and Arabidopsis class XI Myosin and Exo70 proteins using MEGA (www.megasoftware.net).
Accession numbers
Sequence data for the rice genes described in this study can be found in the databases of the Rice Resource Center (http://113.54.11.180/) under the following accession numbers: OsZH11G0305452000 (OsMYA1), OsZH11G1127022600 (OsExo70H1) and OsZH11G0305781300 (OsSyp121), LOC_Os03g58840 (OsVAMP721), LOC_Os02g51430 (OsSec. 6); Sequence for the Arabidopsis genes used in phylogenetic analysis was downloaded from the TAIR database (https://www.arabidopsis.org/) under the following accession numbers: At3g19960 (AtATM), At5g54280 (AtATM2), At1g50360 (AtVIIIA), At4g27370 (AtVIIIB), At1g17580 (AtMYA1), At5g43900 (AtMYA2), At1g04600 (AtXIA), At1g04160 (AtXIB), At1g08730 (AtXIC), At2g33240 (AtXID), At1g54560 (AtXIE), At2g31900 (AtXIF), At2g20290 (AtXIG), At4g28710 (AtXIH), At4g33200 (AtXI-I), At3g58160 (AtXIJ), At5g20490 (AtXIK), At5g03540 (AtExo70A1), At5g52340 (AtExo70A2), At5g52350 (AtExo70A3), At5g58430 (AtExo70B1), At1g07000 (AtExo70B2), At5g13150 (AtExo70C1), At5g13990 (AtExo70C2), At1g72470 (AtExo70D1), At1g54090 (AtExo70D2), At3g14090 (AtExo70D3), At3g29400 (AtExo70E1), At5g61010 (AtExo70E2), At5g50380 (AtExo70F1), At4g31540 (AtExo70G1), At1g51640 (AtExo70G2), At3g55150 (AtExo70H1), At2g39380 (AtExo70H2), At3g09530 (AtExo70H3), At3g09520 (AtExo70H4), At2g28640 (AtExo70H5), At1g07725 (AtExo70H6), At5g59730 (AtExo70H7) and At2g28650 (AtExo70H8).
ACKNOWLEDGEMENTS
This work was funded by the National Key Research and Development Program of China (2022YFF1001500); the National Natural Science Foundation of China (grant numbers 31970284, 31900385); the Natural Science Foundation of Fujian Province, China (2023J01483, 2022J01616); the Distinguished Young Scientists Fund of Fujian Agriculture and Forestry University of China (xjq202121); and the Fujian Provincial Science and Technology Key Project (2022NZ030014).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Y.B.L. performed most of the research and drafted the manuscript. C.Y.L., N.N.S., S.Z., X.Y.D. and Z.X.L. participated in experiments. L.B.H. carried out electron microscopy analysis and contributed to manuscript writing and revising. D.Z.T. designed the experiments, supervised the study, and revised the manuscript. All authors read and approved of its content.






