Light-stabilized GIL1 suppresses PIN3 activity to inhibit hypocotyl gravitropism
Edited by: Hongtao Liu, Center for Excellence in Molecular Plant Sciences, Institute of Plant Physiology and Ecology, CAS, China
ABSTRACT
Light and gravity coordinately regulate the directional growth of plants. Arabidopsis Gravitropic in the Light 1 (GIL1) inhibits the negative gravitropism of hypocotyls in red and far-red light, but the underlying molecular mechanisms remain elusive. Our study found that GIL1 is a plasma membrane-localized protein. In endodermal cells of the upper part of hypocotyls, GIL1 controls the negative gravitropism of hypocotyls. GIL1 directly interacts with PIN3 and inhibits the auxin transport activity of PIN3. Mutation of PIN3 suppresses the abnormal gravitropic response of gil1 mutant. The GIL1 protein is unstable in darkness but it is stabilized by red and far-red light. Together, our data suggest that light-stabilized GIL1 inhibits the negative gravitropism of hypocotyls by suppressing the activity of the auxin transporter PIN3, thereby enhancing the emergence of young seedlings from the soil.
INTRODUCTION
Gravity is a ubiquitous physical force on earth, acting on all organisms, including plants. Generally, plant shoots show upward growth, and roots show downward growth, which are named as negative gravitropism and positive gravitropism, respectively (Barlow, 1995; Vandenbrink and Kiss, 2019). Gravitropism regulates many agricultural traits via controlling the growth direction and architecture of shoots and roots (Yu et al., 2007; Uga et al., 2013; Huang et al., 2018). Plant gravitropism can be divided into three sequential processes: gravity sensing, signal transduction, and differential growth (Morita and Tasaka, 2004; Morita, 2010; Vandenbrink and Kiss, 2019). Two widely accepted theories, the starch–statolith hypothesis and the Cholodny–Went theory, respectively explain gravity sensing and responses in plants (Chin and Blancaflor, 2022). Recent works from us and others revealed that amyloplasts sedimentation guides LAZY proteins to repolarize on the plasma membrane of statocytes to control gravitropism, which provides molecular interpretation for the starch–statolith hypothesis (Chen et al., 2023; Nishimura et al., 2023). The Cholodny–Went theory proposed that an asymmetric auxin distribution between the two sides of the organs in plants triggers differential growth and thus bending (Went and Thimann, 1937). The auxin exporter PIN3 was demonstrated to be required for both shoot and root gravitropism (Friml et al., 2002; Rakusová et al., 2011). It has been well established that PIN-mediated auxin fluxes play a major role in the asymmetric distribution of auxin and organ bending during gravitropism (Wisniewska et al., 2006; Kleine-Vehn et al., 2010; Su et al., 2017). The asymmetric expression of Small Auxin Up RNA (SAUR) and METHYL ESTERASE17 (MES17) genes mediated by auxin promotes the gravitropism of plant hypocotyls (Wang et al., 2020; Zhang et al., 2022).
Light is another key environmental factor, and red light exposure led to the randomization of hypocotyl orientation (RHO) (Nagashima et al., 2008; Vandenbrink et al., 2014), which benefits the survival of young seedlings during their emergence from the soil (Schepens et al., 2008). Mutation of phytochromes A (phyA) and B resulted in lacking RHO phenotype in red light (Poppe et al., 1996; Robson and Smith, 1996). At low fluence rates of blue light, suppression of gravitropism is also due to the action of phyA (Lariguet and Fankhauser, 2004). Phytochrome kinase substrate 4 (PKS4) was proposed to inhibit changes in growth orientation under red or far-red light based on the characterization of pks4 mutants (Schepens et al., 2008). Phytochrome-interacting factors (PIFs) suppress conversion of gravity-sensing starch-filled amyloplasts into other plastids in darkness, and light-activated phytochromes promote the degradation of PIFs to inhibit hypocotyl negative gravitropism by regulating the development of amyloplasts (Kim et al., 2011; Kim et al., 2016). Our previous study showed that light inhibits the negative gravitropism of shoots and promotes the positive gravitropism of roots through organ-specific PIFs and ELONGATED HYPOCOTYL 5 (HY5) regulation of LAZY4 expression (Yang et al., 2020).
The crosstalk between light and gravity signaling clearly exists, but the underlying mechanisms are still waiting to be further studied. Genetic screening isolated the mutant gil1 (for gravitropic in the light), which showed better negative gravitropism compared to wild type under red or far-red light irradiation (Allen et al., 2006). When seeds were sown on moist soil with mulch, the number of gil1 seedlings that emerged was significantly reduced compared to the wild type. This suggests that the light-mediated randomization of hypocotyl growth orientation, which depends on GIL1, may confer a fitness advantage to Arabidopsis seedlings emerging from beneath ground cover into the light (Allen et al., 2006). However, the biochemical activity of GIL1 involved in light-mediated gravitropism remains totally unclear. Therefore, revealing the underlying molecular function of GIL1 will deepen our understanding of the intricate crosstalk between light and gravity signaling.
In this study, we examined the molecular mechanism of GIL1 in regulating gravitropism and discovered that GIL1 is stabilized by light and suppresses the auxin transport activity of PIN3. This suppression results in the inhibition of negative gravitropism in hypocotyls. Consequently, our research uncovers a new level of coordination between light and gravity signaling.
RESULTS
Expression pattern and subcellular localization of GIL1
To elucidate the expression pattern of GIL1, we constructed a pGIL1:GUS transgenic plant. In both darkness and red light, GIL1 was ubiquitously expressed in cotyledon, hypocotyl and root (Figure 1A, B). Further, we compared the expression of GIL1 under darkness, red light, far-red light and blue light using real-time polymerase chain reaction (PCR). Using 1.5×-fold change as the threshold (Darwish et al., 2022), the transcriptional levels of GIL1 after different light treatments were not obviously different compared to under darkness (Figure 1C). Compared to Col wild type, gil1-2 exhibited significantly better negative gravitropism when the seedlings were grown vertically in red light, but not in dark, far-red light, or blue light conditions (Figure S1). Additionally, gil1-2 showed an enhancement of negative hypocotyl gravitropism when exposed to far-red light pulses (Figure S2). The phenotypes of gil1-2 under red light and far-red light are consistent with previous results (Allen et al., 2006). Further studies were carried out mainly in red light, in which GIL1 showed the most obvious effect on gravitropism. GIL1-GFP driven under its native promoter was expressed in the gil1-2 mutant, and the transgene rescued the mutant phenotype under red light (Figure 1D, E). Previous studies have showed that mutation of red/far-red light receptors phyA and phyB resulted in better negative gravitropism of hypocotyls in red light (Poppe et al., 1996; Robson and Smith, 1996). We compared the gravitropic phenotype of hypocotyls in Col, gil1-2, pGIL1:GIL1-GFP/gil1-2, phyA, phyB and phyA phyB seedlings in parallel under red light conditions (Figure 1D, E). The gravitropic phenotype of gil1-2 is similar to that of phyA phyB double mutant, suggesting that GIL1 plays a crucial role in the RHO induced by red light.
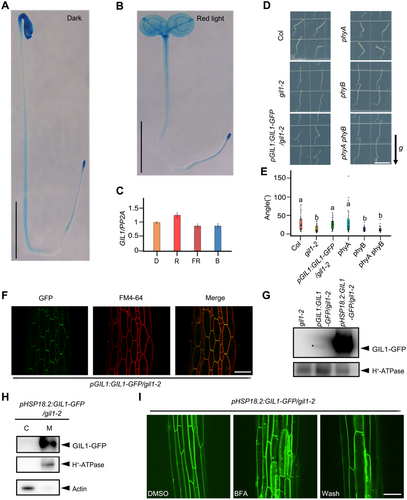
The expression pattern and subcellular localization of Gravitropic in the Light 1 (GIL1)
(A, B) Histochemical staining of 4-d-old pGIL1:GUS seedlings growing under darkness (A), or red light (B). Scale bars, 0.5 cm. (C) The transcriptional levels of GIL1 in wild type Col seedlings under different light conditions. Data are mean ± SEM. D, darkness; R, red light; FR, far-red light; B, blue light. (D, E) Col, gil1-2, pGIL1:GIL1-GFP/gil1-2, phyA, phyB and phyA phyB seedlings were grown vertically in red light. (D) Representative seedlings. Scale bar, 1 cm. The arrow labeled “g” indicates the direction of gravity. (E) One-way analysis of variance (ANOVA) was used to assess the statistical significance of the angles between the directions of the hypocotyls and negative gravity vector (alpha = 0.05; n > 24). (F) Hypocotyls of 3-d-old pGIL1:GIL1-GFP/gil1-2 seedlings were stained with FM4-64 (5 μmol/L). The fluorescence was collected by confocal microscopy. Scale bar, 50 μm. (G) Abundance of GIL1 protein in 4-d-old seedlings grown in red light. pHSP18.2:GIL1-GFP/gil1-2 seedlings were treated in 37°C for 3 h. Membrane proteins were extracted and subjected to immunoblot analysis using antibodies against green fluorescent protein (GFP) and H+-ATPase (adenosine triphosphatase). gil1-2 was used as a control. The asterisk indicates the position of GIL1-GFP protein. (H) Four-d-old pHSP18.2:GIL1-GFP/gil1-2 seedlings were treated in 37°C for 3 h. Membrane and cytosolic proteins were extracted separately, and immunoblot assay was carried out using antibodies against GFP, H+-ATPase, and actin. H+-ATPase, and actin are representatives of membrane and cytoplasmic proteins, respectively. C, cytosolic fraction; M, membrane fraction. (I) Subcellular localizations of GIL1-GFP in the hypocotyls of the seedings with dimethylsulfoxide (control) or Brefeldin A (BFA) (100 μmol/L) treatment for 1 h. BFA treated seedlings were then washed using liquid 1/2 Murashige and Skoog medium for another 1 h. Scale bar, 50 μm.
Consistent with the β-glucuronidase (GUS) expression results, the fluorescence signal of GIL1-GFP (green fluorescent protein) was widely detected in cotyledon, hypocotyl and root (Figure S3). We stained pGIL1:GIL1-GFP/gil1-2 seedlings with the membrane dye FM4-64 and found that GIL1-GFP co-localized with FM4-64 on the plasma membrane of hypocotyl cells (Figure 1F). Due to the low expression of GIL1-GFP under its native promoter, we generated GIL1-GFP driven by heat shock promoter pHSP18.2, which significantly increased the GIL protein level (Figure 1G). We preformed cell fractionation experiments using pHSP18.2:GIL1-GFP/gil1-2 seedlings and found that GIL1-GFP was specifically detected in the membrane pellet (Figure 1H). Moreover, pHSP18.2:GIL1-GFP/gil1-2 seedlings were treated with the vesicle trafficking inhibitor, Brefeldin A (BFA), and GIL1-GFP fluorescence was found to be aggregated in BFA bodies (Figure 1I). When the BFA was washed out, GIL1-GFP signal in BFA bodies almost disappeared (Figure 1I). Together these results suggest that GIL1 is a membrane-localized protein expressed widely in plant organs, and that its subcellular localization is mediated by the BFA-sensitive secretion pathway.
GIL1 inhibits the negative gravitropism of hypocotyls in endodermal cells of the upper part of hypocotyls
To identify the localization of GIL1 essential for its function in inhibiting negative hypocotyl gravitropism, we generated transgenic lines expressing GIL1-GFP under the control of different promoters in the gil1-2 mutant background. Based on previous studies, pCAB3, pPKS4 and pCASP1 promoters were selected to drive GIL1-GFP, which are mainly expressed in the cotyledons, upper part of hypocotyls and lower part of hypocotyls, respectively (Endo et al., 2007; Schepens et al., 2008; Roppolo et al., 2011). Although the fluorescence of GIL1-GFP in these three transgenic lines was too low to be detected, we found that transformation with GIL1 driven by the PKS4 promoter complemented the gravitropic phenotype of gil1-2, whereas GIL1 driven by CAB3 and CASP1 promoters did not (Figure 2A, B). These results indicate that GIL1 expressed in the upper part of hypocotyls can inhibit the negative hypocotyl gravitropism under red light. Endodermal cells are essential for the gravitropism of hypocotyls, and scr and shr mutants showed defects in forming endodermal cells and lost the response to gravistimulation (Tasaka et al., 1999). To investigate the function of GIL1 in endodermal cells, we generated transgenic plants expressing pSCR:GIL1 in the gil1-2 mutant background. The pSCR:GIL1/gil1-2 plants exhibited reduced gravitropic hypocotyl responses compared to gil1-2 under red light (Figure 2C, D), indicating that GIL in endodermal cells can inhibit the negative gravitropism of hypocotyls. Together, our data suggest that the function of GIL1 during inhibition of hypocotyl gravitropism is dependent on its localization in endodermal cells of the upper part of hypocotyls.

Gravitropic in the Light 1 (GIL1) expressed in the upper part of hypocotyls or endodermal cells complements the hypocotyl gravitropic response of gil1-2 mutant
(A, C) Seedlings grown vertically in red light for 3 d. GIL1 driven by the promoters of CAB3, PKS4 and CASP1 were mainly expressed in the cotyledons, upper and lower parts of the hypocotyls of gil1-2, respectively (A). GIL1 driven by the promoter of SCR was specifically expressed in the endodermal cells of gil1-2 (C). Scale bars, 0.75 cm. (B, D) One-way ANOVA was used to assess the statistical significance of the angles between the directions of the hypocotyls and negative gravity vector in A and C (alpha = 0.05; n > 30). In A and C, arrows labeled “g” indicate the direction of gravity.
GIL1 interacts with PIN3 and inhibits its activity to interrupt gravitropism
To identify the possible working mechanism of GIL1, we studied whether GIL1 regulates the development of amyloplasts, which are involved in gravity sensing (Caspar and Pickard, 1989; Kiss et al., 1989). Previous studies have shown that PIFs sustain the starch accumulation in amyloplasts, which is very important for the negative gravitropism of hypocotyls (Shin et al., 2009; Kim et al., 2011). In red light, epidermal phyB induced the degradation of PIF1 and inhibited the negative gravitropic growth of hypocotyls (Kim et al., 2016). To determine whether GIL1 also inhibits negative hypocotyl gravitropism through the suppression of starch accumulation, we compared the amyloplast morphology in the hypocotyls of Col and gil1-2 by transmission electron microscope (TEM). We found that the morphology of amyloplasts is similar in the endodermal cells of the hypocotyls of Col and gil1-2 growing in red light (Figure S4), which indicates that GIL1 may not affect hypocotyl negative gravitropism by mediating the development of amyloplasts.
Further, we investigated whether GIL1 inhibits the gravitropism via targeting factors mediating auxin distribution. As an auxin efflux carrier, PIN3 is essential for the asymmetric auxin distribution controlling negative hypocotyl gravitropism. PIN3 is localized on the plasma membrane, which is mediated by BFA-sensitive membrane protein recycling (Rakusová et al., 2011; Rakusová et al., 2016). Also, GIL1 and PIN3 could colocalize on the plasma membrane in the hypocotyls of Arabidopsis (Figure 3A). Based on these similar cellular characteristics, we tested the direct interaction between GIL1 and PIN3. Yeast two-hybrid assay showed that GIL1 interacted with the hydrophilic PIN3 loop (PIN3-HL) (Figure 3B). In addition, in in vitro pull-down assays, the maltose-binding protein (MBP)-tagged PIN3-HL was able to pull down glutathione S-transferase (GST)-tagged full-length GIL1, whereas MBP alone did not (Figure 3C). To confirm this interaction in vivo, we extracted membrane proteins from 4-day-old pPIN3:PIN3-GFP/pHSP18.2:GIL1-mCherry and pPIN3:PIN3-GFP seedlings growing under red light and carried out a co-immunoprecipitation (Co-IP) assay. The results revealed that PIN3-GFP associates with GIL1-mCherry in plants (Figure 3D). Moreover, we performed a firefly luciferase (LUC) complementation imaging (LCI) assay. Co-expression of GIL1-nLUC with cLUC-PIN3-HL in tobacco (Nicotiana benthamiana) leaves resulted in high LUC activity, whereas control combinations showed no signal (Figure 3E). These results indicate that GIL1 directly interacts with PIN3.
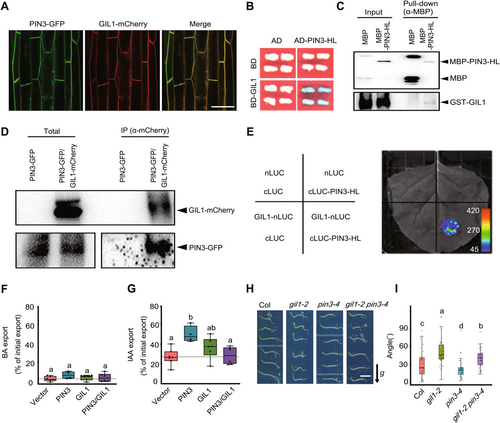
Gravitropic in the Light 1 (GIL1) interacts with PIN3 and inhibits the auxin export activity of PIN3
(A) GIL1 and PIN3 colocalize on the plasma membrane in the hypocotyls of Arabidopsis. Four-d-old pPIN3:PIN3-GFP/pHSP18.2:GIL1-mCherry seedlings grown in red light were treated in 37°C for 3 h, and the fluorescence was collected by confocal microscopy. Scale bar, 50 μm. (B) GIL1 interacts with PIN3-HL (hydrophilic loop) in yeast two-hybrid assay. GIL1 was fused with DNA binding domain (BD) as the bait. PIN3-HL fragment was fused with activated domain (AD) as the prey. Blue indicates positive interactions. Reporter: LacZ; substrate, X-Gal. (C) GIL1 interacts with PIN3-HL in an in vitro pull-down assay. Maltose-binding protein (MBP) or MBP-tagged PIN3-HL were mixed with glutathione S-transferase (GST)-GIL1 proteins. Then the anti-MBP antibody was used to pull down MBP and MBP-PIN3-HL, and the precipitates and inputs were subjected to immunoblot analyses with antibodies against MBP and GST. (D) GIL1 associates with PIN3 in vivo. PIN3-GFP and PIN3-GFP/GIL1-mCherry seedlings were grown in red light for 4 d, and then membrane proteins were extracted and incubated with beads that can bind mCherry-tagged proteins. The total extracts and precipitates were subjected to immunoblot analyses with antibodies against mCherry and green fluorescent protein (GFP). (E) GIL1 interacts with PIN3 in luciferase (LUC) complementation imaging (LCI) assay using tobacco leaves grown in red light. GIL1 and PIN3-HL were fused with nLUC and cLUC, respectively. Nucleotides ATG was added into pCAMBIA-nLUC vector to make the nLUC translatable. The pseudo color bar shows the range of luminescence intensity. (F, G) GIL1 inhibits PIN3-mediated indolylacetic acid (IAA) but not benzoic acid (BA) export out of protoplast prepared from tobacco leaves transfected with PIN3 and GIL1. BA was used as a control. One-way ANOVA was used to assess the statistical significance of PIN3 export activity (alpha = 0.05; n = 4 to 6 independent transfections and protoplast preparations). (H, I) Mutation of PIN3 suppresses the gravitropic responses of gil1 mutant in red light. (H) Seedlings of Col, gil1-2, pin3-4 and gil1-2 pin3-4 were grown vertically in darkness for 2 d, and then were transferred into red light and rotated 90° to the horizontal position for gravistimulation for 24 h. Scale bar, 0.5 cm. The arrow labeled “g” indicates the direction of gravity. (I) One-way ANOVA was used to assess the statistical significance of the hypocotyl curvature angles relative to the horizontal direction (alpha = 0.05; n > 35).
To study a possible regulatory impact of GIL1 on PIN3 activity, we expressed PIN3-RFP (red fluorescent protein) and GIL1-GFP independently and together in tobacco protoplasts by using Agrobacterium-mediated leaf transfection (Henrichs et al., 2012), and quantified efflux of radiolabeled indolylacetic acid (IAA). Benzoic acid (BA) served as the diffusion control, and its export was unaffected by PIN3 and GIL1 (Figure 3F). As anticipated, PIN3 exhibited notably heightened auxin export activity in comparison to the vector control (Figure 3G). The co-expression of GIL1 alongside PIN3 led to comparable IAA export levels as those observed in the vector control, suggesting that GIL1 significantly diminished PIN3-facilitated IAA export activity (Figure 3G).
To analyze the genetic relationship between GIL1 and PIN3, we crossed gil1-2 with pin3-4 and analyzed the negative hypocotyl gravitropism of the gil1-2 pin3-4 double mutant. Upon gravistimulation in red light, gil1-2 and pin3-4 showed increased and decreased hypocotyl gravitropic responses compared to wild type, respectively. The double mutant gil1-2 pin3-4 exhibited slower kinetics of hypocotyl bending compared to gil1-2 in red light (Figure 3H, I), indicating that mutation of PIN3 suppresses gravitropic response of the gil1 mutant. These data suggest that GIL1 may work upstream of PIN3 in mediating gravitropic responses in hypocotyls.
Light stabilizes GIL1 protein
Since the effects of GIL1 on the negative gravitropism of hypocotyls were various under different light conditions (Figures S1, S2), while its transcriptional levels did not show obvious change under various light conditions (Figure 1C), we investigated whether the abundance of GIL1 protein is regulated by light conditions. By comparing to under darkness, the abundance of GIL1 protein was dramatically increased in seedlings grown in red or far-red light, whereas slightly increased in blue light (Figure 4A, B). The abundance of GIL1 is consistent with the gravitropic phenotype of gil1 mutant under these light conditions. During darkness to red light transition, GIL1 was gradually accumulated with prolonged red light treatment (Figure 4C). Further, we used the heat shock promoter driven GIL1-GFP (pHSP18.2:GIL1-GFP) to study the protein stability of GIL1. After heat shock at 37°C, GIL1 protein abundance in pHSP18.2:GIL1-GFP/gil1-2 seedlings could be accumulated in both darkness and under red light. When the heat shocked seedlings were placed back to regular temperature (22°C) for 5 h, most of the GIL1 protein in seedlings grown under darkness was degraded, whereas GIL1 proteins in seedlings grown in red light remained stable (Figure 4D). These data suggest that GIL1 protein is unstable in the dark, whereas light can enhance its stability. Together, these data indicate that light stabilizes GIL1 protein to inhibit the negative gravitropism of hypocotyls.
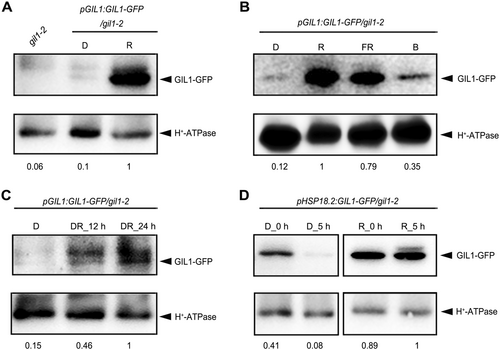
Light stabilizes Gravitropic in the Light 1 (GIL1) protein
(A) GIL1 protein is accumulated more in red light compared to in darkness. Membrane proteins were extracted from 4-d-old pGIL1:GIL1-GFP/gil1-2 seedlings grown in darkness (D) or red light (R) conditions, and subjected to immunoblot analysis with antibodies against green fluorescent protein (GFP) and H+-ATPase (adenosine triphosphatase). gil1-2 grown in red light was used as a control. (B) Abundance of GIL1 protein under various light conditions. Membrane proteins were extracted from 4-d-old pGIL1:GIL1-GFP/gil1-2 seedlings grown in darkness (D), red light (R), far-red light (FR) and blue light (B), and subjected to immunoblot analysis with antibodies against GFP and H+-ATPase. (C) Abundance of GIL1 protein increases during dark to red light transition. pGIL1:GIL1-GFP/gil1-2 seedlings were grown in darkness (D) for 4 d, and then transferred into red light and kept for 12 h (DR_12 h) or 24 h (DR_24 h). Membrane proteins were extracted and subjected to immunoblot analysis with antibodies against GFP and H+-ATPase. (D) Red light promotes the stability of GIL1 protein. Four-d-old pHSP18.2:GIL1-GFP/gil1-2 seedlings grown in darkness (D) or red light (R) were treated with 37°C for 3 h and put back to 22°C. Membrane proteins were extracted from the seedlings collected immediately after 37°C treatment (0 h), or after being kept in 22°C for another 5 h (5 h), and subjected to immunoblot analysis with antibodies against GFP and H+-ATPase. In A to D, H+-ATPase was used as a loading control. The intensities of GIL1-GFP were quantified using ImageJ and normalized relative to H+-ATPase. Then, the highest intensity of GIL1-GFP in each panel was set to 1, and relative intensities of GIL1-GFP under other conditions were calculated and indicated by numbers below blots.
DISCUSSION
Based on our and previous studies, we propose a model that describes how GIL1 mediates gravitropism (Figure 5). In dark conditions, GIL1 is unstable and difficult to accumulate, while PIN3 promotes polar auxin transport, which is crucial for the negative gravitropism of hypocotyls. Under light, particularly red light, GIL1 protein is stabilized. The stabilized GIL1 interacts with PIN3 and inhibits its auxin efflux activity, ultimately leading to gravitropic defects of the hypocotyl (Figure 5).
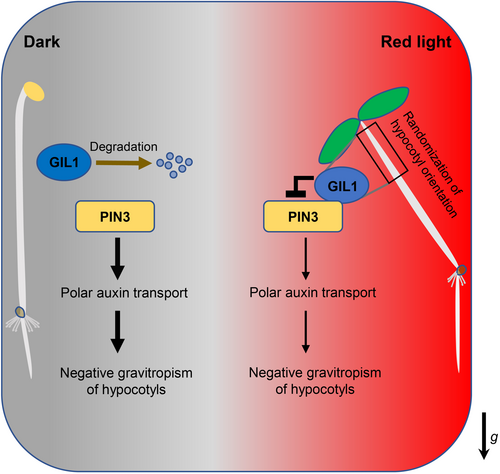
The model of Gravitropic in the Light 1 (GIL1) mediating gravitropism of hypocotyls in Arabidopsis
In darkness, GIL1 protein is unstable and difficult to accumulate. Auxin transporter PIN3 promotes polar auxin transport to maintain the negative gravitropism of hypocotyls. In light, especially red light, GIL1 protein becomes stabilized and accumulates. GIL1 interacts with PIN3 and inhibits its transporting activity, leading to suppression of polar auxin transport and negative gravitropism of hypocotyls. The impairment of negative gravitropism in hypocotyls causes randomization of hypocotyl orientation (RHO). The arrow labeled “g” indicates the direction of gravity.
Our study revealed that GIL1 protein is unstable in darkness, but the underlying mechanism is unclear. Under dark condition, the 26S proteasome pathway mediates degradation of many light-stimulated proteins (Xu et al., 2015). For example, E3 ligases containing CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) promotes the degradation of transcriptional factor HY5 in darkness, whereas light inhibits COP1 activity to stabilize HY5 protein (Osterlund et al., 2000; Chen et al., 2010). Identifying the E3 ligase or other factors responsible for the degradation of GIL1 protein would reveal more about the underlying mechanisms of GIL1 stability regulation.
Light stabilizes GIL1 in a wavelength-dependent manner, and red and far-red light showed much stronger stabilization capability than blue light (Figure 4B), which correlates with the phenotypes well (Figures S1, S2). The RHO phenotype is mediated by photoreceptor phyA in far-red light, and by the action of phyA and phyB redundantly in red light (Poppe et al., 1996; Robson and Smith, 1996). It is plausible that these photoreceptors protect GIL1 from being degraded under red or far-red light, and the detailed mechanism needs to be studied in the future.
Gravity-induced PIN proteins polarization is a critical step in asymmetric auxin distribution and organ bending (Friml et al., 2004; Kleine-Vehn et al., 2010; Roychoudhry et al., 2023). In roots, the polarity of PIN3 occurs in columella cells after gravistimulation, with more proteins localized at the lower side of columella cells (Friml et al., 2002). In shoots, gravistimulation polarizes PIN3 to the bottom side of hypocotyl endodermal cells, which mediate the asymmetric distribution of auxin for gravitropic shoot bending (Rakusová et al., 2011). Comparing the localization of PIN3 in shoot endodermal cells between wild type plants and gil1 mutants after gravistimulation in red light could potentially reveal whether GIL1 mediates PIN3 polarization. This might uncover an additional regulatory mechanism, beyond the inhibition of PIN3 auxin efflux activity by GIL1 that has already been established in this study.
The light-mediated RHO phenotype decreases the negative gravitropism of hypocotyls and reinforces hypocotyl phototropism, ultimately improving the survival rate of young seedlings as they emerge from the soil (Schepens et al., 2008). A previous report showed that when mulch was present, the number of gil1-1 seedlings that emerge was much reduced compared with Ws wild type (Allen et al., 2006). Our repeated experiments using gil1-2 in the Col background confirmed that mutating GIL1 reduced the percentage of seedling emergence when the seeds were covered with soil (Figure S5A–C). Further, we measured the light intensity penetrating through soil, and found that the penetration of red or far-red light through soil is significantly stronger than that of blue light (Figure S5D), which is consistent with a previous report that longer wavelength lights show greater penetration through soil (Bliss and Smith, 1985). Together, in nature, red or far-red light penetrate the soil much more easily than blue light, stabilizing the GIL1 proteins to inhibit the negative gravitropism of the seedling hypocotyls, and thus enabling them to emerge better from the soil.
There are several revealed mechanisms regarding how red light suppresses negative gravitropism of hypocotyls. Phytochromes may enhance hypocotyl bending by suppressing the expression of PGP19, which is involved in auxin transport (Nagashima et al., 2008). PIF1 regulates plastid development by repressing photosynthetic genes in the endodermis (Kim et al., 2016). Hence, phytochromes, when activated by light, facilitate the degradation of PIFs, thereby inhibiting hypocotyl negative gravitropism. This occurs through the promotion of the conversion of gravity-sensing, starch-filled amyloplasts into other types of plastids (Kim et al., 2011). Our previous studies revealed that light promotes the degradation of PIF proteins to inhibit the expression of LAZY4, which is a key positive regulator in gravity sensing, resulting in inhibition of gravitropic response of hypocotyls (Yang et al., 2020; Chen et al., 2023). This study reveals that light stabilizes GIL1 protein to inhibit gravitropism via inhibiting the auxin efflux activity of PIN3, thus bringing new insights about the inhibition of light on negative gravitropism of hypocotyls. Together, light may target multiple components involved in gravity signaling, such as gravity sensing and auxin transport, to mediate gravitropism.
MATERIALS AND METHODS
Plant materials and growth conditions
The Arabidopsis thaliana gil1-2 (SALK_041346) and pin3-4 (SALK_005544) are in the Col ecotype. Seeds were sterilized by 10% NaClO for 10 min, washed in sterile distilled water at least three times, and cold-treated at 4°C for 3 to 4 d in darkness. The seeds were sown on Murashige and Skoog (MS) medium (4.4 g/L MS, 0.56 g/L 2-[N-morpholino]ethanesulfonic acid, and 8 g/L agar, pH 5.7–5.9), and treated in white light (70 μmol/m2/s) at 22°C for 12 h to induce germination. Then, then seedlings were grown in darkness, red light (5 μmol/m2/s), far-red light (5 μmol/m2/s) or blue light (5 μmol/m2/s). Seedling emergence tests were conducted according to the methods previously described (Allen et al., 2006). Seeds of Col or gil1-2 were spread on the surface of soil or covered with 1 cm nutrient soil. The pots were placed at 4°C for 4 d in darkness and then transferred to a greenhouse. Seedling emergence was analyzed after 1 week.
Generation of transgenic plants
To generate the GIL1-GFP expressing construct, the full-length coding sequence (CDS) of GIL1 (without stop codon) and native promoter of GIL1 (2052 bp) were cloned and inserted into the Xba I/Xho I and Sbf I/Xba I sites of pJim19-GFP (Sun et al., 2016), yielding pGIL1:GIL1-GFP. Further, the GIL1 promoter was replaced by HSP18.2 promoter (720 bp), CAB3 promoter (1537 bp), PKS4 promoter (1606 bp), and CASP1 promoter (1144 bp), respectively. For the construction of pSCR:GIL1, CDS of GIL1 was inserted into the Xho I/Afl II sites of pJim19 vector, and SCR promoter (1627 bp) was digested by Sbf I/Xho I and ligated into pJim19-GIL1. All the promoter sequences were amplified from genomic DNA. To generate stable transgenic plants, these constructs in Agrobacterium tumefaciens GV3101 were transformed into gil1-2 plants via the floral dip method (Clough and Bent, 1998). Transgenic plants were selected on MS medium containing 20 mg/L Basta. To obtain pPIN3:PIN3-GFP/pHSP18.2:GIL1-mCherry (abbr.:PIN3-GFP/GIL1-mCherry), the construct of pHSP18.2:GIL1-mCherry was transformed into pPIN3:PIN3-GFP plants (Zadnikova et al., 2010).
β-glucuronidase histochemical assay
β-glucuronidase activity assays were performed following the previous protocol (Li, 2011). In brief, plants were submerged in 90% acetone for 20 min on ice and subsequently washed by staining buffer (50 mmol/L sodium phosphate buffer pH 7.2, 0.2% Triton X-100, 2 mmol/L K4[Fe(CN)6] 3H2O, 2 mmol/L K3[Fe(CN)6]). X-Gluc was added into staining buffer to a final concentration of 2 mmol/L. Plants were immersed, vacuum infiltrated in the staining buffer for 1 h, and then incubated at 37°C for 24 h. After staining, chlorophyll was removed by immersing the seedlings in 70% ethanol for 30 min.
Measurement of hypocotyl negative gravitropism
For vertically growing seedlings, the angles between hypocotyl growth orientation and negative gravity vector were measured by ImageJ software (https://imagej.nih.gov/ij/index.html). For gravistimulation assay, seedlings were grown vertically in darkness for 2 d, and then transferred into red light and rotated 90° to the horizontal position. The angles between hypocotyl growth orientation and horizontal direction were measured by ImageJ software. One-way analysis of variance was used to assess the statistical significance.
Confocal microscopy
Fluorescence of GFP tagged protein was excited with a 488 nm laser and emission signal was collected in the 500–550 nm range using a Zeiss LSM 710 confocal microscope or spinning-disk confocal microscopy (Andor Dragonfly 200). FM4-64 was used to label the plasma membrane, which was excited with a 514 nm laser and the emission signal was collected in the 580–640 nm range. Brefeldin A treatment and wash out was performed as previously described (Huang and Zhang, 2020).
Membrane protein extracts
Arabidopsis seedlings were ground into powder in liquid nitrogen and homogenized with 1× EB buffer (100 mmol/L Tris-HCl pH 7.5, 10 mmol/L ethylenediaminetetraacetic acid pH 8.0, 10 mmol/L ethyleneglycoltetraacetic acid pH 8.0, 5 mmol/L KCl, 25% sucrose, 5% glycerol, 20 mmol/L disodium β-glycerophosphate, 20 mmol/L Na3VO4, 50 mmol/L NaF) containing 1 mmol/L phenylmethylsulfonyl fluoride and 1× cocktail of protease inhibitors. After incubation on ice for 5 min, the mixture was centrifuged at 600 g for 3 min at 4°C. The supernatant was further isolated by ultracentrifugation at 55,000 g for 1 h at 4°C to obtain pellet microsomes. Microsomal membranes were then fully resolved by 1× RIPA buffer (Abcam, Cambridge, UK) containing 1 mmol/L phenylmethylsulfonyl fluoride and 1× cocktail of protease inhibitors. After centrifugation at 850 g for 5 min at 4°C, the supernatant was transferred into a new tube and 1× sodium dodecyl sulfate (SDS) was added as a loading buffer for immunoblots.
Yeast two-hybrid assay
The yeast two-hybrid assays were carried out as previously described (Serino et al., 1999). The GIL1 CDS was subcloned into the EcoR I/Xho I sites of pLexA vector, and PIN3 hydrophilic loop (PIN3-HL, 157–493 a.a.) was subcloned into the EcoR I/Xho I sites of pB42AD vector. The bait and prey constructs were co-transformed into yeast strain EGY48[p8op-lacZ] (CLONTECH, Kyoto, Japan), and clones containing both constructs were selected and β-Galactosidase activity was tested on medium containing X-gal.
In vitro pull-down assay
The EcoR I/Xho I DNA fragment encoding the full-length GIL1 CDS was cloned into the pGEX-4T-1 vector. PIN3-HL was digested by EcoR I/Sal I and ligated into the pMAL-c2x vector. GST-GIL1 (2 μg) was mixed with MBP or MBP-PIN3-HL fusion proteins (2 μg) in 1 mL binding buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 1% Triton X-100) containing 1 mmol/L phenylmethylsulfonyl fluoride and 1× cocktail of protease inhibitors. The mixture was incubated at 4°C for 2 h. The MBP resin was washed three times with binding buffer and then added into the mixture. Another incubation was performed for 2 h at 4°C. MBP resin was washed three times with binding buffer and boiled with 1× SDS loading buffer to elute the binding proteins. Immunoblots were performed with anti-MBP and anti-GST antibodies.
Co-immunoprecipitation assay
The microsomal membranes were resolved by 1× RIPA buffer (added with 20 mmol/L disodium β-glycerophosphate, 20 mmol/L Na3VO4, 50 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride and 1× cocktail of protease inhibitors). After centrifugation (850 g , 5 min, 4°C), the supernatant was transferred into a new tube and 20 μL prewashed anti-RFP-conjugated beads (ChromoTek, Martinsried, Germany) were added and incubated at 4°C for 6 h under red light. The anti-RFP-conjugated beads can bind mCherry-fused proteins. Eluted proteins were analyzed by immunoblotting using anti-GFP and anti-mCherry antibodies. Immunoblots were performed as previously described (Dong et al., 2014).
Luciferase complementation imaging assay
The CDS of GIL1 was inserted into BamH I/Sal I sites of pCAMBIA-nLUC vector (Zhou et al., 2018). PIN3-HL was inserted into Kpn I/Sal I sites of pCAMBIA-cLUC vector (Zhou et al., 2018). Nucleotides ATG was added into BamH I/Sal I sites of pCAMBIA-nLUC vector using Gibson assemble kit, yielding translatable ATGnLUC construct. nLUC- and cLUC-fused constructs were transformed into Agrobacterium strain GV3101 and the paired constructs were injected into 4-week-old N. benthamiana leaves. The plants were subsequently grown in darkness for 24 h and then transferred into red light for another 24-h growth. The LUC activity was analyzed by LB985 (Berthold Technologies, Bad wildbad, Germany).
Auxin export assay
Simultaneous 3H-3-indolylacetic acid (3H-IAA; specific activity 20 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO) and 14C-benzoic acid (14C-BA; specific activity of 50 Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO) export from mesophyll protoplasts was analyzed as previously described (Henrichs et al., 2012). In short, tobacco (Nicotiana benthamiana) mesophyll protoplasts were prepared 4 d after Agrobacterium-mediated transfection of combinations of indicated plasmids or plasmid combinations. Relative export from protoplasts was calculated from exported radioactivity into the supernatant as follows: ((radioactivity in the supernatant at time t = 15 min) − (radioactivity in the supernatant at time t = 0)) × (100%)/(radioactivity in the supernatant at t = 0 min); presented are mean values from four to six independent protoplast preparations derived from independent transfections.
ACKNOWLEDGEMENTS
We thank Prof. Jing Zhang for the seeds of pPIN3:PIN3-GFP and pin3-4, Prof. Jigang Li for the seeds of phyA, phyB and phyA phyB, Dr. Yufan Li for project design, and Dr. Yangyang Zhou for figure organization. We thank the Core Facilities of Life Sciences of Peking University, particularly Dr. Yingchun Hu for technical assistance with the TEM experiment. This study was supported by the National Natural Science Foundation of China (32350001, 32370306, 32022005), Tsinghua University Dushi Program, and the Tsinghua-Peking Center for Life Sciences. M. G. was funded by grants from the Swiss National Funds (project 31003A_165877 and 310030_197563).
CONFLICTS OF INTEREST
The authors declare no conflict of interest
AUTHOR CONTRIBUTIONS
H.C. and X.W.D. conceived and supervised the study. Y.Y. and X.W. performed most of the experiments. L.C. and M.G. carried out auxin export assay. Z.D. analyzed some of the data. X.W., Y.Y., Z.D., M.G., X.W.D. and H.C. analyzed data and wrote the manuscript. All authors read and approved this manuscript.






