The TaGW2-TaSPL14 module regulates the trade-off between tiller number and grain weight in wheat
Edited by: Zhongfu Ni, China Agricultural University, China
ABSTRACT
IDEAL PLANT ARCHITECTURE1 (IPA1) is a pivotal gene controlling plant architecture and grain yield. However, little is known about the effects of Triticum aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14), an IPA1 ortholog in wheat, on balancing yield traits and its regulatory mechanism in wheat (T. aestivum L.). Here, we determined that the T. aestivum GRAIN WIDTH2 (TaGW2)-TaSPL14 module influences the balance between tiller number and grain weight in wheat. Overexpression of TaSPL14 resulted in a reduced tiller number and increased grain weight, whereas its knockout had the opposite effect, indicating that TaSPL14 negatively regulates tillering while positively regulating grain weight. We further identified TaGW2 as a novel interacting protein of TaSPL14 and confirmed its ability to mediate the ubiquitination and degradation of TaSPL14. Based on our genetic evidence, TaGW2 acts as a positive regulator of tiller number, in addition to its known role as a negative regulator of grain weight, which is opposite to TaSPL14. Moreover, combinations of TaSPL14-7A and TaGW2-6A haplotypes exhibit significantly additive effects on tiller number and grain weight in wheat breeding. Our findings provide insight into how the TaGW2-TaSPL14 module regulates the trade-off between tiller number and grain weight and its potential application in improving wheat yield.
INTRODUCTION
Grain yield in cereal crops like rice and wheat is determined by three major agronomic traits: effective tiller number per plant, grain number per panicle and grain weight. In breeding practices, there is often a mutual balance and limitation among these three yield traits. An increase in a single yield factor may lead to a decrease in other yield factors, which in turn limits the overall improvement in crop yield. The optimization of plant architecture is crucial to overcoming these limitations. In rice, the IDEAL PLANT ARCHITECTURE1 (IPA1) quantitative trait locus, which encodes Oryza sativa SQUAMOSA PROMOTER-BINDING-LIKE 14 (OsSPL14), is a pivotal gene for improving plant architecture and grain yield. A point mutation in OsSPL14 enhances its transcription, leading to an ideal plant architecture with fewer unproductive tillers, increased lodging resistance, and higher grain yield per panicle (Jiao et al., 2010). Interestingly, removing a 54-bp cis-regulatory region in IPA1 optimizes its expression levels, mitigates the negative effect on tiller number reduction, and effectively balances the trade-off between tiller number and grains per panicle, significantly boosting grain yield per plant (Song et al., 2022). Triticum aestivum SPL14 (TaSPL14, also referred to as TaSPL17 in some studies), the closest ortholog of OsSPL14 located on wheat group 7 chromosomes, has a conserved role in regulating plant architecture. Overexpression of TaSPL14-7A (TraesCS7A02G246500) in wheat notably alters plant architecture and yield traits, including a decrease in tiller number and an increase in grain size and weight (Cao et al., 2023). Therefore, SPL14 is integral to regulating plant architecture and yield, and fine-tuning its expression is key to achieving optimal plant architecture and enhancing grain yield in cereal crops.
As a central gene in the regulation of plant architecture and yield traits, understanding the function mechanism and regulatory network of SPL14 is crucial for establishing optimal plant architecture, such as tillering. In rice, OsSPL14 has been shown to intricately regulate tiller number through interactions with multiple factors, forming a complex regulatory network. For instance, the expression of OsSPL14 is suppressed by micro RNA 156 (miRNA156) and DNA methylation at both transcriptional and post-transcriptional levels (Jiao et al., 2010; Zhang et al., 2017). At the protein level, OsSPL14 is modulated by the E3 ubiquitin ligase IPI1 and the deubiquitinase OsOTUB1. IPI1 mainly polyubiquitinates the K63 site of OsSPL14 at the shoot apex, whereas OsOTUB1 can deubiquitinate this site, dynamically maintaining the protein stability and activity of OsSPL14 (Wang et al., 2017a, 2017b). Additionally, OsSPL14 can regulate the expression of several downstream genes involved in tillering, including TB1, D53, OsPIN1b and PILS6b (Lu et al., 2013; Song et al., 2017; Li et al., 2022). D53, which is degraded by ubiquitination in response to strigolactones, also interacts with OsSPL14 to negatively regulate its own gene expression, establishing a feedback loop (Song et al., 2017). In wheat, TaSPL14 exhibits a conserved functional mechanism in certain aspects, including shared downstream genes and interaction partners (Liu et al., 2017). Thus, the multifaceted regulatory network of SPL14 is essential for precisely controlling key architecture traits like tillering. Nevertheless, identifying new regulatory factors of SPL14 and fully assessing the application potential of SPL14 and its regulatory components to improve crop architecture require further exploration.
GRAIN WIDTH2 (GW2) is a major gene controlling grain width and weight. This gene encodes a RING-finger E3 ubiquitin ligase involved in the ubiquitin proteasome pathway, regulating the proliferation and filling of grain cells to ultimately determine grain size and weight (Song et al., 2007). Loss of GW2 function in rice leads to increased cell numbers, larger glume shells, and a higher grain filling rate, which results in wider and heavier grains, indicating that GW2 acts as a negative regulator of grain width and weight (Song et al., 2007). GW2 is highly conserved across different crops, and its homologs have been found to influence grain size and weight in maize and wheat (Li et al., 2010; Su et al., 2011). Knocking down or out TaGW2 in wheat significantly increases grain weight and enhances yield, supporting a conserved role of GW2 as a negative regulator of grain size and weight (Simmonds et al., 2016; Zhang et al., 2018; Liu et al., 2020). Recent advancements have been made in understanding the functional mechanism of GW2 in grain size and weight regulation and identifying substrate proteins in rice and wheat, including EXPLA1 (Choi et al., 2018), TaDA1 (Liu et al., 2020), WG1 (Hao et al., 2021) and TaAGPS (Lv et al., 2022). Beyond grain development, the ubiquitous expression of GW2 in various tissues suggests important roles in other plant life processes. The potential of GW2 to control other important traits such as plant architecture is still under investigation.
In this study, we found that both overexpression (OE) and clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9 -(Cas9) gene editing of TaSPL14 altered tiller number and grain weight in wheat, demonstrating a function similar to rice IPA1. Through yeast two-hybrid (Y2H) screening, we identified several proteins interacting with TaSPL14, including E3 ubiquitin ligase TaGW2. We further confirmed that TaGW2 could mediate the ubiquitination and degradation of TaSPL14. Additionally, both OE and knockout of TaGW2 influenced tiller number and grain weight, showing effects opposite to those of TaSPL14. Our findings offer new insights into the TaGW2-TaSPL14 regulatory module and its potential for improving plant architecture and enhancing yield in wheat.
RESULTS
TaSPL14 negatively regulates tillering and positively regulates grain weight
To investigate the role of TaSPL14 in wheat development, we generated TaSPL14-7A OE lines in the hexaploid wheat cultivar Fielder (Cao et al., 2023). Using reverse-transcription quantitative polymerase chain reaction (RT-qPCR), we detected the total expression levels of TaSPL14 in the transgenic lines. The transcript levels of TaSPL14 showed a significant increase in all five representative OE lines (Figure S1A). Simultaneously, TaSPL14 knockout (KO) lines were developed utilizing CRISPR/Cas9 technology. Sequencing confirmed the successful gene editing of the three TaSPL14 homoeologs in the KO lines (Figure S1B). Two independent OE and KO lines were selected for further phenotypic evaluation.
The phenotypes of TaSPL14-OE and TaSPL14-KO lines were assessed in two environments (greenhouse and field). We observed that both TaSPL14 OE and KO induced significant changes in tiller number. In the greenhouse, for 30-d-old plants at the tillering stage, the average tiller numbers for OE lines ranged from 4.3 to 4.7, markedly lower than that of the wild-type (WT) control, which had an average tiller number of 20.2 (Figure 1A, B). Conversely, the KO lines exhibited a significantly higher tiller number compared to the WT (Figure 1A, B). Similar trends were noted in the field under natural conditions. Relative to WT, the OE lines demonstrated lower effective tiller numbers (ETN), whereas the KO lines had higher ETN (Figure 1C, D). Post-harvest analysis showed that the thousand-grain weight (TGW) was significantly higher in OE plants but lower in KO plants compared to WT (Figures 1E, F). Notably, the differences in TGW among WT, OE and KO lines were primarily attributed to grain width (GW), not grain length (GL) (Figure 1E, S2). Thus, TaSPL14 acts as a conserved negative regulator of tiller number and a positive regulator of TGW in wheat.
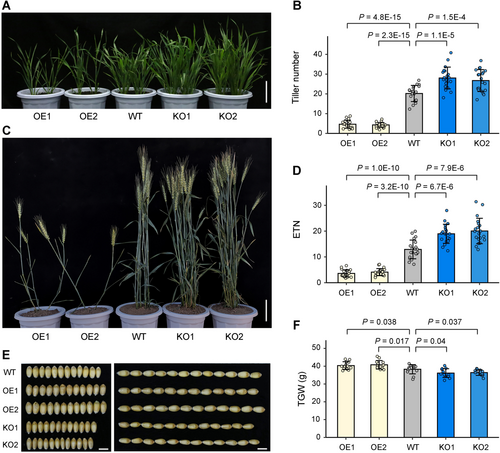
Triticum aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14) negatively regulates tillering and positively regulates grain weight in wheat
(A, B) Phenotypic comparison of tiller numbers at the tillering stage among wild-type (WT), TaSPL14 overexpression (OE), and knockout (KO) lines in the greenhouse. Scale bar, 10 cm. (C, D) Phenotypic comparison of effective tiller numbers (ETNs) of mature plants among WT, TaSPL14 OE and KO lines in the field. Scale bar, 10 cm. (E, F) Comparison of grain phenotypes and thousand-grain weight (TGW) among these genotypes. Scale bars, 5 mm. All values are presented as mean ± SD, and the actual P-values (Student's t-test) are indicated in the figures.
TaSPL14 physically interacts with TaGW2
To elucidate the molecular mechanism of TaSPL14 in regulating tillering and grain weight, we conducted Y2H screening to identify potential interactors of TaSPL14 in wheat. Following screening, seven potential interacting proteins were identified, including TaGW2-6A (TraesCS6A02G189300), a known negative regulator of GW and grain weight (Table S1). The interaction between TaSPL14 and TaGW2 was confirmed via Y2H assay (Figure 2A). Additionally, a pull-down assay demonstrated that maltose-binding protein (MBP)-TaSPL14, but not the MBP control, directly interacted with His-TaGW2 in vitro (Figure 2B). To further verify that TaSPL14 interacts with TaGW2 in plants, we performed firefly luciferase (LUC) complementation imaging (LCI) and bimolecular fluorescence complementation (BiFC) assays in Nicotiana benthamiana leaves. Co-infiltration of TaSPL14-nLUC and cLUC-TaGW2 resulted in luminescence generated by the complemented LUC (Figure 2C), and co-expression of TaSPL14-nYFP and TaGW2-cYFP produced strong yellow fluorescent protein (YFP) signals predominantly in the nuclei of N. benthamiana leaf epidermal cells (Figure 2D), confirming that TaSPL14 interacts with TaGW2 in plant cells. These findings suggest that TaSPL14 is associated with TaGW2 both in vitro and in vivo.
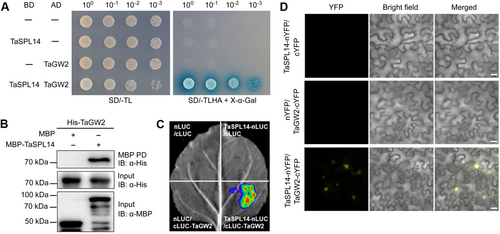
Triticum aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14) physically interacts with T. aestivum GRAIN WIDTH2 (TaGW2)
(A) Yeast two-hybrid (Y2H) assay showing the interaction between TaSPL14 and TaGW2. BD, GAL4 DNA binding domain; AD, GAL4 activation domain. The yeast strains were serially diluted (100–10−3) before spotting on selection medium. SD/-TL, synthetic dextrose medium lacking Trp and Leu; SD/-TLHA, synthetic dextrose medium lacking Trp, Leu, His and Ade. (B) Pull-down assay revealing that maltose-binding protein (MBP)-SPL14, but not MBP itself, can pull down His-TaGW2 in vitro. (C) Luciferase (LUC) complementation imaging (LCI) assay demonstrating that TaSPL14 interacts with TaGW2 in Nicotiana benthamiana leaves. nLUC, N-terminal part of LUC; cLUC, C-terminal part of LUC. (D) Bimolecular fluorescence complementation (BiFC) assay showing the in vivo interaction between TaSPL14 and TaGW2 predominantly in the nuclei of N. benthamiana leaf epidermal cells. nYFP, N-terminal part of yellow fluorescent protein (YFP); cYFP, C-terminal part of YFP. Scale bars, 20 μm.
Previous studies have indicated that GW2 possesses a transactivation activity in the yeast system (Choi et al., 2018). We discovered that this transactivation activity is conserved, as GW2-family proteins, including TaGW2 homoeologs, OsGW2 and AtDA2; all exhibited similar transactivation activity (Figure S3). To identify the TaGW2 transactivation domain, we created a series of deletion constructs and evaluated the transactivation activity of each. Assays with these TaGW2 deletion constructs revealed that the C-terminal region (residues 205–424) possesses the transactivation activity, whereas the N-terminal region (residues 1–204), containing the RING domain, does not (Figure 3A). Furthermore, we tested the interaction of TaSPL14 with different regions of TaGW2 via Y2H, and the result indicated that the C-terminal region (residues 205–424), which has transactivation activity, is responsible for the interaction with TaSPL14 (Figure 3B).
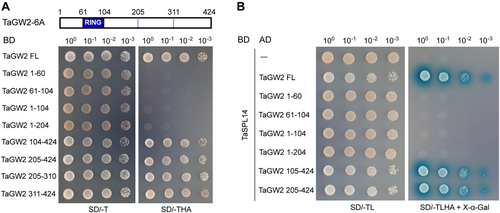
The C-terminal portion of Triticum aestivum GRAIN WIDTH2 (TaGW2) with transactivation activity is responsible for the interaction with T. aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14)
(A) Transactivation activity assay of full-length (FL) and truncated TaGW2-6A in the yeast system. The protein structure of TaGW2-6A is depicted with the RING-finger domain highlighted. SD/-T, synthetic dextrose medium lacking Trp; SD/-THA, synthetic dextrose medium lacking Trp, His and Ade. (B) Yeast two-hybrid (Y2H) assay demonstrating the C-terminal portion of TaGW2 responsible for the interaction with TaSPL14. BD, GAL4 DNA binding domain; AD, GAL4 activation domain. The yeast strains were serially diluted (100–10−3) before spotting on selection medium. SD/-TL, synthetic dextrose medium lacking Trp and Leu; SD/-TLHA, synthetic dextrose medium lacking Trp, Leu, His and Ade.
TaGW2 mediates the ubiquitination and proteasomal degradation of TaSPL14
As TaGW2 is a known RING-type E3 ubiquitin ligase, we first conducted an in vitro ubiquitination assay to demonstrate that purified His-TaGW2 recombinant protein possesses E3 ubiquitin ligase activity (Figure S4). Given that TaGW2 directly interacts with TaSPL14, we next investigated its role in mediating the ubiquitination of TaSPL14. The ubiquitination assay revealed that MBP-TaSPL14 was ubiquitinated in vitro in the presence of E1, E2, Ub and His-TaGW2, while the MBP control was not (Figure 4A). The absence of E1, E2, Ub or His-TaGW2 all abolished the ubiquitination of TaSPL14 (Figure 4A). Our results confirmed that TaGW2 can mediate the ubiquitination of TaSPL14.
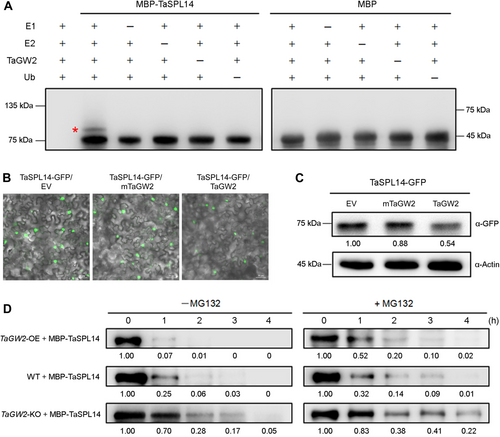
Triticum aestivum GRAIN WIDTH2 (TaGW2) mediates the ubiquitination and degradation of T. aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14)
(A) In vitro ubiquitination assay illustrating TaGW2-mediated ubiquitination of TaSPL14. Recombinant maltose-binding protein (MBP)-TaSPL14 protein was ubiquitinated by His-TaGW2 in the presence of E1, E2 and ubiquitin (Ub). An anti-MBP antibody was used to detect both non-ubiquitinated and ubiquitinated forms of MBP-tagged proteins. The red asterisk indicates ubiquitinated MBP-TaSPL14. (B, C) Transient assays demonstrating that TaGW2 promotes the degradation of TaSPL14 in Nicotiana benthamiana leaves. The fluorescence intensity (B) and protein accumulation (C) of TaSPL14-GFP (green fluorescent protein) were significantly reduced in the presence of TaGW2 compared to controls such as the empty vector (EV) and an inactive mutant of TaGW2 (mTaGW2). Relative protein abundances of TaSPL14-GFP are shown below the lane. (D) Cell-free degradation assay indicating that TaGW2 mediates the protein degradation of TaSPL14 via the 26 S proteasome pathway. Purified MBP-TaSPL14 protein was subjected to degradation by protein extracts from wild-type (WT), TaGW2 overexpression (OE), and knockout (KO) plants for varying durations with or without 50 μmol/L MG132, and detected by immunoblotting using an anti-MBP antibody. Relative amounts of MBP-TaSPL14 are indicated below each lane.
Given the close relationship between protein ubiquitination and proteasomal degradation, we hypothesized that TaGW2 could promote the protein degradation of TaSPL14 through ubiquitination. To test this hypothesis, we performed transient assays in N. benthamiana leaves by co-expressing TaSPL14-GFP (green fluorescent protein) and TaGW2 proteins. A C-to-A substitution at the 100th amino acid of TaGW2, known to result in the loss of E3 ligase activity (Lv et al., 2022), allowed us to show that this mutant form of TaGW2 (mTaGW2) could still interact with TaSPL14 but did not mediate its ubiquitination (Figure S5). In contrast, the WT TaGW2 effectively promoted the degradation of TaSPL14, as evidenced by a significant reduction in TaSPL14-GFP fluorescence intensity (Figure 4B) and a marked decrease in TaSPL14-GFP protein levels (Figure 4C), compared to the empty vector (EV) and mTaGW2 controls. Additionally, cell-free degradation assays demonstrated that the MBP-tagged TaSPL14 protein was rapidly degraded when incubated with protein extracts from TaGW2 OE plants, whereas degradation was notably slower with extracts from TaGW2 KO plants (Figure 4D). Moreover, the addition of MG132, a specific inhibitor of the 26 S proteasome, significantly reduced the degradation rates of MBP-TaSPL14 under these conditions (Figure 4D). Our findings confirm that TaGW2 mediates the ubiquitination of TaSPL14 and targets it for degradation via the 26 S proteasome pathway.
TaGW2 positively regulates tillering and negatively regulates grain weight
As a negative regulator of TaSPL14 at the protein level, TaGW2 likely also influences wheat tillering. We generated and phenotypically evaluated representative TaGW2 OE and KO lines (Figure S6). In the greenhouse, OE lines exhibited average tiller numbers 2.5–4.5 higher than WT, while KO lines had average tiller numbers 4.7–5.0 lower than WT (Figure 5A, B). In field conditions, the average ETNs of OE lines were significantly higher than that of WT, whereas the average ETNs of KO lines were significantly lower than that of WT (Figure 5C, D). After harvest, the average TGWs of OE lines were 2.3–2.8 g lower than that of WT, while the average TGWs of KO lines were 2.5–3.7 g higher than that of WT (Figure 5E, F). Although both GL and GW were significantly decreased in OE lines and increased in KO lines compared to WT (Figures 5E, S7), TaGW2 exerted a stronger effect on GW, aligning with our prior findings (Liu et al., 2020). These results suggest TaGW2 acts as a positive regulator of tiller number and a negative regulator of TGW in wheat, demonstrating an effect opposite to TaSPL14.
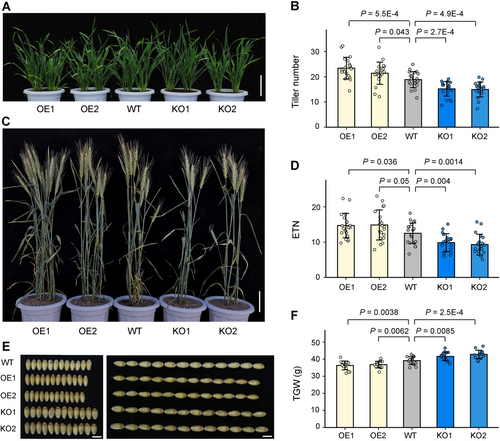
Triticum aestivum GRAIN WIDTH2 (TaGW2) positively regulates tillering and negatively regulates grain weight in wheat
(A, B) Phenotypic comparison of tiller numbers at the tillering stage among wild-type (WT), TaGW2 overexpression (OE), and knockout (KO) lines in the greenhouse. Scale bar, 10 cm. (C, D) Phenotypic comparison of effective tiller numbers (ETNs) of mature plants among WT, TaGW2 OE and KO lines in the field. Scale bar, 10 cm. (E, F) Comparison of grain phenotypes and thousand-grain weight (TGW) among these genotypes. Scale bars, 5 mm. All values are presented as mean ± SD, and the actual P-values (Student's t-test) are shown in the figures.
TaSPL14 activates the transcription of TaTB1 and TaPIN1
Previous studies have illustrated that IPA1 suppresses rice tillering by upregulating certain downstream genes including O. sativa TEOSINTE BRANCHED 1 (OsTB1) and O. sativa PIN-FORMED 1 (OsPIN1), which negatively regulate tillering (Lu et al., 2013; Li et al., 2022). In wheat, SPL transcription factors also inhibit tillering by binding to GTAC elements in the TaTB1 promoter to activate its expression (Liu et al., 2017). Thus, we explored whether TaSPL14 could similarly influence TaTB1 and TaPIN1 expression and consequently affect tillering. Promoter analysis revealed multiple GTAC elements within the 2-kb promoter regions of TaTB1 (TraesCS4B02G042700) and TaPIN1 (TraesCS6B02G337300), and electrophoretic mobility shift assay (EMSA) confirmed that MBP-TaSPL14 recombinant protein, unlike the MBP tag alone, could directly bind to the promoter sequences (TaTB1-P1 and TaPIN1-P1) containing GTAC elements in vitro (Figure 6A, B).
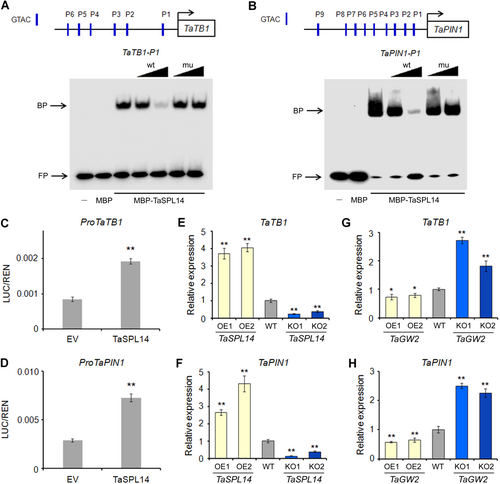
Triticum aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14) positively regulates the expression of T. aestivum TEOSINTE BRANCHED 1 (TaTB1) and T. aestivum PIN-FORMED 1 (TaPIN1)
(A, B) Electrophoretic mobility shift assay (EMSA) showing that TaSPL14 binds to promoter sequences of TaTB1 (TaTB1-P1) and TaPIN1 (TaPIN1-P1) containing GTAC elements in vitro. Multiple GTAC elements are present in these promoters. Competition assays were performed using unlabeled 10× and 100× wild-type (wt) and motif-mutated (mu) probes. (C, D) Transient expression assay in Nicotiana benthamiana leaves showing that TaSPL14 activates the transcription of TaTB1 and TaPIN1. Luciferase (LUC)/Renilla luciferase (REN) ratio was quantified at 60 h after infiltration. EV, empty vector. (E, F) Relative expression of TaTB1 (E) and TaPIN1 (F) in shoot base (at the third-leaf stage) of wild-type (WT), TaSPL14 overexpression (OE), and knockout (KO) plants. (G, H) Relative expression of TaTB1 (G) and TaPIN1 (H) in shoot base (at the third-leaf stage) of WT, TaGW2 OE, and KO plants. All values are presented as mean ± SD. *P < 0.05; **P < 0.01 (Student's t-test).
Next, transient expression assays were carried out to investigate the effects of TaSPL14 on TaTB1 and TaPIN1 expression. We constructed LUC reporter plasmids driven by 2-kb promoter regions of TaTB1 and TaPIN1. Co-infiltration of TaSPL14 effector and LUC reporters in N. benthamiana leaves led to a 2.2–2.5 fold increase in LUC reporter activity (Figure 6C, D), indicating that TaSPL14 activates the transcription of TaTB1 and TaPIN1. Furthermore, RT-qPCR analysis showed that TaTB1 and TaPIN1 expression levels were significantly elevated in TaSPL14 OE lines but reduced in KO lines compared to WT (Figure 6E, F), confirming that TaSPL14 positively regulates TaTB1 and TaPIN1 expression. Notably, TaGW2 transgenic lines exhibited opposite TaTB1 and TaPIN1 expression patterns to TaSPL14 transgenic lines. TaTB1 and TaPIN1 expression levels were significantly lower in TaGW2 OE lines but higher in KO lines compared to WT (Figure 6G, H), consistent with the role of TaGW2 as a negative regulator of TaSPL14.
The TaSPL14-7A and TaGW2-6A haplotypes exhibit additive effects on ETN and TGW
After genotyping 348 modern Chinese wheat cultivars with molecular markers previously developed (Su et al., 2011; Cao et al., 2023), we found that TaSPL14-7A and TaGW2-6A haplotypes are associated with both ETN and TGW, and can be categorized into high-TGW/low-ETN haplotypes (TaSPL14-7A-Hap-1/2 and TaGW2-Hap-6A-A) and low-TGW/high-ETN haplotypes (TaSPL14-7A-Hap-3 and TaGW2-Hap-6A-G) (Figure 7A, B). Further, these haplotypes can be divided into four combinational haplotypes: HapC1–C4 (Figure 7B).
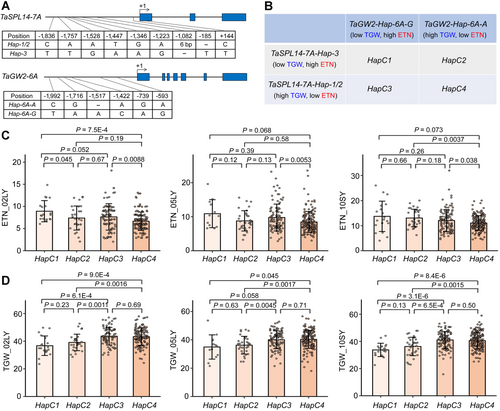
Combinations of Triticum aestivum SQUAMOSA PROMOTER-BINDING-LIKE 14 (TaSPL14)-7A and T. aestivum GRAIN WIDTH2 (TaGW2)-6A haplotypes exert additive effects on effective tiller number (ETN) and thousand-grain weight (TGW) in modern Chinese wheat cultivars
(A) Schematic diagrams of the TaSPL14-7A and TaGW2-6A haplotypes. The ATG start codon is designated as position +1 bp, and the polymorphic sites are shown below. (B) Combinations of TaSPL14-7A and TaGW2-6A haplotypes (HapC1–C4). The effects of each haplotype on ETN and TGW are indicated. (C, D) Association of combinational haplotypes (HapC1–C4) with ETN (C) and TGW (D) in 348 modern Chinese wheat cultivars. The agronomic traits of this population were collected from three environments: Luoyang, 2002 (02LY); Luoyang, 2005 (05LY); Shunyi, 2010 (10SY). The values are presented as mean ± SD, and the actual P-values (analysis of variance) are shown in the figures.
Among these combinations, HapC1 (TaSPL14-7A-Hap-3/TaGW2-Hap-6A-G) was associated with the highest ETN but the lowest TGW in the modern Chinese cultivars across all three environments. Conversely, HapC4 (TaSPL14-7A-Hap-1/2/TaGW2-Hap-6A-A) exhibited the highest TGW but the lowest ETN (Figure 7C, D), illustrating the additive effects of the TaSPL14-7A and TaGW2-6A haplotypes. Moreover, these haplotypes had a minor impact on grain number per spike (GN) (Figure S8), indicating a trade-off between ETN and TGW. Notably, the ETN of HapC3 was significantly higher than that of HapC4, yet the TGW of HapC3 was almost identical to that of HapC4 (Figure 7C, D). During the wheat breeding process in China, HapC4 with highest TGW, was positively selected, whereas HapC1, with the lowest TGW, was nearly eliminated. However, HapC3 consistently maintained a significant proportion due to its superior comprehensive yield traits (Figure S8).
DISCUSSION
The TaGW2-TaSPL14 module regulates trade-off between tiller number and grain weight
In cereal crops such as rice and wheat, IPA1 (OsSPL14) and its orthologs are central regulators of plant architecture and yield, exhibiting pleiotropic effects on three major yield traits: tiller number per plant, grain number per panicle and grain weight (Jiao et al., 2010; Miura et al., 2010). IPA1 represses tillering but promotes panicle branching and grain productivity, highlighting the importance of understanding the balance and limitations among these yield components. In this study, we explored the function of TaSPL14, an ortholog of IPA1 in wheat, through transgenic approaches. Findings suggest that both OE and KO of TaSPL14 lead to significant changes in tiller number and grain weight in wheat. The TaSPL14-OE lines exhibited reduced tiller number and increased TGW, while the TaSPL14-KO lines showed decreased TGW and increased tiller number compared to the WT (Figure 1). Thus, TaSPL14 plays a conserved role in balancing tiller number and TGW. Furthermore, we identified TaGW2 as a novel interacting protein of TaSPL14, mediating its ubiquitination and protein degradation (Figures 2, 4), and discovered that TaGW2 OE and KO also led to alterations in tiller number and grain weight. The TaGW2-OE lines had an increased tiller number and decreased TGW, while the TaGW2-KO lines exhibited increased TGW and decreased tiller number compared to the WT (Figure 5), showing effects opposite to those of TaSPL14. Collectively, our data reveal that the TaGW2-TaSPL14 module is a novel regulatory unit determining the trade-off between tiller number and grain weight. It may be possible to overcome the mutual limitation between tiller number and TGW by optimizing the expression levels of TaSPL14 and TaGW2 and fine-tuning their tissue-specific expression patterns, offering a viable solution for achieving high-yield goals in the future.
TaGW2 has pleiotropic effects on multiple important traits in wheat
As a well-established regulator of GW and grain weight, GW2 plays a conserved role in controlling cell proliferation and grain filling processes, ultimately determining grain size and weight. In wheat, TaGW2 interacts with the ubiquitin receptor TaDA1 and negatively regulates grain weight, contributing an additive effect (Liu et al., 2020). TaGW2 specifically mediates the degradation of adenosine diphosphate-glucose pyrophosphorylase TaAGPS through the 26 S proteasome pathway, affecting starch synthesis and thereby regulating grain size (Lv et al., 2022). Given that TaGW2 encodes a RING-finger E3 ubiquitin ligase and is constitutively expressed across various tissues, it has the potential to target multiple important substrates for degradation. Recently, TaGW2 has been implicated in the regulation of other important traits in addition to grain size and weight. For example, TaGW2 mediates the ubiquitination and degradation of the B-type ARR transcription factor TaARR12, positively regulating wheat drought tolerance (Li et al., 2024). Furthermore, TaGW2 targets the defense immune factor TaSGT1 for ubiquitination, thereby negatively regulating wheat leaf rust resistance (Liu et al., 2024). In this study, we also provide convincing evidence that TaGW2 positively regulates wheat tillering by mediating the ubiquitination and degradation of TaSPL14. Therefore, TaGW2 plays multifaceted roles in plant development and responses to various abiotic and biotic stresses. The pleiotropic effects make TaGW2 an important target for maintaining the balance among yield components and environmental adaptation. With the increasing identification and characterization of novel interacting proteins or substrates, new regulatory networks and functional mechanisms of TaGW2 will be further elucidated in wheat.
Potential application of TaSPL14 and TaGW2 haplotypes in wheat improvement
Wheat is a major staple crop for approximately 40% of the world's population. To date, breeding high-yield varieties has been the primary goal for wheat breeders to feed an increasing population. Given the strict restrictions on genetically modified approaches, marker-assisted selection (MAS) has emerged as a highly efficient method for the genetic improvement of wheat. The TaGW2-TaSPL14 module, with its strong effects on plant architecture and yield traits including ETN and TGW, offers potential for yield improvement in wheat. Our association analysis indicated that the TaSPL14-7A and TaGW2-6A haplotypes were correlated with both ETN and TGW in the modern Chinese cultivars, and their combinational haplotypes (HapC1–C4) exhibited strong additive effects on ETN and TGW (Figure 7). HapC4 (TaSPL14-7A-Hap-1/2/TaGW2-Hap-6A-A) had the highest TGW but the lowest ETN, and underwent strong positive selection during the wheat breeding process in China. In contrast, HapC1 (TaSPL14-7A-Hap-3 and TaGW2-Hap-6A-G) was associated with the highest ETN but the lowest TGW, and was gradually eliminated over the course of the wheat breeding process (Figure S8). Therefore, the strong selection pressure in wheat breeding primarily focuses on TGW, not ETN. However, pyramiding favorable haplotypes on TGW may lead to over-reduction of ETN due to their trade-off. HapC3 represents an intermediate combinational haplotype with a higher ETN but similar TGW compared with HapC4. This haplotype combination may represent desirable plant architecture and superior comprehensive yield traits, and therefore holding potential for application in MAS breeding for higher yield in wheat.
MATERIALS AND METHODS
Plant materials and transformation
The wheat (Triticum aestivum L.) variety Fielder was used for genetic transformation and gene expression analysis. The TaSPL14-7A (TraesCS7A02G246500) and TaGW2-6A (TraesCS6A02G189300) OE lines were developed in our previous studies (Cao et al., 2023; Liu et al., 2024). To disrupt the TaSPL14 gene, a KO construct was designed using a pair of single guide RNAs (sgRNAs) that specifically target its homoeologs. The sgRNA sequences, GGGGACGACCAGCAGCGCCACGG and GGGAAGGCCGTGGCCGCCGGGGG, were cloned into the pBUN421 vector for CRISPR/Cas9-mediated genome editing (Zhang et al., 2022b). Similarly, a specific sgRNA sequence (GCTCGCGCCCTGCTACCCGGGGG) was engineered to disrupt the TaGW2 homoeologs and was incorporated into the pBUN421 vector for the TaGW2 KO (Liu et al., 2024). All constructs were then introduced into Agrobacterium tumefaciens strain EHA105 and transformed into immature embryos of wheat (T. aestivum L.) cv Fielder using an Agrobacterium-mediated transformation method (Liu et al., 2020).
Phenotypic assessment
The phenotypes of the T3 generation of TaSPL14 and TaGW2 OE and KO lines were evaluated in two different environments: greenhouses under long-day conditions (16-h light/8-h dark photoperiod) at 23°C and the Chinese Academy of Agricultural Sciences (CAAS) experimental field in Shunyi, Beijing (116°65′E, 40°13′N) under natural conditions. The tiller number trait was assessed during the tillering stage for 30-d-old plants grown in greenhouses. The ETN and grain traits were measured for mature plants grown in the CAAS experimental field following established protocols (Jiao et al., 2023). For each line, approximately 20 plants were subjected to phenotypic assessment and statistical analyses.
RNA extraction and RT-qPCR
Total RNA was isolated using the RNAiso Plus Reagent (Takara Bio, China) according to the manufacturer's instructions. For complementary DNA (cDNA) synthesis, the FastQuant RT Kit (Tiangen, China) was used, and RT-qPCR analysis was conducted on a LightCycler 96 Real-Time PCR system (Roche Applied Science, Germany) using SYBR Premix Ex Taq (Takara Bio, China). The TaActin gene served as the internal reference, and the fold-change value for the relative expression level of each gene was calculated using the comparative cycle threshold (CT) method. Each assay was independently conducted three times. Primers used in this study are listed in Table S2.
Yeast two-hybrid assay
Yeast two-hybrid screening involved using the full-length TaSPL14 as bait and a yeast library comprising a mixture of wheat cDNA previously assembled (Cao and Yan, 2013). After screening, clones exhibiting strong interaction with TaSPL14 were identified and selected for subsequent sequencing. To investigate the potential interaction between TaSPL14 and TaGW2, their coding sequences (CDSs) were introduced into the pGBKT7 and pGADT7 vectors, respectively. These constructs were used as bait and prey plasmids for co-transformation into Y2H Gold cells. The interaction was assessed based on the growth and blue coloration of the co-transformants on selective medium (synthetic dextrose/-Trp/-Leu/-His/-Ade/X-α-gal).
Luciferase complementation imaging and BiFC assays
For the LCI assay, the full-length CDSs of TaSPL14 and TaGW2 were fused to the N- and C-terminal parts of the LUC reporter gene to construct TaSPL14-nLUC and cLUC-TaGW2 plasmids, respectively. These constructs were introduced into A. tumefaciens strain GV3101, and then co-infiltrated into N. benthamiana leaves (Ma et al., 2023). After 60 h of cultivation, LUC activities were visualized and assessed using the Night SHADE LB 985 plant imaging system (Berthold Technologies, Germany).
For the BiFC assay, the TaSPL14 and TaGW2 CDSs were independently fused to the N- and C-terminal portions of the YFP gene, transformed into A. tumefaciens strain GV3101, and co-infiltrated into N. benthamiana leaves (Ma et al., 2023). The YFP fluorescence signal was observed 72 h after infiltration under an LSM880 laser-scanning confocal microscope (Carl Zeiss, Germany).
Pull-down assay
The full-length TaGW2 and TaSPL14 CDSs were cloned into the pET-32a and pMAL-c2x vectors, respectively. The recombinant His-TaGW2 and MBP-TaSPL14 proteins were expressed in Escherichia coli BL21 (DE3) cells and purified following the manufacturer's instructions. The pull-down assay was performed as previously described (Jian et al., 2022). Briefly, 10 μg of His-TaGW2 protein was incubated with 10 μg of either MBP-TaSPL14 or MBP protein in 500 μL of binding buffer, then subjected to pull-down using amylose resin (New England Biolabs, USA) at 4°C for 3 h. The eluted proteins were separated on 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS-PAGE) gels and analyzed by immunoblotting using anti-His and anti-MBP antibodies (ABclonal, China).
In vitro ubiquitination assay
The in vitro ubiquitination assay was carried out following a previously established protocol with minor modifications (Zhang et al., 2022a). In brief, a mixture containing 0.15 μg of E1, 0.5 μg of E2, 9 μg of ubiquitin (Ub), 1 μg of His-TaGW2, and 1 μg of either MBP-TaSPL14 or MBP protein alone was incubated in 30 μL of reaction buffer at 30°C for 3 h. Subsequently, the samples were separated on 12% SDS-PAGE gels, and ubiquitinated proteins were detected through immunoblotting using an anti-MBP antibody (ABclonal, China). Human E1 (UBE1) and Ub were sourced from R&D Systems, while the recombinant His-tagged E2 (TraesCS3A02G238400) protein was produced and purified in the E. coli system.
Electrophoretic mobility shift assay
The EMSA was conducted as previously described (Jian et al., 2022). The 5′-biotin-labeled probes (100 fmol) were incubated with 1 μg of MBP-TaSPL14 protein in a 20 μL reaction mixture containing 1× binding buffer, 2.5% glycerol, 5 mmol/L MgCl2, and 1 μg of poly (dI-dC) for 30 min at room temperature. Subsequently, the protein–DNA complexes were separated via electrophoresis on 0.5× Tris-borate-ethylenediaminetetraacetic acid 6% polyacrylamide gels, transferred onto Hybond-N+ nylon membranes (Amersham, USA), and detected using a Light Shift Chemiluminescent EMSA Kit (Thermo Scientific, USA) following the manufacturer's instructions.
Transient assays in N. benthamiana leaves
Transient assays to assess transcriptional activity in N. benthamiana leaves utilized a dual-LUC reporter system (Hellens et al., 2005). Approximately 2-kb promoter regions of downstream genes were cloned into the pGreenII 0800-LUC reporter plasmid, and the TaSPL14 CDS was inserted into the pGreenII 62-SK effector plasmid. The resulting reporter and effector constructs were introduced into A. tumefaciens strain GV3101 cells with the help of the pSoup-p19 plasmid, followed by co-infiltration into N. benthamiana leaves. After 60 h of incubation, relative LUC activity (LUC/Renilla) was quantified using the Dual-Luciferase Reporter Assay System with a GloMax-Multi luminescence reader (Promega, USA).
Transient assays were performed in N. benthamiana leaves to investigate the TaGW2-mediated degradation of substrate proteins. An inactive mutant of TaGW2 (mTaGW2) harboring a C-to-A substitution at the 100th amino acid position was used as a negative control (Lv et al., 2022). The CDSs of TaGW2 and mTaGW2 were cloned into a modified pCAMBIA1300 vector to construct the 35S-TaGW2 and 35S-mTaGW2 vectors. Additionally, the CDS of TaSPL14 was fused to GFP gene to construct the 35S-TaSPL14-GFP vector. Subsequently, 4-week-old N. benthamiana leaves were infiltrated with A. tumefaciens carrying 35S-TaSPL14-GFP and either 35S-TaGW2 or 35S-mTaGW2 vectors. GFP fluorescence signal was observed at 72 h after infiltration, and detected using immunoblot analysis with an anti-GFP antibody (ABclonal, China).
Cell-free protein degradation assay
Cell-free extracts were prepared from 15-d-old seedlings of WT, TaGW2 OE and KO plants following an established protocol (Rubio et al., 2005). The cell-free protein degradation assay was carried out as described (Li et al., 2024). Briefly, 1 μg of purified MBP-TaSPL14 protein was incubated with 300 μg protein extracts in a 100 μL reaction mixture containing 25 mmol/L Tris-HCl (pH 7.5), 100 mmol/L NaCl, 10 mmol/L MgCl2, 5 mmol/L dithiothreitol, 10 mmol/L adenosine triphosphate, and either 50 μmol/L MG132 or dimethylsulfoxide at 30°C. The reaction was stopped at different time points (0, 1, 2, 3, 4 h), and the reaction mixture was subjected to immunoblotting using an anti-MBP antibody (ABclonal, China).
Haplotype association analysis
A natural population including 348 Chinese modern cultivars was used for the haplotype association analyses (Liu et al., 2020). Agronomic traits of these wheat cultivars were collected from three environments: Luoyang (111°60′E, 33°80′N), Henan Province, China in 2002 and 2005, and Shunyi (116°65′E, 40°13′N), Beijing, China in 2010. Haplotype identification and marker development of TaGW2-6A and TaSPL14-7A were described by Su et al. (2011) and Cao et al. (2023). After genotyping, the variance analyses were performed using SPSS software (version 16.0; SPSS Inc., USA), and statistical comparisons of phenotypic traits associated with different haplotypes were conducted using a one-way analysis of variance (ANOVA) according to Tukey test with significance set at P < 0.05.
ACKNOWLEDGEMENTS
We are grateful to Mr. Kang Zhang (Genovo Biotechnology Co. Ltd., Tianjin, China) for his help with wheat transformation. This research was financially supported by Beijing Natural Science Foundation (6242032), the Innovation Program of Chinese Academy of Agricultural Sciences (CAAS-CSCB-202401), and the Natural Science Foundation of Ningxia Province (2022AAC02056).
CONFLICTS OF INTEREST
The authors declare they have no conflict of interest.
AUTHOR CONTRIBUTIONS
T.L., C.H. and J.H. planned and designed the research. C.J., Y. P., S. L., M.G., Y.H. and L.C. performed the experiments. T.L., C.H., J.H, W.Z., L.Y. and X.Z. analyzed the data. T. L., C. J., and S.L. wrote the article. T.L. and C. H. supervised and revised the writing of the article. All authors read and approved the contents of this paper.






