A transcriptional cascade involving BBX22 and HY5 finely regulates both plant height and fruit pigmentation in citrus
Edited by: Hong-Quan Yang, Shanghai Normal University, China
ABSTRACT
Dwarfing is a pivotal agronomic trait affecting both yield and quality. Citrus species exhibit substantial variation in plant height, among which internode length is a core element. However, the molecular mechanism governing internode elongation remains unclear. Here, we unveiled that the transcriptional cascade consisting of B-BOX DOMAIN PROTEIN 22 (BBX22) and ELONGATED HYPOCOTYL 5 (HY5) finely tunes plant height and internode elongation in citrus. Loss-of-function mutations of BBX22 in an early-flowering citrus (Citrus hindsii “SJG”) promoted internode elongation and reduced pigment accumulation, whereas ectopic expression of BBX22 in SJG, sweet orange (C. sinensis), pomelo (C. maxima) or heterologous expression of BBX22 in tomato (Solanum lycopersicum) significantly decreased internode length. Furthermore, exogenous application of gibberellin A3 (GA3) rescued the shortened internode and dwarf phenotype caused by BBX22 overexpression. Additional experiments revealed that BBX22 played a dual role in regulation internode elongation and pigmentation in citrus. On the one hand, it directly bound to and activated the expression of HY5, GA metabolism gene (GA2 OXIDASE 8, GA2ox8), carotenoid biosynthesis gene (PHYTOENE SYNTHASE 1, PSY1) and anthocyanin regulatory gene (Ruby1, a MYB DOMAIN PROTEIN). On the other hand, it acted as a cofactor of HY5, enhancing the ability of HY5 to regulate target genes expression. Together, our results reveal the critical role of the transcriptional cascade consisting of BBX22 and HY5 in controlling internode elongation and pigment accumulation in citrus. Unraveling the crosstalk regulatory mechanism between internode elongation and fruit pigmentation provides key genes for breeding of novel types with both dwarf and health-beneficial fortification in citrus.
INTRODUCTION
Internode length is a key determinant of plant height and architecture, influencing yield and biomass (Hedden, 2020; Song et al., 2023). Internode elongation and plant height are primarily influenced by environmental factors and endogenous hormones (Feng et al., 2008; Kendall et al., 2011; Davière and Achard, 2016), with gibberellin (GA) playing a pivotal role (Peng et al., 1999). Endogenous GA levels are tightly controlled through the regulation of GA biosynthesis and catabolism. Loss-of-function mutations of GA biosynthesis genes or overexpression of GA catabolism genes lead to shortened internodes and dwarfism (Schomburg et al., 2003; Chen et al., 2014; Wang et al., 2021; Yang et al., 2024). An increasing number of transcription factors (TFs) regulating internode elongation through the GA pathway have been reported in model plants and crop species (Weller et al., 2009; Chen et al., 2019; Dong et al., 2022; Lyu et al., 2024). For example, in Arabidopsis (Arabidopsis thaliana), the GA-induced homeobox TF HB40 controls GA homeostasis during plant growth by directly activating the GA biosynthesis repressor JUNGBRUNNEN1 and GA2-oxidase (GA2ox) involved in GA catabolism (Dong et al., 2022). However, in citrus, only the ACC SYNTHASE4 (ACS4)–ETHYLENE RESPONSE FACTOR3 complex has been identified; it binds directly to GA20ox1 and GA20ox2, decreasing their transcription and thus inhibiting GA biosynthesis, leading to a dwarf phenotype (Chu et al., 2023). To aid in molecular breeding efforts, it is important to further elucidate the transcriptional regulatory networks involved in internode elongation and plant development in citrus.
For many crops, fruit pigmentation plays a crucial role in both appearance quality and nutritional value, as demonstrated in plants such as “purple endosperm rice” (Oryza sativa L.) (Zhu et al., 2017b), “red-fleshed apple” (Malus domestica) (Espley et al., 2009), and “purple pummelo” (Citrus maxima) (Huang et al., 2018). Considerable progress has been made in understanding the regulatory networks controlling the metabolism of pigment, such as carotenoids and anthocyanins, and their biosynthetic pathways have been elucidated in diverse plant species (Nisar et al., 2015; Huang et al., 2018, 2019). Carotenoids and anthocyanins in citrus fruits profoundly influence their appearance quality, nutritional value and economic significance. In our previous study, we identified ELONGATED HYPOCOTYL 5 (HY5) as a pivotal factor in light-regulated anthocyanin accumulation in citrus (Huang et al., 2019). However, research on cross-regulation between carotenoids and anthocyanins is limited. In Medicago truncatula, the R2R3-MYB protein WP1 functions as a transcriptional activator to directly regulate both carotenoid and anthocyanin biosynthetic genes (Meng et al., 2019). However, no TFs with similar effects on both types of pigments have been identified yet in citrus. It is crucial to further elucidate the complex interplay between carotenoid and anthocyanin metabolism, along with the underlying molecular mechanisms that govern their cross-regulation in citrus fruits.
The TF HY5 plays a crucial role in integrating GA and light signals to control hypocotyl elongation and mediate light-dependent regulation of pigment accumulation (Weller et al., 2009; Gangappa and Botto, 2016; Burman et al., 2018). As a key gene in both light and hormone signaling pathways, HY5 interacts with many cofactors or antagonists, collaboratively regulating downstream pathways. In Arabidopsis, HY5 antagonizes ABI4, forming a feedback loop that controls GA2ox expression, leading to the regulation of GA content and hypocotyl elongation (Xiong et al., 2023). In addition, B-box zinc-finger TFs are essential partners in HY5-dependent modulation of transcriptional regulation in Arabidopsis (Bursch et al., 2020). However, in citrus, the cofactors or antagonists of HY5 remain unclear, and the contribution of HY5 in modulating plant growth and carotenoid metabolism remains unknown.
In this study, we observed significantly higher expression of B-BOX DOMAIN PROTEIN 22 (BBX22) and HY5 in a short-internode and dwarf sour orange (Citrus aurantium) than in a long-internode and tall sour orange. The expressions of BBX22 and HY5 were also higher in fruits exposed to light, which accumulated high amounts of pigments, than in fruits that were bagged. This study unveils a previously uncharacterized transcriptional cascade regulatory network in which BBX22 not only independently regulates internode elongation and pigment accumulation, but also acts as a cofactor of HY5, thereby finely tuning targeted genes in light-mediated internode elongation and pigment biosynthesis. BBX22 and HY5 emerge as crucial targets for molecular breeding efforts aimed at reducing plant height and enhancing pigment accumulation in citrus.
RESULTS
Phenotype of a short-internode citrus
We identified a naturally occurring short-internode sour orange (C. aurantium; “SISO”) that has significantly shorter internodes than other citrus genotypes (Figure 1A). A phylogenetic tree of 11 sour orange genotypes indicated that SISO and Chengdu sour orange (C. aurantium; “CDSO”) had a close relationship (Figure S1A). SISO exhibited a dwarf and prostrate growth habit with significantly shorter internodes than CDSO (Figure 1B). In SISO, each shoot internode showed a significant reduction compared to CDSO (Figure 1C). Consequently, CDSO was selected as a control for investigating the molecular mechanisms underlying internode shortening in SISO.
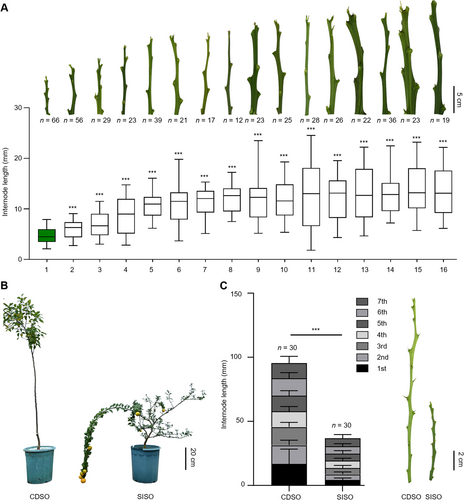
The identification of a citrus genotype with a short internode length
(A) Representative image and internode length of shoots in 16 citrus materials. Arranged by internode length. 1, short-internode sour orange (Citrus aurantium; “SISO”); 2, Huanglinmiaoju (C. reticulata; “HLMJ”); 3, Dahong sweet orange (C. sinensis; “DHTC”); 4, Yunnan citron (C. medica; “YNXY”); 5, ZM ponkan (C. reticulata; “ZMPG”); 6, Foshou citron (C. medica; “FS”); 7, Juzi (C. reticulata; “JZ”); 8, Linwuyeju (C. linwuensis; “LWYJ”); 9, Wanbaiyou (C. maxima; “WBY”); 10, Chengdu sour orange (C. aurantium; “CDSO”); 11, Juyuan (C. medica; “JY”); 12, Zigui sour orange (C. aurantium; “ZGSO”); 13, Zipiyou (C. maxima; “ZPY”); 14, Daoxianyeju (C. reticulata; “DXYJ”); 15, Guanximiyou (C. maxima; “GXMY”); 16, Aiwanyou (C. maxima; “AWY”). Scale bar, 5 cm. n > 10 biological replicates. Each box represents the median and interquartile range (IQR). The whiskers extend from the minimum to the maximum values. (B) Morphology of CDSO and SISO. Scale bar, 20 cm. (C) Internode length of shoots in CDSO and SISO. n = 30 biological replicates. Data are the mean (± SEM); asterisks represent significant difference by Student's t-test: ***P < 0.001.
SISO seedlings had significantly shorter hypocotyls and internodes than CDSO (Figures 2A–C, S1B). To investigate the differences in cellular development between CDSO and SISO internodes, the cellular basis cells in a longitudinal section from the internode were measured. The longitudinal parenchyma cell size in SISO internodes was significantly smaller than in CDSO (Figure 2D, E), while the cell numbers per unit area in SISO internodes were significantly higher compared to CDSO (Figure 2F). Additionally, the number of cells per internode in SISO significantly decreased compared to CDSO (Figure S1C).
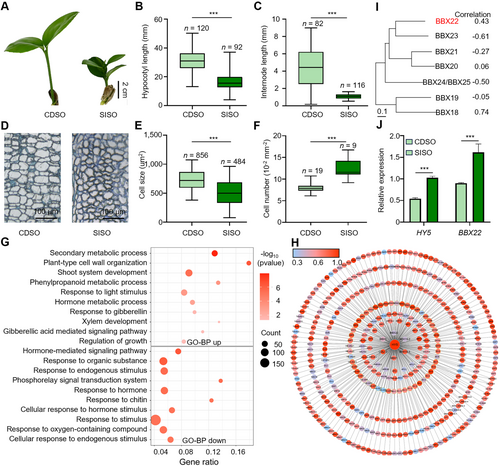
ELONGATED HYPOCOTYL 5 (HY5) and B-BOX DOMAIN PROTEIN 22 (BBX22) are potential regulators of internode elongation in citrus
(A) Representative image of seedlings in 1-month-old Chengdu sour orange (Citrus aurantium; “CDSO”) and short-internode sour orange (C. aurantium; “SISO”). Scale bar, 2 cm. (B) Hypocotyl length of seedlings in 1-month-old CDSO and SISO. Each box represents the median and interquartile range (IQR). The whiskers extend from the minimum to the maximum values. (C) Internode length of seedlings in 2-month-old CDSO and SISO. Each box represents the median and IQR. The whiskers extend from the minimum to the maximum values. (D) Representative images of longitudinal sections from the internodes of seedlings in 3-month-old CDSO and SISO. Scale bar, 100 μm. (E) Cell size of longitudinal sections from the internodes of seedlings in 3-month-old CDSO and SISO. n > 9 biological replicates. At least 50 cells were measured from each biological replicate. (F) Cell numbers of longitudinal sections from the internodes of seedlings in 3-month-old CDSO and SISO. (G) Gene Ontology (GO) analysis derived from the upregulated 1,395 genes and downregulated 836 genes in RNA-seq on the hypocotyls of SISO and CDSO. Genes were selected based on fold change ≥1.5. Gene ratio (number of genes in the specific GO item against all the genes used for GO analysis) is indicated at the bottom. (H) Interaction network diagram of transcription factors (TFs) co-expressed with HY5 in the hypocotyl RNA-seq data of SISO and CDSO. TFs were predicted using PlantTFDB v5.0. Visualization of the correlation between HY5 and the identified TFs was carried out using Cytoscape software, with gene colors indicating the strength of correlation. (I) Phylogenetic tree analysis of the correlation between HY5 and BBX transcription factors in group IV. BBX18 (Cs_ont_1g027600), BBX19 (Cs_ont_8g020180), BBX20 (Cs_ont_8g020600), BBX21 (Cs_ont_3g002820), BBX22 (Cs_ont_5g034100), BBX23 (Cs_ont_5g025030), BBX24 (Cs_ont_5g018090). Scale bar, 0.1. (J) Relative expression of HY5 and BBX22 in the hypocotyls of 1-month-old CDSO and SISO seedlings. The expression level of HY5 in SISO was set to 1. n = 3 biological replicates. Data are the mean (±SEM); asterisks represent significant difference by Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001.
RNA sequencing analysis indicates HY5 and BBX22 as candidate genes regulating internode elongation
We performed RNA sequencing (RNA-seq) on the hypocotyls of SISO and CDSO to identify TFs that regulate hypocotyl and internode elongation. Among the 2,229 differentially expressed genes (DEGs), 1,394 genes were upregulated and 835 genes were downregulated. Gene Ontology (GO) analysis revealed that DEGs were correlated with light response, secondary metabolic, shoot development, and the gibberellic acid signaling pathway (Figures 2G, S2A, B). HY5, a critical positive regulator associated with plant dwarfing and hypocotyl shortening (Gangappa and Botto, 2016), exhibited significantly higher expression in SISO than in CDSO. However, in Arabidopsis, previous reports indicated that overexpression of the full-length HY5 has no significant effect on hypocotyl development (Ang et al., 1998). Given that the HY5 protein lacks a transactivation domain (TAD) (Oyama et al., 1997), we conducted gene co-expression analysis to identify potential proteins that interact with HY5. A total of 6,247 genes co-expressed with HY5 were identified. GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that these genes were primarily enriched in light response and pigment metabolism (Figure S3A). Notably, two TFs (BBX18 and BBX22) from the group IV BBX family were found among the 412 co-expressed TFs (Figures 2H, I). Some BBXs from group IV have been reported to converge on HY5 in regulating photomorphogenesis through distinct regulatory mechanisms (Song et al., 2020). Further analysis revealed that BBX22 exhibited significantly higher expression than BBX18 in the hypocotyls of CDSO and SISO (Figure S3B), and only BBX22 exhibited a pattern of co-expression with HY5 both in hypocotyls (Figure 2J) and internodes, showing significantly higher expression levels in SISO compared with other citrus genotypes (Figure S3C). Therefore, we considered both HY5 and BBX22 as candidate regulators of internode elongation in citrus.
BBX22 limits internode elongation and hence represses plant height in citrus and tomato
Based on the above results, we hypothesized that BBX22 and HY5 are involved in the regulation of internode elongation in citrus. We used clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology to generate bbx22 mutants in an early-flowering citrus (C. hindsii; “SJG”), facilitating a comprehensive examination of the role of BBX22 in the development of internodes (Figures 3A, S4A). We measured plant height and internode length to evaluate the impact of BBX22 protein on plant growth and development. The bbx22 mutants were significantly taller than wild-type (WT) plants, exhibiting a 1.67-fold increase in plant height compared with WT plants (Figure 3B). The internode length of bbx22 mutants was significantly longer than that of WT plants (Figure 3C).
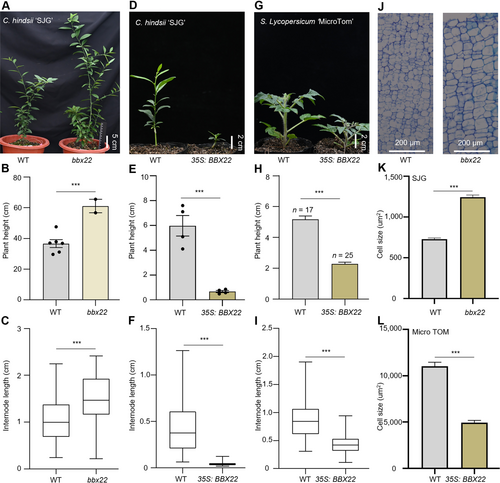
B-BOX DOMAIN PROTEIN 22 (BBX22) regulates internode elongation
(A) Representative image of 19-month-old wild-type (WT) and bbx22 mutant early-flowering citrus (Citrus hindsii; “SJG”). Scale bar, 5 cm. (B) Plant height of WT and bbx22 mutant (bbx22#13 and #32) SJG plants. (C) Internode length of WT and bbx22 mutant (bbx22#13 and #32) SJG plants. (D) Representative image of WT and BBX22-overexpression line (35 S:BBX22) in 4-month-old SJG. Scale bar, 2 cm. (E) Plant height of WT and 35 S:BBX22 (lines #21, #22, #24, and #26) SJG plants. (F) Internode length of WT and 35 S:BBX22 (lines #21, #22, #24, and #26) SJG plants. (G) Representative image of 6-week-old WT and 35 S:BBX22 MicroTom tomato (Solanum lycopersicum) plants. Scale bar, 2 cm. (H) Plant height of WT and 35 S:BBX22 (lines #15, #22, and #24) tomato plants. n = 3 biological replicates. (I) Internode length of WT and 35 S:BBX22 (lines #15, #22, and #24) tomato plants. (J) Representative images and sizes of cells in a longitudinal section from the shoot internode of WT and bbx22 plants. Scale bar, 50 μm. (K) One hundred cells were measured each from bbx22#13 and bbx22#32. (L) The sizes of cells in a longitudinal section from the internode of 8-week-old WT and 35 S:BBX22 tomato plants. One hundred cells were measured each from lines #15, #22, and #24. Each box represents the median and interquartile range (IQR). The whiskers extend from the minimum to the maximum values. Data are the mean (±SEM); asterisks represent significant difference by Student's t-test: ***P < 0.001.
In contrast to the phenotype of bbx22 mutants, all citrus plants’ ectopic expression of BBX22 (BBX22-OE) were dwarf with shorter internode length than the corresponding WT plants (Figures 3D, S4B, C, F). BBX22-OE citrus plants were shorter than their corresponding WT plants, measuring 88.8% shorter in SJG (Figure 3E), 50.1% shorter in sweet orange (C. sinensis; “AL”) (Figure S4D), and 22.1% shorter in pomelo (C. maxima; “MJY”) (Figure S4G). BBX22-OE citrus plants also had significantly shorter internodes than the corresponding WT plants (Figures 3F, S4E, H). Heterologous expression of BBX22 in MicroTom tomato (Solanum lycopersicum) also resulted in dwarf plants with shorter internodes compared with WT plants (Figure 3G–I).
We investigated the underlying cellular basis of the short internodes and dwarf phenotype caused by BBX22 overexpression. In comparison with those in WT plants, longitudinal parenchyma cell size in internodes of bbx22 mutants was significantly larger (Figure 3J, K). By contrast, BBX22-OE tomato plants displayed a significant reduction in the size of longitudinal parenchyma cells (Figure 3L). Collectively, these results indicate that BBX22 plays a crucial role in internode elongation, consequently repressing plant height.
BBX22 regulates gibberellin catabolism by directly targeting GA2ox8
To identify potential targets of BBX22, we compared the global gene expression profiles of the apical meristem tissue of BBX22-OE SJG and bbx22 mutant lines with those of the WT via RNA-seq analysis. As shown in a Venn diagram (Figure 4A), 1,576 DEGs overlapped and exhibited opposite expression patterns in the BBX22-OE and bbx22 mutant lines. GO analysis revealed that these overlapping genes were enriched in terms primarily associated with plant development, secondary metabolic process (flavonol and apocarotenoid), and hormone metabolic process (brassinosteroid, indolebutyric acid, abscisic acid, and GA) (Figure 4B). As hormones play crucial roles in plant growth and development, we examined the genes associated with this term. Notably, the gene with the highest upregulation showed an 8.2-fold increase in the BBX22-OE lines, and its expression showed a 2.3-fold decrease in bbx22 mutants. This gene encodes GA2-oxidase 8 (GA2ox8). GA2ox8 expression was significantly lower in all bbx22 mutants than in the WT (Figure 4C). By contrast, GA2ox8 expression was significantly higher in BBX22-OE citrus and tomato apical meristem tissues than in the corresponding WT (Figures 4D, S5A–C). Thus, GA2ox8 is the potential target of BBX22 in mediating GA catabolism and inhibiting internode elongation. An electrophoretic mobility shift assay (EMSA) demonstrated that BBX22 bound directly to the G-box element in the GA2ox8 promoter (Figure 4E). Luciferase (LUC) experiments revealed that BBX22 activates GA2ox8 transcription (Figure 4F). These results revealed that BBX22 inhibits internode elongation and reduces plant height by directly binding to and activating GA2ox8, which is involved in the GA catabolism pathway.
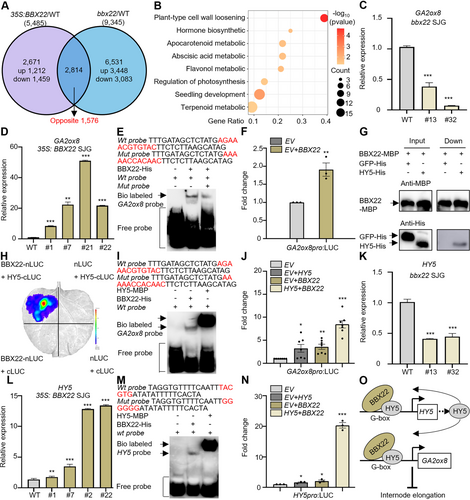
B-BOX DOMAIN PROTEIN 22 (BBX22) targets GIBBERELLIC ACID 2 OXIDASE 8 (GA2ox8) in the regulation of internode elongation
(A) Venn diagram illustrating the overlap of differentially expressed genes (DEGs) between the BBX22-overexpression lines (35 S:BBX22) versus the wild-type (WT) (fold change ≥ 1.5) and bbx22 mutants versus the WT (fold change ≥ 1.5) in the apical meristem tissue of early-flowering citrus (Citrus hindsii, “SJG”). (B) Gene Ontology (GO) analysis derived from the 1,578 genes with opposite expression patterns in the BBX22-overexpression lines and bbx22 mutants. (C) Relative expression of GA2ox8 in the apical meristem tissue of WT and bbx22 SJG plants (lines #13 and #32). GA2ox8 expression in the WT was set to 1. n = 3 biological replicates. (D) Relative expression of GA2ox8 in the leaves of WT and BBX22-overexpression SJG plants (lines #1, #7, #21, and #22). GA2ox8 expression in the WT was set to 1. n = 3 biological replicates. (E) Electrophoretic mobility shift assay (EMSA) showing the binding of BBX22-His protein to the G-box motif in the GA2ox8 promoter. The + and − symbols indicate the presence and absence of protein or probe, respectively. The wt probe is labeled with biotin (Bio labeled); the competitor is unlabeled. (F) Transient dual-luciferase (LUC) assay in tobacco examining the interaction between the BBX22 and the GA2ox8 promoter. n = 3 independent replicates. (G) Pull-down assays confirmed the interaction between BBX22 with ELONGATED HYPOCOTYL 5 (HY5). The + and − symbols indicate the presence and absence of protein. Green fluorescent protein (GFP)-His was used as the negative control. (H) Bimolecular fluorescence complementation assay (BiFC) in tobacco leaves showing the interaction between BBX22 and HY5. (I) EMSA showing the binding of HY5-MBP (maltose-binding protein) and BBX22-His protein to the G-box motif in the GA2ox8 promoter. The + and − symbols indicate the presence and absence of protein or probe, respectively. The wt probe is labeled with biotin (Bio labeled) and 5-carboxyfluorescein (FAM labeled), respectively. (J) Transient dual- LUC assay in tobacco examining the interaction between the BBX22, HY5 and the GA2ox8 promoter. n = 6 independent replicates. (K) Relative expression of HY5 in the apical meristem tissue of WT and bbx22 SJG plants (lines #13 and #32). HY5 expression in the WT was set to 1. n = 3 biological replicates. (L) Relative expression of HY5 in the leaves of WT and BBX22-OE SJG plants (lines #1, #7, #21, and #22). HY5 expression in the WT was set to 1. n = 3 biological replicates. (M) EMSA showing the binding of HY5-MBP protein and BBX22-His protein to the G-box motif in the HY5 promoter. The + and − symbols indicate the presence and absence of protein or probe, respectively. The wt probe was labeled with biotin (Bio labeled) and FAM (FAM labeled), respectively. (N) LUC assay in tobacco examining the interaction between the BBX22, HY5 and HY5 promoter. n = 3 independent replicates. (O) A proposed working model of BBX22 and HY5 inhibiting internode elongation and reducing plant height. The brown ellipses represent the BBX22 protein, the gray ellipses represent the HY5 protein, and the white boxes represent the genes (HY5, GA2ox8). Data are the mean (± SEM); asterisks represent significant difference by Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001.
Due to the co-expression trend of HY5 and BBX22, we aimed to elucidate the association between HY5 and BBX22. Yeast two-hybrid (Y2H) analysis (Figure S5D), pull-down (Figure 4G) and bimolecular fluorescence complementation (BiFC) assays (Figure 4H) verified that BBX22 physically interacts with HY5. Therefore, we hypothesized that HY5 may act as a cofactor of BBX22, enhancing the ability of BBX22 to regulate downstream genes. EMSA demonstrated that HY5 bound directly to the G-box element in the GA2ox8 promoter, albeit with higher affinity compared to BBX22's direct binding to the same G-box element in the GA2ox8 promoter (Figures 4I, S5E). Further, LUC experiments revealed that both BBX22 and HY5 activate GA2ox8 transcription; the BBX22–HY5 complex activated GA2ox8 transcription to a greater extent than BBX22 or HY5 alone (Figure 4J).
At the same time, we observed that HY5 expression was significantly lower in all bbx22 mutants than in the WT (Figure 4K). By contrast, HY5 expression was significantly higher in BBX22-OE citrus and tomato apical meristem tissues than in the corresponding WT (Figures 4L, S5F–H). EMSA indicated that BBX22 and HY5 could directly bind to the same G-box element in the HY5 promoter (Figure S5I, J). EMSA demonstrated that HY5 bound directly to the G-box element in the HY5 promoter, albeit with higher affinity compared to BBX22's direct binding to the same G-box element in HY5 promoter (Figure 4M). LUC experiments revealed that both BBX22 and HY5 activated HY5 transcription, and the BBX22–HY5 complex activated HY5 transcription to a greater extent than BBX22 or HY5 alone (Figure 4N). The above experiments reveal that BBX22 and HY5 worked independently, and BBX22 also acted as a cofactor of HY5, inhibiting internode elongation and reducing plant height by directly binding to and activating GA2ox8, which is involved in the GA catabolism pathway (Figure 4O).
Exogenous gibberellin application rescues the shortened internode phenotype in BBX22-overexpressing citrus and tomato
GA2ox8 inactivates bioactive GAs and plays a crucial role in the GA catabolism pathway. To establish whether the internode shortening and dwarfism resulting from BBX22 overexpression were due to a decrease in endogenous bioactive GA content, we measured the GA3 content in transgenic lines and found that the GA3 content in bbx22 mutants was significantly higher than in the WT (Figure 5A). Additionally, the GA3 content in BBX22-OE citrus and tomato plants showed a significant decrease (Figures 5B–D). Furthermore, we investigated whether the exogenous application of GA3 could rescue or alleviate the dwarf phenotype. We thus treated the BBX22-OE citrus and tomato plants with GA3. After GA3 treatment, the BBX22-OE citrus and tomato plants increased in height (Figures 5E–H), primarily through a notable elongation of their internodes. Specifically, plant height was 6.2-, 3.4-, 2.4-, and 3.1-fold higher in SJG (Figure 5I), AL (Figure 5J), MJY (Figure 5K), and tomato (Figure 5L), respectively, after GA3 treatment. Furthermore, the internodes of the BBX22-OE citrus that grew after the GA3 treatment were significantly longer than the internodes that grew before the treatment. The newly formed internodes were 17.26-, 3.8-, and 6.0-fold longer than those that formed before GA3 treatment in SJG (Figure 5M), AL (Figure 5N), and MJY (Figure 5O) plants, respectively. Similarly, in tomato, the internodes after the GA3 treatment were significantly longer than those before the treatment (Figure 5P). Together, these results indicate that exogenous GA3 application rescues the shortened internode phenotype and alleviates the dwarf phenotype caused by BBX22 overexpression in citrus and tomato.

Exogenous gibberellin application rescues the shortened internode phenotype in B-BOX DOMAIN PROTEIN 22 (BBX22)-overexpressing (OE) citrus and tomato
(A) Gibberellic acid 3 (GA3) content in bbx22 mutants of early-flowering citrus (Citrus hindsii; “SJG”). (B) GA3 content in BBX22-OE Majiayou pomelo (C. maxima; “MJY”). (C) GA3 content in BBX22-OE Anliu sweet orange (C. sinensis; “AL”). (D) GA3 content in BBX22-OE MicroTom tomato (Solanum lycopersicum; “MicroTom”). (E–H) Phenotypes of BBX22-OE) early-flowering citrus (Citrus hindsii; “SJG”) (E), OE Anliu sweet orange (C. sinensis; “AL”) (F), OE Majiayou pomelo (C. maxima; “MJY”) (G), and OE MicroTom tomato (S. lycopersicum; “MicroTom”) (H) before GA3 treatment and after GA3 treatment. Scale bar, 2 cm. (I–L) Plant height of SJG-OE (I), AL-OE (J), MJY-OE (K), and MicroTom-OE (L) before GA3 treatment and after GA3 treatment. (M–O) Internode length before GA3 treatment and the length of newly formed internodes in SJG-OE (M), AL-OE (N), and MJY-OE (O) after GA3 treatment. (P) Internode length of MicroTom-OE MicroTom before GA3 treatment and after GA3 treatment. Each box represents the median and interquartile range (IQR). The whiskers extend from the minimum to the maximum values. Data are the mean (±SEM); asterisks represent significant difference by Student's t-test: ns represents non-significant (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001.
BBX22 promotes carotenoid and anthocyanin accumulation in citrus and tomato
Carotenoid accumulation was reduced in the fruit of bbx22 mutants (Figure 6A) and apocarotenoids were among the enriched terms identified in our GO analysis of the DEGs with opposite expression patterns in the BBX22-OE lines and bbx22 mutants (Figure 4B). Most genes in the carotenoid metabolic pathway exhibited significantly higher expression in BBX22-OE SJG plants than in the WT and significantly lower expression in bbx22 mutants than in the WT (Figures 6B, S6A). The BBX22-OE tomato fruits were a noticeably deeper red color (Figure 6C) with significantly higher total levels of carotenoids, especially lycopene, compared to the WT at the full ripening stage (Figure 6D). Reverse transcription – quantitative polymerase chain reaction (RT-qPCR) analysis indicated that the expression of key carotenoid pathway genes (PSY1 and PDS1) in full ripening fruits was significantly higher in BBX22-OE tomato plants than in the WT (Figure S6B).
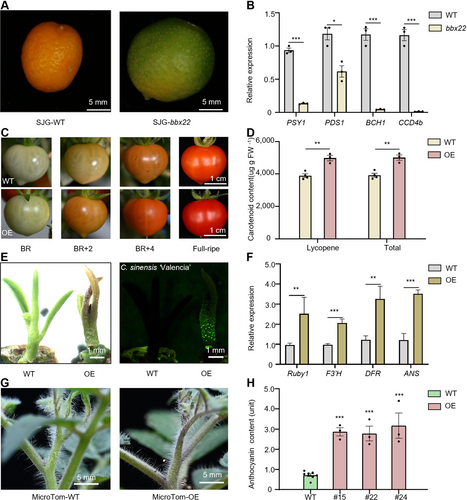
B-BOX DOMAIN PROTEIN 22 (BBX22) promotes pigmentation accumulation in citrus and tomato
(A) Representative images of fruits from the wild-type (WT) and bbx22 mutant in early-flowering citrus (Citrus hindsii ‘SJG’). Scale bar, 1 cm. (B) Relative expression levels of genes involved in the carotenoid biosynthesis pathway, including PSY1, PDS1, BCH1, and CCD4b, in the fruits of SJG-WT and the bbx22 mutant. PSY1, phytoene synthase 1; PDS1, phytoene desaturation 1; BCH1, beta-carotenoid hydroxylase 1; and CCD4b, carotenoid cleavage dioxygenase 4b. The expression level of each gene in WT was set to 1. n = 3 biological replicates. (C) Representative images of WT and BBX22-overexpression (OE) lines in MicroTom tomato (Solanum lycopersicum) (OE) at breaker (BR), 2 d post-breaker (BR + 2), 4 d post-breaker (BR + 4), and full-ripe stage. Scale bar, 1 cm. (D) Total carotenoid and lycopene content of fully ripe fruits in tomato from WT and OE tomato plants. n = 3 biological replicates. (E) Representative image of bud from the wild-type (WT) and BBX22- OE sweet orange (Citrus sinensis, cv Valencia) lines. Scale bar, 1 mm. (F) Relative expression levels of genes involved in anthocyanin accumulation, including Ruby1, F3′H, DFR, and ANS, in the apical meristem tissue of WT and BBX22-OE lines in SJG. Ruby1, a key MYB regulatory gene involved in anthocyanin accumulation in citrus; F3′H, flavonoid 3′ hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase. The expression level of each gene in WT was set to 1. n = 3 biological replicates. (G) Representative shoots of WT and BBX22-OE MicroTom tomato lines. Scale bar, 1 cm. (H) Anthocyanin content of apical meristem tissue from MicroTom-WT and MicroTom-OE (OE#15, OE#22, and OE#24). n = 3 biological replicates. Data are the mean (±SEM); asterisks represent significant difference by Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001.
A sweet orange (C. sinensis cv. Valencia) bud transgenically expressing green fluorescent protein (GFP)-labeled BBX22 appeared to accumulate more anthocyanin than the non-transformed control (Figure 6E). RT-qPCR analysis of apical meristem tissue showed that, compared with the corresponding WT controls, Ruby1, F3′H, DFR and ANS were expressed at significantly higher levels in BBX22-OE SJG plants (Figure 6F). Similarly, BBX22-OE tomato apical buds appeared to accumulate more anthocyanins than the WT (Figure 6G). Anthocyanin content was significantly higher in the apical buds of BBX22-OE tomato plants than in those of the WT (Figure 6H), and ANT1, F3′H, DFR, and ANS were expressed at significantly higher levels in tomato plants (Figure S6C). The above experiments revealed that BBX22 promotes the accumulation of carotenoid and anthocyanin in citrus and tomato.
BBX22 directly activates PSY1 and Ruby1 transcription, contributing to the light-regulated pigmentation in citrus fruits
In our previous study, we identified HY5 as a key factor in light-regulated anthocyanin accumulation in citrus (Huang et al., 2019). Hence, we investigated whether BBX22 is also involved in light-induced accumulation of pigments in citrus. Bagging of blood orange (C. sinensis) fruits growing on the same branch of the same tree caused fruits to turn light yellow (with low carotenoid and no anthocyanin accumulation), while the fruits accumulated high levels of carotenoids and anthocyanins under natural light conditions (Figure 7A). When the bags were removed, fruits recovered carotenoid and anthocyanin accumulation. These pigment measurement experiments indicated that light induces the accumulation of carotenoids and anthocyanins in blood orange fruits (Figures 7B, C, S7).
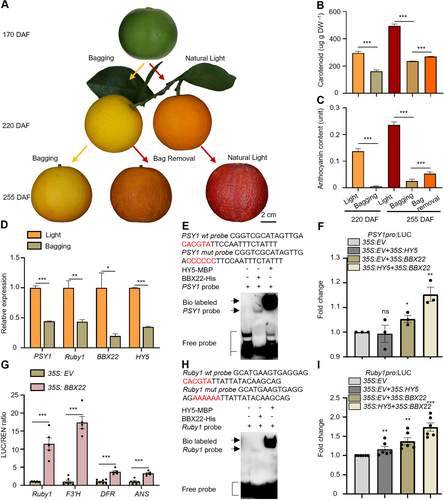
ELONGATED HYPOCOTYL 5—B-BOX DOMAIN PROTEIN 22 (HY5-BBX22) is also involved in the light-regulated fruit pigmentation in citrus
(A) Representative image of blood orange fruits (Citrus sinensis) during bagging treatment (without light) and bag-removal treatment (with natural light) at 170, 220, and 255 d after flowering (DAF). Scale bar, 2 cm. (B) Total carotenoid content of blood orange pulps under light and bagging treatment at 220 DAF and 255 DAF, and under bag-removal treatment at 255 DAF, respectively. n ≥ 3 biological replicates. (C) Anthocyanin content of blood orange pulps under light and bagging treatment at 220 DAF and 255 DAF, and under bag-removal treatment at 255 DAF, respectively. n ≥ 3 biological replicates. (D) Relative expression of phytoene synthase 1 (PSY1), Ruby1, HY5 and BBX22 in pulps of blood oranges grown under light and bagging treatments at 195 DAF. The expression level of each gene under light was set to 1. n = 3 biological replicates. (E) Electrophoretic mobility shift assay (EMSA) showing the binding of HY5-MBP (maltose-binding protein) and BBX22-His protein to the G-box motif in the PSY1 promoter. The + and − symbols indicate the presence and absence of protein or probe, respectively. The wt probe was labeled with biotin (Bio labeled) and FAM (FAM labeled), respectively; the competitor and mutated probe (mut) were unlabeled. (F) Transient dual-luciferase (LUC) assay in tobacco examining the interaction between the BBX22, HY5and PSY1 promoter. (G) LUC assay in tobacco examining the interaction between the BBX22 and Ruby1, F3′H, DFR, ANS promoter. The LUC/REN ratio of 35 S:EV + pro:LUC was set to 1. (H) Electrophoretic mobility shift assay (EMSA) showing the binding of HY5-MBP protein and BBX22-His protein to the G-box motif in the Ruby1 promoter. The + and − symbols indicate the presence and absence of protein or probe, respectively. The wt probe was labeled with biotin (Bio labeled) and 5-carboxyfluorescein (FAM labeled), respectively; the competitor and mutated probe (mut) were unlabeled. (I) Luciferase (LUC) assay in tobacco examining the interaction between the BBX22, HY5 and Ruby1 promoter. Data are the mean (±SEM); asterisks represent significant difference by Student's t-test: ns represents non-significant (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001.
Interestingly, we observed BBX22 exhibited a pattern of co-expression with PSY1 (r = 0.76) and Ruby1 (r = 0.96) in the RNA-seq on blood orange fruits under natural light and subjected to bagging treatment at 255 d after flowering (DAF) (Figure 7D). This result indicates that HY5 and BBX22 may also participate in the light-induced accumulation of carotenoids and anthocyanins in fruits.
An EMSA indicated that BBX22 and HY5 bound directly to the G-box element in the PSY1 promoter (Figures 7E, S8A, B) and PDS1 promoter (Figures S8C, D), which encodes rate-limiting enzymes for carotenoids biosynthesis. However, HY5 showed a stronger binding affinity to the PSY1 promoter and PDS1 promoter compared to BBX22 (Figures 4E, S8E). LUC experiments revealed that BBX22 activated PSY1 transcription, and the BBX22–HY5 complex activated PSY1 transcription to a greater extent than BBX22 alone (Figure 7F).
BBX22 also strongly activated Ruby1, F3′H, DFR and ANS transcription, which are key genes involved in anthocyanin biosynthesis (Figure 7G). EMSA indicated that BBX22 and HY5 could directly bind to the G-box element in the Ruby1 promoter (Figure S9A, B), F3′H promoter (Figure S9C, D) and DFR promoter (Figure S9F, G). HY5 showed a stronger binding affinity to these target genes compared to BBX22 (Figures 7H, S9E, H). The LUC experiments indicated that the addition of BBX22 significantly increased Ruby1 transcription in the presence of HY5 (Figure 7I).
DISCUSSION
BBX22 and HY5 form a cascade regulation involved in GA catabolism to repress internode elongation and plant height
Reduced internode length and plant height are significant objectives in achieving an ideal plant architecture (Kaneko et al., 2003; Hollender et al., 2015). While much is known about the transcriptional modulation of internode elongation in model plants, the molecular regulatory networks that govern internode elongation in perennial fruit tree crops remain largely elusive. GA plays a key role in determining internode length and plant height in diverse plant species (Peng et al., 1999; Sun, 2010). HY5 integrates GA and light signals to control hypocotyl elongation and plant development in model plants (Weller et al., 2009; Burman et al., 2018; Xiong et al., 2023). HY5 is widely recognized as the primary and direct upstream regulator of GA pathway genes. OsbZIP48, a HY5 homolog, participates in GA metabolism by directly inhibiting the expression of ent-kaurene oxidase 2 in rice (Burman et al., 2018). LONG1, another HY5 homolog, participates in the light regulation of GA metabolism by promoting the expression of GA2ox2 in pea (Weller et al., 2009). In pear, PyBBX24ΔN14 acts as a negative regulator of plant height by directly enhancing the transcription of PyGA2ox8 (Yang et al., 2024), indicating that the BBX family can directly bind to and activate genes in the GA catabolism pathway.
In this study, we identified HY5 and BBX22 in a comparative analysis of citrus varieties with short and long internodes. Our analysis of bbx22 mutants in citrus and BBX22-OE lines in citrus and tomato provided evidence that BBX22 is the key gene responsible for internode elongation and plant development (Figure 2). The internode shortening and dwarfing phenotype induced by BBX22 overexpression is stronger than the dwarfing phenotype caused by ACS4 overexpression in citrus (Chu et al., 2023).
We demonstrated that BBX22 not only worked independently by directly binding to and activating GA2ox8 but also facilitated the regulation of GA2ox8 by activating HY5 transcription. Moreover, BBX22 interacting with HY5 forms a cascade regulation, creating a positive feedback loop that boosts HY5 and GA2ox8 transcription (Figure 4), differing from the negative feedback regulation of HY5 by BBX20/BBX21 in tomato (Yang et al., 2022). The regulation module of BBX22 an HY5 also differed from the report that BBXs are dependent on HY5 in modulation of transcriptional regulation in Arabidopsis (Bursch et al., 2020). In our study, the function of BBX22 was independent of HY5. Additionally, it also acted as a cofactor of HY5, enhancing HY5's transcriptional regulatory capacity. Exogenous GA application rescued the shortened internode and dwarf phenotype induced by BBX22 overexpression (Figure 5), suggesting that the transcriptional cascade consisting of BBX22 and HY5 finely tuned GA-mediated citrus internode elongation and plant development.
BBX22 and HY5 play a role in the cross-regulation between GA and carotenoids
Geranylgeranyl pyrophosphate (GGPP) is the biosynthetic precursor for GA, carotenoids and chlorophyll. Mutation of a key enzyme in the carotenoid biosynthesis pathway resulted in a dwarf phenotype due to gibberellin deficiency in Arabidopsis (Qin et al., 2007), whereas overexpression of a key enzyme in the carotenoid biosynthesis pathway had the opposite effect in tobacco (Nicotiana tabacum) (Moreno et al., 2020). In citrus, the combined treatment of GA and prohydrojasmon (PDJ) inhibited carotenoid biosynthesis during the fruit ripening process (Ma et al., 2021). Additionally, the application of GA resulted in decreased carotenoid content and the downregulation of carotenoid biosynthesis genes (Keawmanee et al., 2022). These findings suggest that GA and pigment biosynthesis pathways are cross-regulated. The carotenoid metabolic pathway relates to various hormone pathways, possibly triggering feedback regulation in response to metabolic disruptions. In this study, BBX22 and HY5 expression and pigment accumulation were higher in fruit grown under natural light conditions than in fruit that had been bagged on the tree. Our analysis of bbx22 mutant and BBX22- overexpression lines suggested that BBX22 is involved in carotenoid regulation in citrus. The expression levels of the 2-C-methyl-D-erythritol 4-phosphate pathway gene 1-DEOXY-D-XYLULOSE 5-PHOSPHATE SYNTHASE 1 (DXS1) was significantly upregulated in BBX22-OE citrus (Figure S7A), and may supply more substrates for the metabolism of GA and carotenoids. However, BBX22 directly activated GA catabolism genes (GA2ox8) and carotenoid biosynthesis genes (PSY1 and PDS1). Consequently, in BBX22-OE lines, the balance between GA and carotenoid metabolism was disrupted, resulting in shortened internodes, dwarfism, and an increased carotenoid accumulation phenotype. Application of exogenous gibberellin rescues the shortened internode phenotype and suppresses pigment accumulation, signifying the restoration of balance in GA and carotenoid metabolic flux.
BBX22 and HY5 play a pivotal role in light-regulated pigments accumulation
Light influences plant photomorphogenesis, growth, development, and pigment metabolism. In the model plant Arabidopsis, as part of photomorphogenesis, light-induced anthocyanin accumulation involves HY5 and several BBX proteins (Gangappa and Botto, 2016; Bursch et al., 2020). In the cultivation of perennial fruit tree crops, the application of light serves as a crucial method to improve fruit appearance quality by promoting fruit coloration. Research on cross-regulation between carotenoids and anthocyanins is very limited. Based on our previous research on light-regulated anthocyanins (Huang et al., 2019), in this study, we discovered that light also induces the accumulation of carotenoids. BBX22 was identified as co-expressed with PSY1 and Ruby1, indicating BBX22 cross-regulation between carotenoids and anthocyanins in citrus. The introduction of BBX22 significantly enhanced the HY5-mediated activation of pigment accumulation-related gene transcription. EMSA and LUC demonstrated that HY5 bound directly to and activated these genes. In contrast, BBX22 exhibited weaker binding but stronger activation compared to HY5. This indicated that physical interaction between BBX22 and HY5 is crucial for transcriptional activation. BBX22 and HY5 exerted multi-target regulation on the metabolism of carotenoids (PSY1 and PDS1) and anthocyanins (Ruby1, F3′H, DFR, ANS), finely tuning the metabolic flux. These results demonstrate that BBX22 and HY5 worked independently in the regulation of pigmentation in citrus fruits, and BBX22 also acted as a crucial cofactor of HY5 in light-regulated pigmentation. Furthermore, the amino acid sequence of BBX22 was conserved all citrus species (Figure S10), indicating a conserved transcriptional cascade regulation mechanism consisting of BBX22 and HY5 in light-induced carotenoids and anthocyanin biosynthesis. Hence, our findings strongly indicated that BBX22 served as a previously unknown and potent transcription factor involved in the regulation of internode elongation and plant development in citrus. Achieving shortened internodes and increased pigment accumulation are critical and economically beneficial objectives in the artificial selection and modern breeding of citrus, as these goals can enhance yield by enabling denser planting, reducing labor costs, and improving quality. We showed here that the BBX22 was a strong target for molecular breeding aimed at achieving dwarfism and enhanced pigment accumulation in citrus.
MATERIALS AND METHODS
Plant materials
Citrus materials, including short-internode sour orange, referred to as Xiaoye sour orange (C. aurantium, “SISO”), Chengdu sour orange (C. aurantium, “CDSO”), blood orange (C. sinensis); Anliu sweet orange (C. sinensis, “AL”), early-flowering citrus (Citrus hindsii, “SJG”), Huanglinmiaoju (C. reticulata, “HLMJ”), Dahong sweet orange (C. sinensis, “DHTC”), Yunnan citron (C. medica, “YNXY”), ZM ponkan (C. reticulata, “ZMPG”), Foshou citron (C. medica, “FS”), Juzi (C. reticulata, “JZ”), Linwuyeju (C. linwuensis, “LWYJ”), Wanbaiyou (C. maxima, “WBY”), Juyuan (C. medica, “JY”), Zigui sour orange (C. aurantium, “ZGSO”), Zipiyou (C. maxima, “ZPY”), Daoxianyeju (C. reticulata, “DXYJ”), Guanximiyou (C. maxima, “GXMY”), Aiwanyou (C. maxima, “AWY”), were cultivated in the germplasm resource nursery at Huazhong Agricultural University (Wuhan, China). Majiayou (C. maxima, “MJY”) were cultivated in Science and Technology Innovation Research Center of Majia Pummelo, Guangfeng (Shangrao, China).
Seedlings of SISO and CDSO, as well as transgenic citrus, transgenic MicroTom tomato (S. lycopersicum), and tobacco (N. benthamiana), were cultivated in a growth chamber at 26°C under a 16-h light and 8-h dark photoperiod.
The pulps were obtained from at least three fruits at four different developmental stages of blood orange: 170 DAF, 195 DAF, 220 DAF, and 255 DAF. The pulps were immediately frozen in liquid nitrogen and stored. Bagging treatment (without light) and bag-removal treatment (with natural light) were performed at 170 DAF and 220 DAF.
Phylogenetic tree of sour orange
The Illumina reads of 11 sour oranges, including Huangpi sour orange (C. aurantium, “HPSO”), Bianhong sour orange (C. aurantium, “BHSO”), Pushi sour orange (C. aurantium, “PSSO”), Chenggu sour orange (C. aurantium, “CGSO”), Daidai sour orange (C. aurantium, “DDSO”), CDSO, SISO, Defule sour orange (C. aurantium, “DFLSO”), Baxi sour orange (C. aurantium, “BXSO”) and Banggan sour orange (C. aurantium, “BGSO”), were mapped to the sweet orange genome using Burrows-Wheeler Aligner (Li and Durbin, 2009). Single nucleotide variations (SNVs) among these accessions were identified using vcftools (Danecek et al., 2011). Subsequently, these SNVs were filtered using the parameters “--max-missing 1 --max-alleles 2 --minDP 3 --minQ 30.” SNVs occurring at the four-fold degenerate sites were extracted and used to construct a phylogenetic tree using RaxmlHPC (Stamatakis, 2015). Mangshan (C. mangshanensis, “17 M”) was used as the outgroup.
Expression profiling analysis
The RNA-seq data from the hypocotyl of 1-month-old SISO and CDSO seedlings were aligned to the sweet orange reference genome (http://citrus.hzau.edu.cn/). The RNA-seq data from blood orange fruits under natural light and subjected to bagging treatment at 255 DAF were aligned to the sweet orange reference genome (http://citrus.hzau.edu.cn/). Similarly, the RNA-seq data from the apical meristem of shoots from bbx22 mutants and BBX22 overexpressing lines were aligned to the early-flowering citrus reference genome using Hisat2 in the first step. Subsequently, normalization to fragments per kilobase of transcript per million mapped reads for each predicted transcript was conducted using Cufflink. Cluster Profiler (v4.4.4) was used for GO and KEGG enrichment.
RNA extraction and RT-qPCR
The total RNA was extracted with RNAiso Plus (Takara, China). Complementary DNA (cDNA) was obtained from 1 mg total RNA using the HiScriptII Q RT SuperMix Kit (Vazyme, China). RT-qPCR was carried out with SYBR-Green PCR Master Mix (YEASEN, China) using gene-specific primers on the Roche LightCycler® 480 system (Roche, China). The classic 2−∆∆Ct method was employed to calculate the relative expression levels. RT-qPCR for each sample was conducted with three biological replicates and three technical replicates. The primers are given in Table S1.
Plant transformation
The coding sequences of BBX22 and HY5 were amplified from blood orange pulp and subsequently cloned into the pK7WG2D vector driven by the 35 S promoter with BP and LR enzymes to generate 35 S: BBX22 and 35 S: HY5 (Gateway BP Clonase kit, Gateway LR Clonase kit, Invitrogen, CA). For CRISPR/Cas9-based constructs, small-guide RNA (sgRNA) sequence was designed according to the exon sequences of BBX22 using Citrus Pan-genome to Breeding Database (http://citrus.hzau.edu.cn/crispr/query.php). The AtU6-26-sgRNA-SK vector was amplified using primers containing the corresponding sgRNA and then cloned into the CRISPR/Cas9 vector (pCAMBIA1300-pYAO:: Cas9), as previously described (Tang et al., 2021). The CRISPR/Cas9 gene editing construct and the 35 S:BBX22 construct were transformed into hypocotyls of SJG, AL, and MJY using Agrobacterium tumefaciens following (Tang et al., 2021). The 35 S:BBX22 construct was transformed into cotyledons of MicroTom tomato (S. lycopersicum) using Agrobacterium tumefaciens as described by (Sun et al., 2006). The overexpression lines (OE) of tomato and citrus were confirmed by PCR and RT-qPCR, and the mutations in SJG were confirmed by Hi-TOM sequencing. Two mutants (#13, and #32), and four OE SJG (#1, #7, #21 and #22), three OE AL (#1, #2, and #3), two OE MJY (#1, and #2) and three OE tomato (#15, #22, and #24) were used for further analyses. The primers used in the vector construction are shown in Table S1.
Microscopy procedures
The internodes of BBX22-OE lines in 6-week-old tomato, in 19-month-old bbx22 mutant SJG shoots, and in 3-month-old CDSO and SISO seedlings, were observed for longitudinal sectional parenchyma cells. The internodes of bbx22 mutants were fixed in FAA solution (5% formaldehyde, 50% absolute alcohol, 5% acetic acid) and then embedded in paraffin using a HistoCore Arcadia machine (Leica Biosystems); sections (9 μm in thickness) were obtained using a HistoCore MULTICUT (Leica Biosystems). The internodes of tomato, CDSO and SISO seedlings were fixed in FAA solution and then embedded in cold-curing resin (Technovit 7100); sections (3.5 μm in thickness) were obtained using a semi-thin microtome (Leica RM2265). The sections were observed and photographed using a microscope (Olympus BX51) with a DP72 camera (Olympus). The cell sizes were determined by measuring 50–100 cells in each sample, with 3–6 biological replicates. The cell numbers per unit area of longitudinal sections from the internodes of CDSO and SISO seedlings were determined by measuring at least three areas in each sample, with 3–6 biological replicates.
Anthocyanin and carotenoids extraction and measurement assays
Anthocyanin extraction and measurement followed Huang's protocol (Huang et al., 2018). Fresh powder from blood orange pulps (collected described above) and the apical meristem of 6-week-old transgenic tomato plants (0.1 g each sample) were used for extraction. The extracted samples were then subjected to absorbance measurements at 530 nm and 657 nm using a spectrophotometer (SHIMADZU, Japan; UV-1600). Anthocyanin content was calculated using the formula (A530 - 0.25 × A657)/fresh weight (FW). Each sample was carried out with three biological replicates and three technical replicates.
Carotenoids extraction and quantification were conducted based on a previously reported method (Liu et al., 2007). Approximately 1–3 g power from blood orange pulps (collected as described above) and tomato fruits at full-ripe stage were used. Carotenoid contents were analyzed by high-performance liquid chromatography (Waters 2695, Milford, MA, USA) with a C30 carotenoid column (250 × 4.6 mm, 5 μm; YMC, Wilmington, NC, USA). The mobile phases were composed of acetonitrile : methanol (3:1, v/v) and 100% methyl tert-butyl ether. The identification of the individual carotenoids was performed by comparing the retention times and UV-visible spectral peaks between the samples and standards. Carotenoid levels were calculated according to calibration curves based on authentic standards and are expressed as μg/g FW or dry weight (DW). Each sample was carried out with three biological replicates and three technical replicates.
Yeast two-hybrid assay
To generate the prey and bait vector, AD-BBX22, AD-HY5, BD-BBX22 and BD-HY5, the coding sequences of BBX22 and HY5 were cloned into the PGADT7 vector and PGBKT7 vector using Clone Express II OneStep Kit, respectively (Vazyme, China.).
The Y2H assay was carried out according to the manufacturer's instructions of the Clontech Takara Kit (Takara, China). The AD and BD fusion constructs were co-transformed into the yeast strain AH109 and the positive yeast colonies were cultivated on synthetic defined (SD) medium lacking tryptophan and leucine (-T-L), lacking T, L, His, Ade (-T-L-H-A), and -T-L-H-A containing 20 µg/mL X-α-Gal plates to validate the interaction. Empty vectors were used as negative controls. The primers used in the vector construction are shown in Table S1.
Bimolecular fluorescence complementation assays
For BiFC assays, the JW771-nLUC and JW772-cLUC vectors were used. The full-length coding sequence of BBX22 and HY5 was cloned into JW771-nLUC and JW772-cLUC respectively, using Clone Express II OneStep Kit (Vazyme, China). The assigned combinations of nLUC and cLUC fusion proteins were transiently co-expressed in 5-week-old tobacco (N. benthamiana). Luminescence signals were observed 3 d after infiltration using a living imaging apparatus (NightShade, LC985) following the instructions. The primers used in the vector construction are shown in Table S1.
Transient dual-LUC assay
For a dual-LUC reporter assay, the 35 S:BBX22, 35 S:HY5 and empty vector (EV) were used for the effector vectors. To generate the HY5pro-LUC, GA2ox8-LUC and PSY1pro-LUC reporter vectors, the putative promoters (approximately 1.5 kb ~2 kb upstream of the ATG start codon of these genes) were amplified by PCR using genomic DNA of blood orange as templates. The Ruby1pro-LUC, F3′Hpro-LUC, DFRpro-LUC, ANSpro-LUC reporter vectors were previously constructed in our laboratory (Huang et al., 2018, 2019). The primers used in the vector construction are shown in Table S1.
The corresponding fragments were inserted into pGreen0800-LUC using Clone Express II OneStep Kit, respectively (Vazyme, China). The assigned combinations of effector and reporter were transiently co-expressed in 5-week-old tobacco (N. benthamiana). Luminescence signals were observed 3 d after infiltration using a living imaging apparatus (NightShade, LC985) following the instructions.
Protein purification and EMSA
The coding sequence encoding BBX22 was cloned into pMAL-C2X vector (maltose-binding protein (MBP)-tag) and PET32a vector (His-tag), respectively, and the coding sequence encoding HY5 was cloned into pMAL-C2X vector (MBP-tag) and PET28a vector (His-tag), respectively. These constructs were transformed into Escherichia coli BL21 (DE3), and protein expression was induced by 0.1 mmol/L isopropyl β-D-1-thiogalactopyranoside. Protein purification was conducted using Ni NTA Beads 6FF (for His-tag protein) or Dextrin Beads 6FF (for MBP-tag protein) following the protocol by Smart Life Sciences (Changzhou, China). The primers used in the vector construction are shown in Table S1.
For EMSA, approximately 40-bp promoters containing G-box from the HY5 promoter, GA2ox8 promoter, PSY1 promoter, PDS1 promoter, Ruby1 promoter, F3′H promoter, DFR promoter and ANS promoter were synthesized as single-stranded oligonucleotides (5ʹ 5-carboxyfluorescein (5′ FAM)-labeled or 3′ Biotin-labeled) by Sangon Biotech (Shanghai, China). Competitive probes and mutated G-box probes were unlabeled. 5′ FAM-labeled EMSA were performed as previously described (Zhu et al., 2017a) and 3′ Biotin-labeled EMSA were performed according to the manufacturer's instructions of the Chemiluminescent EMSA Kit (GS009, Beyotime Biotechnology, China). Probes information is given in Table S1.
Pull-down assays
The constructs of BBX22-MBP and HY5-His were co-transformed into E. coli BL21 (DE3) cells and inducted for 16 h at 16°C. GFP was cloned into the PET28a vector (His-tag) and was used as a negative control. Protein purification was conducted using Dextrin Beads 6FF following the protocol by Smart Life Sciences (Changzhou, China). Proteins were detected using anti-HIS and anti-MBP antibodies (ABclonal, China) for BBX22-MBP, HY5-His, GFP-His proteins by western blotting.
The quantification of GA3 and GA3 treatment
The quantification of GA3 in plant materials (apical meristem tissue with some new leaves from bbx22 citrus mutants, BBX22-OE citrus and tomato) was conducted following the manufacturer's instructions of Plant GA3 Enzyme-Linked Immunosorbent Assay Kit (ml077232, Shanghai Enzyme-linked Biotechnology, China).
The GA3 treatment was conducted following the protocol by Chu et al. (2023) with modifications. Four-month-old citrus plants, including four BBX22- OE SJG lines (#21, #22, #21, and #22), three OE AL lines (#1, #2, and #3), and two OE MJY lines (#1, and #2), were treated with 20 μmol/L GA3 (PH102-X; Colaber, China) on d 0, d 5, and d 10. Internode length and plant height were measured on d 0 and d 30. Additionally, the GA3 treatment was carried out in 6-week-old OE tomato (OE#15, #22, #24) with 20 μmol/L GA3 on d 0, d 3, and d 7. Each treatment group included 10 plants with similar growth states, and internode length and plant height were measured on d 0 and d 14.
Statistical analysis
Plant height, internode length, and cell size were measured using ImageJ. The analysis of significant differences was conducted using two-sample Student's t-test with GraphPad Prism 8.3.0: *P < 0.05, **P < 0.01, ***P < 0.001.
ACKNOWLEDGEMENTS
This research was supported by funding from the National Natural Science Foundation of China granted to Q.X. (number 31925034), the National Key Research and Development Program of China granted to Q.X. (number 2022YFF1003100), Major Special Projects and Key R&D Projects in Yunnan Province granted to Q.X. (202102AE090054), the Key Research and Development Program of Hubei granted to X.W. (number 2022BBA155), the Foundation of Hubei Hongshan Laboratory granted to Q.X. (number 2021hszd016), and Key Project of Hubei Provincial Natural Science Foundation granted to Q.X. (number 2021CFA017). We are grateful to Dr. Qi Xie from the Institute of Genetics and Development, Chinese Academy of Sciences, for providing the YAO promoter driven CRISPR/Cas9 vector.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Q.X. supervised the research; J.L.F designed the experiments and drafted the manuscript; Q.X. revised the manuscript; J.L.F performed most of the experiments and analyzed the data; L.L. conducted microscopy procedures and FAM-EMSA experiment; assisted in Y2H assay, LUC assay and plant transformation; J.J.J. assisted in Biotin-EMSA and provided BBX22-His protein; J.S. assisted in plant transformation, vectors construction and LUC assay; Z.H.L., L.Z.S.,Y.H., and S.J.L. conducted the RNA-seq data; Z.H.L., X.W., D.H., Y.T.X. and J.X.H. provided guidance on experimental design and manuscript writing; B.H. assisted in protein purification an pull-down assays; Y.Q.Z. assisted in material management; F.F.W. provided the seeds of Majiayou pomelo; X.X.D. provided critical comments on this project. All authors read and approved the contents of this paper.
Biographies
Open Research
Data availability statement
RNA-seq data from the hypocotyl of 1-month-old SISO and CDSO seedlings, RNA-seq data from blood orange fruits under natural light and subjected to bagging treatment at 255 DAF, and RNA-seq data from the apical meristem of shoots from bbx22 mutants and BBX22 overexpressing lines in this study are available from the National Center for Biotechnology Information under the BioProject PRJNA1116257.






