The processed C-terminus of AvrRps4 effector suppresses plant immunity via targeting multiple WRKYs
Edited by: Dingzhong Tang, Fujian Agriculture and Forestry University, China
ABSTRACT
Pathogens generate and secrete effector proteins to the host plant cells during pathogenesis to promote virulence and colonization. If the plant carries resistance (R) proteins that recognize pathogen effectors, effector-triggered immunity (ETI) is activated, resulting in a robust immune response and hypersensitive response (HR). The bipartite effector AvrRps4 from Pseudomonas syringae pv. pisi has been well studied in terms of avirulence function. In planta, AvrRps4 is processed into two parts. The C-terminal fragment of AvrRps4 (AvrRps4C) induces HR in turnip and is recognized by the paired resistance proteins AtRRS1/AtRPS4 in Arabidopsis. Here, we show that AvrRps4C targets a group of Arabidopsis WRKY, including WRKY46, WRKY53, WRKY54, and WRKY70, to induce its virulence function. Indeed, AvrRps4C suppresses the general binding and transcriptional activities of immune-positive regulator WRKY54 and WRKY54-mediated resistance. AvrRps4C interferes with WRKY54's binding activity to target gene SARD1 in vitro, suggesting WRKY54 is sequestered from the SARD1 promoter by AvrRps4C. Through the interaction of AvrRps4C with four WRKYs, AvrRps4 enhances the formation of homo-/heterotypic complexes of four WRKYs and sequesters them in the cytoplasm, thus inhibiting their function in plant immunity. Together, our results provide a detailed virulence mechanism of AvrRps4 through its C-terminus.
INTRODUCTION
Induced innate immunity in plants is a two-tier system (Chisholm et al., 2006; Jones and Dangl, 2006; Dangl et al., 2013). Pattern-trigger immunity (PTI), the first layer of plant immune response, relies on recognizing pathogen-associated molecular patterns (PAMPs) by plant pattern-recognition receptors (Jones and Dangl, 2006; Dangl et al., 2013). In turn, pathogens have developed various effectors to suppress or bypass PTI responses (Lapin and Van den Ackerveken, 2013; Su et al., 2018). As plants and pathogens have co-evolved for their domination, plants have elaborated the second immune layer, effector-triggered immunity (ETI), which utilizes the specific recognition of pathogen effectors by plant resistance proteins (Andolfo and Ercolano, 2015; Cesari, 2018; Nguyen et al., 2021). Once ETI is activated, plants induce robust immune responses and localized cell death, known as hypersensitive response (HR), to eliminate pathogens and prevent pathogen invasion (Jones and Dangl, 2006; Balint-Kurti, 2019).
The bacterial effector AvrRps4 from Pseudomonas syringae pv. pisi was identified and cloned by Hinsch and Staskawicz (1996). As the name indicates, AvrRps4 was characterized to have an avirulence function in Arabidopsis by activating the corresponding resistance protein RPS4, a Toll/interleukin-1 receptor nucleotide-binding leucine-rich repeat receptor (TNL) (Hinsch and Staskawicz, 1996; Gassmann et al., 1999). In later studies, AvrRps4 was demonstrated to target TNL RRS1, leading to the activation of TNL RPS4 (Narusaka et al., 2009; Williams et al., 2014; Sarris et al., 2015). In Arabidopsis, two linked pairs, RRS1/RPS4 and RRS1B/RPS4B, are required for AvrRps4 recognition (Saucet et al., 2015). In planta, AvrRps4 is processed to form 133 amino acid N-terminal and 88 amino acid C-terminal peptides (Sohn et al., 2009), as shown in Figure S1. Several pieces of evidence showed that the avirulence function of full-length AvrRps4 in Arabidopsis and turnip is mostly due to the C-terminus (Sohn et al., 2009; Sohn et al., 2012; Li et al., 2014). First, conditionally overexpressed AvrRps4C in the Arabidopsis accession Columbia-0 (Col-0) induced a cell death response as strong as full-length AvrRps4-mediated ETI (Li et al., 2014). Second, turnip induced a robust HR when AvrRps4C was transiently expressed (Sohn et al., 2009). Nevertheless, when delivered at native levels by bacteria, both AvrRps4N and AvrRps4C needed to be present to trigger an immune response in Arabidopsis (Halane et al., 2018).
In Arabidopsis, the WRKY transcription factor super-family contains 74 members and is classified into three groups (Eulgem, 2005). This family is characterized by the presence of a WRKY domain comprised of 60 amino acid residues and consisting of a highly conserved N-terminal WRKYGQK motif and a C-terminal zinc-finger structure (Wu et al., 2005; Llorca et al., 2014). WRKY transcription factors require both the WRKYGQK motif and the zinc-finger structure for DNA-binding activity to W-box promoter sequences of target genes (Ulker and Somssich, 2004). Using the WRKY domain, the WRKY transcription factor can bind to many target genes for negative or positive regulation in multiple pathways in planta (Rushton et al., 2010).
Several reports have shown that WRKY members of group III are involved in plant immunity. Overexpression of WRKY46 elevates basal defense in Arabidopsis protoplasts and bacterial resistance in Nicotiana benthamiana (Sheikh et al., 2016). Further, WRKY46 acts as an early ETI marker gene, as shown in the high upregulation in plants expressing avirulence effectors (Gao et al., 2013). WRKY46 redundantly functions with WRKY53 and WRKY70 in basal defense against the bacterial pathogen P. syringae (Hu et al., 2012). During senescence, the expression of some senescence regulators, such as WRKY30, WRKY53, WRKY54, and WRKY70, is partially induced by salicylic acid (SA) (Besseau et al., 2012). WRKY30/53, but not WRKY54/70, respond to reactive oxygen species (ROS) treatment (Besseau et al., 2012). Moreover, WRKY54 and WRKY70 were reported to negatively regulate senescence and resistance to necrotrophic pathogens (Li et al., 2017). Indeed, SA and H2O2 accumulated in the atwrky54 atwrky70 double mutant plant, resulting in accelerated leaf senescence and enhanced immune responses to Botrytis cinerea and Pectobacterium carotovorum. In 2018, Zhou et al. showed that WRKY70 binds to SARD1, an activator of plant immunity in the SA-dependent pathway, and suppresses its expression in the absence of pathogen invasion (Zhou et al., 2018). Later, Liu and co-workers found that WRKY70 is a molecular switch in plant immunity and growth (Liu et al., 2021). CHYR1 ubiquitinates phosphorylated WRKY70 for its degradation. By controlling the degradation of WRKY70, CHYR1 regulates the balance between immunity and growth in planta.
While the avirulence function of AvrRps4 was well studied, its actual virulence function still needs to be fully understood. In the RRS1/RPS4-mediated resistance in Arabidopsis, AvrRps4 interacts with the RRS1-WRKY domain, which functions as an integrated decoy to trap the effector (Sarris et al., 2015). From these observations, it was reasonable to presume that AvrRps4 targets WRKY transcription factors through its C-terminus for initial immunity suppression. In a study on AvrRps4 and PopP2 in 2015, data showed that AvrRps4/AvrRps4C physically interacts with WRKY33, WRKY41, WRKY60, and WRKY70 in planta (Sarris et al., 2015). These WRKYs had been selected because of their previously reported involvement in plant defenses. The targeting of several WRKYs by AvrRps4C and the disparate functions in immunity by individual WRKYs raised the question whether a specific subset of WRKYs is the main target of AvrRps4C for its virulence function.
In this study, using an unbiased screen, we show that AvrRps4C targets members of the sub-group III of the WRKY family, including WRKY46, WRKY53, WRKY70, and especially WRKY54. Among these four WRKYs, WRKY54 provided a pronounced positive contribution to plant defense against bacterial pathogens. However, our data showed that AvrRps4C suppresses WRKY54-mediated immune responses. Indeed, in the presence of AvrRps4C, the general transcriptional activity of WRKY54 and its DNA-binding activity to artificial W-box and target gene SARD1 were reduced. We also show that AvrRps4C enhanced the formation of WRKY hetero-/homotypic complexes that retain WRKY54 and other WRKYs in the cytoplasm and may prevent them from functioning as transcription factors. Our study uncovered the innate virulence function of AvrRps4 through its C-terminus in the regulation of WRKY-mediated immunity.
RESULTS
AvrRps4C is the determinant for AvrRps4-mediated PTI suppression in planta
First, we characterized the virulence function of the effector AvrRps4. To do this, we introduced the effector AvrRps4 into Nicotiana benthamiana leaf cells using the non-pathogenic bacterium Pseudomonas fluorescens (Pf0-1), then infiltrated the bacterium P. syringae pv. tomato DC3000 (Pto DC3000). Pto DC3000 contains the effector HopQ1 that has an avirulence function and is recognized by the resistance protein Roq1 in tobacco species, resulting in ETI (Figure S2) (Wei et al., 2007; Schultink et al., 2017). Our assay relies on the observation that Pf0-1, while non-pathogenic, is a potent inducer of PTI, which suppresses ETI (Oh and Collmer, 2005; Badel et al., 2013; Le Roux et al., 2015). Indeed, the combination of Pf0-1 expressing empty vector (EV) and Pto DC3000 did not induce HR at 2 d post-infiltration (DPI), showing functional Pf0-1-mediated PTI that suppresses DC3000-mediated ETI. However, the tissue expressing AvrRps4 through Pf0-1 delivery showed strong cell death in response to Pto DC3000 (Figure S2). This data suggested that AvrRps4 suppressed PTI responses in N. benthamiana.
It is well known that AvrRps4 possesses two termini in planta. Next, to specify the determinant of AvrRps4-mediated PTI suppression, the PTI suppression assay was conducted using Pf0-1 expressing AvrRps4 or AvrRps4N. In this assay, since AvrRps4C does not contain a Type III secretion signal, we could not use Pf0-1 to deliver AvrRps4C alone. As shown in Figure 1A, the N. benthamiana tissues containing Pf0-1 (avrRps4) induced HR in response to Pto DC3000, while those harboring Pf0-1 (avrRps4N) did not. From this result, we deduced that the C-terminal domain of AvrRps4 determines the observed virulence function. To confirm this speculation, a growth curve assay with Pto DC3000-∆hopQ1 was performed in N. benthamiana. The growth of Pto DC3000-∆hopQ1 was significantly increased in leaves transiently expressing AvrRps4C or AvrRps4 compared to those expressing EV or AvrRps4N (Figure 1B). In addition, N. benthamiana leaves expressing AvrRps4C supported higher bacterial growth than those expressing EV, virulence-deficient mutant KRVY/AAAA (Sohn et al., 2009), and avirulence-deficient mutant E187A (Sohn et al., 2012) (Figure 1C). Moreover, the same pattern was observed in Arabidopsis. Growth curve assays with Pf0-1 delivering EV, avrRps4, and avrRps4N were conducted in Arabidopsis wild-type Col-0 under 30°C conditions (Figure 1D). Several studies have reported that high temperatures can inhibit ETI signaling and enhance PTI signaling (Cheng et al., 2013; Prasad et al., 2022). To suppress AvrRps4-triggered immunity and to analyze the function of AvrRps4C in terms of virulence function in Col-0, we applied high-temperature conditions in our Pf0-1 growth curve assays. In our experimental conditions, we failed to see differences in Pf0-1 bacterial growth in Col-0 at the higher temperature compared to room temperature (Figure S3). In Figure 1D, the growth curve of Pf0-1 (avrRps4N) was similar to that of Pf0-1 (EV), indicating that the N-terminus is not the determinant of effector-triggered susceptibility. In contrast, the growth of Pf0-1 (avrRps4) was significantly higher than that of Pf0-1 (EV) and Pf0-1 (avrRps4N). Next, to determine whether mutant forms of AvrRps4, producing mutant forms of AvrRps4C, fail to enhance bacterial growth of Pf0-1 in Col-0, we generated new Pf0-1 strains expressing avrRps4N, avrRps4, and its mutant variants for growth curve assays (Figure S4). As expected, only Pf0-1 (avrRps4) showed enhanced bacterial colonization of Pf0-1 in Col-0 (Figure S4A). These data indicated that the presence of AvrRps4C, not its mutants, enhanced the growth of a non-pathogenic bacterium in Arabidopsis, indicating suppression of PTI. To confirm that enhanced bacterial growth was specifically caused by AvrRps4C, we generated Arabidopsis transgenic plants expressing AvrRps4C using a dexamethasone (DEX)-inducible construct. In transgenic plants, unlike wild-type Col-0, bacterial growth levels were elevated after DEX treatment compared to mock treatment (Figure 1E). Taken together, our data indicated that AvrRps4C is the determinant of AvrRps4-mediated immune suppression in planta.
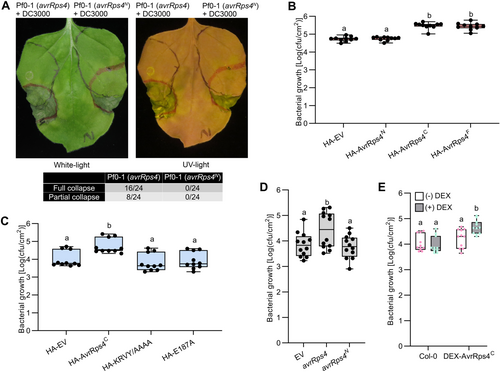
AvrRps4C is the determinant for AvrRps4-mediated bacterial virulence in planta
(A) AvrRps4C inhibits pattern-trigger immunity (PTI) response in Nicotiana benthamiana. Suspensions of Pf0-1 (avrRps4) or Pf0-1 (avrRps4N) were infiltrated into N. benthamiana leaves (black line). After 7 h, a suspension of Pseudomonas syringae DC3000 was infiltrated in an overlapping area (red line). Values indicate the frequency of tissue collapse at 48 h post-inoculation in overlapping areas of leaves from two independent experiments. (B) Growth curve assay of N. benthamiana with DC3000-ΔhopQ1 (pVSP61-EV). Hemagglutinin – empty vector (HA-EV) or HA-tagged effectors were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration at an optical density at 600 nm (OD600) of 0.4. Two d later, DC3000-ΔhopQ1 (pVSP61-EV) was infiltrated into the leaves at an OD600 of 0.0001. Bacterial numbers were measured at 4 d post-inoculation (DPI). Values represent averages from 10 replicates (n = 10). Different letters indicate significant differences (P < 0.0001). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments. (C) Growth curve assay of N. benthamiana with DC3000-ΔhopQ1 (pVSP61-EV). HA-EV or HA-tagged effectors were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration at an OD600 of 0.4. Two d later, DC3000-ΔhopQ1 (pVSP61-EV) was infiltrated in the leaves at an OD600 of 0.0001. Bacterial numbers were measured at 4 DPI. Values represent averages from 10 replicates (n = 10). Different letters indicate significant differences (P < 0.005). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments. (D) Growth curve assay of wild-type Col-0 with non-pathogenic bacteria Pf0-1 (EV), Pf0-1 (avrRps4), and Pf0-1 (avrRps4N) at 30°C. The leaves were infiltrated with Pf0-1 at an OD600 of 0.05. Bacterial numbers were measured at 4 DPI. Values represent averages from 12 replicates (n = 12). Different letters indicate significant differences (P < 0.05). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. This graph represents data combined from four independent experiments. (E) Pf0-1 (EV) growth curve assay under 30°C. The leaves were co-infiltrated with Pf0-1 at an OD600 of 0.05 with or without dexamethasone (DEX) (50 μmol/L). Bacterial numbers were measured at 3 DPI. Values represent averages from 11 replicates (n = 11). Different letters indicate significant differences (P < 0.05). Data were analyzed by two-way ANOVA followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments.
AvrRps4C interacts with WRKY46, WRKY53, WRKY54, and WRKY70
As mentioned previously, in RPS4/RRS1-mediated resistance, AvrRps4C interacts with the WRKY domain of RRS1 (Sarris et al., 2015; Saucet et al., 2015; Mukhi et al., 2021). Since the WRKY domain is well conserved among WRKY transcription factors, we hypothesized that C-terminal AvrRps4 targets one or several WRKYs for the effector's virulence. To test this hypothesis, a yeast two-hybrid (Y2H) screening was performed to check the interaction of AvrRps4C with the WRKY family. Among the WRKY family, we found that AvrRps4C interacts with WRKY46, WRKY53, WRKY54, and WRKY70 in yeast (Figure S5). Interestingly, all four interacting WRKYs belong to sub-group III of the WRKY transcription factor family (Wu et al., 2005), which also contains WRKY52/RRS1 (Kim et al., 2008; Sohn et al., 2014). To confirm the specific interactions of AvrRps4C with WRKY46, WRKY53, WRKY54, and WRKY70, Y2H assays were conducted by co-expressing the effector with these four WRKYs and EV as a negative control. As shown in Figure 2A, AvrRps4C interacted with WRKY46, WRKY53, WRKY54, and WRKY70 in yeast, respectively.
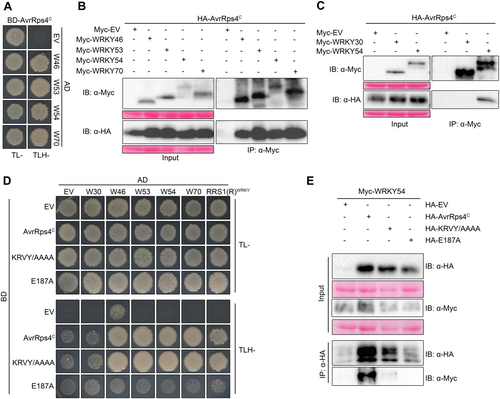
AvrRps4C interacts with WRKY46, WRKY53, WRKY54, and WRKY70
(A) Yeast two-hybrid assay of AvrRps4C and WRKYs. Bait construct (BD-AvrRps4C) and prey constructs (AD-EV or AD-WRKYs) were co-transformed into yeast strain PJ69-4a. The transformed cells were spotted on TL-, medium lacking Trp and Leu (the control medium); TLH-, medium lacking Trp, Leu, and His (the interaction-checking medium). Pictures were taken 4 d after spotting yeast on the media. (B) Co-immunoprecipitation (Co-IP) assay of AvrRps4C and WRKYs. Hemagglutinin (HA)-tagged AvrRps4C controlled by dexamethasone (DEX)-inducible promoter and Myc-tagged WRKYs controlled by 35S-promoter were transiently expressed in Nicotiana benthamiana through Agrobacterium infiltration. Two d post-infiltration, DEX (50 μmol/L) was sprayed on the leaves to induce AvrRps4C induction. Leaves were then sampled 3 h post-DEX treatment, and total protein was extracted for Co-IP assay. This experiment was repeated once with identical results. (C) Co-IP assay of AvrRps4C with WRKY54 and WRKY30. HA-tagged AvrRps4C variants and Myc-tagged WRKYs controlled by 35S-promoter were transiently expressed in N. benthamiana through Agrobacterium infiltration. Two d post-infiltration, leaves were sampled, and total protein was extracted for Co-IP assay. (D) Yeast two-hybrid assay of AvrRps4C variants and WRKYs. Bait construct (BD-EV or BD-AvrRps4C variants) and prey constructs (AD-EV or AD-WRKYs) were co-transformed into yeast strain PJ69-4a. The transformed cells were spotted on TL- (the control medium) and TLH- (the interaction-checking medium). Pictures were taken 5 d after spotting yeast on the media. (E) Co-IP assay of AvrRps4C variants and WRKY54. HA-tagged AvrRps4C variants and Myc-tagged WRKY54 controlled by 35S-promoter were transiently expressed in N. benthamiana through Agrobacterium infiltration. Two d post-infiltration, leaves were sampled, and total protein was extracted for Co-IP assay. This experiment was repeated once with similar pattern.
To further confirm the interaction in planta, we validated these interactions using co-immunoprecipitation (Co-IP) analyses. The combination of proteins (HA-AvrRps4C with Myc-EV, Myc-WRKY46, Myc-WRKY53, Myc-WRKY54, and Myc-WRKY70) were transiently expressed in N. benthamiana leaves. Co-IP, followed by immunoblot analyses, showed that AvrRps4C interacts with all tested WRKYs (Figure 2B). Next, we wondered whether AvrRps4C specifically interacts with four candidate WRKYs, which belong to sub-group III. Therefore, Co-IP of AvrRps4C with WRKY54 or WRKY30, another member from sub-group III (Besseau et al., 2012), was conducted. As shown in Figure 2C, AvrRps4C associated with WRKY54, not with WRKY30, suggesting the interaction of AvrRps4C with candidate WRKYs is specific.
AvrRps4 E187 residue contributes to the interaction of the effector with WRKYs
Several AvrRps4 residues were identified as being essential for its virulence or avirulence functions. In AvrRps4, while the KRVY (135–138 residues) motif is important for AvrRps4-mediated immunity suppression (Sohn et al., 2009), E175 and E187 are required for RRS1/RPS4-mediated HR (Sohn et al., 2012). However, E187, but not E175, contributes to RRS1A/RPS4A-dependent and -independent immunity (Sohn et al., 2012). To determine whether these residues have an impact on the interaction of WRKYs with AvrRps4C, we generated the mutants AvrRps4C (KRVY/AAAA) (KRVY/AAAA) and AvrRps4C (E187A) (E187A) and performed Y2H and Co-IP assays. First, in the Y2H results, wild-type AvrRps4C interacted with WRKY46, WRKY53, WRKY54, and WRKY70, but not WRKY30, which is another member of sub-group III (Figure 2D), as expected. RRS1(R)-WRKY domain was used as a positive control for the interaction in this assay. Interestingly, KRVY/AAAA behaved like AvrRps4C to interact with four candidate WRKY proteins and the RRS1(R)-WRKY domain, but not WRKY30. Unlike wild-type AvrRps4C and the KRVY/AAAA mutant, the E187A mutant showed no interaction with the four candidate WRKYs (Figure 2D). Consistent with the results in the previous study (Mukhi et al., 2021), the E187A mutant failed to interact with the RRS1(R)-WRKY domain. Next, in Co-IP assays, unlike wild-type AvrRps4C, the E187A mutant failed to retain the interaction with WRKY54 in planta (Figure 2E), while the KRVY/AAAA mutant decreased the interaction. We consistently found that the protein expression of E187A was reduced compared to the wild-type and the KRVY/AAAA mutant (Figure S6). We also failed to see any interaction of E187A with WRKY46, WRKY53, and WRKY70 in Co-IP. From these results, we concluded that the E187A mutant does not interact with WRKY46, WRKY53, WRKY54, or WRKY70 in planta, possibly due to the instability of the protein. From this observation, we speculated that E187 contributes to the interaction of AvrRps4 with WRKYs through its protein stability.
AvrRps4C compromises WRKY54-mediated resistance
Since we expected that one/some of the four interacting WRKYs is/are targeted by AvrRps4C due to its virulence function, we wanted to characterize the role of WRKYs in plant defense. For this purpose, we generated transgenic Arabidopsis overexpressing WRKYs (WRKYs-OE), including WRKY46, 53, and 54. WRKY proteins were stably expressed in the transgenic plants (Figure S7).
First, we tested whether these OE lines induce immune changes to Pf0-1. As shown in Figure S8A, only the WRKY54-OE line displayed a reduction in Pf0-1 growth compared to wild-type Col-0 and other WRKY-OE lines at 30°C. In Figure 3A, WRKY54-mediated enhanced resistance in response to Pf0-1 (EV) was also observed in two different independent lines at 23°C, indicating the positive function of WRKY54 in PTI. Furthermore, we also tested the function of WRKYs in N. benthamiana against bacterial pathogens. For this purpose, we transiently expressed WRKYs in N. benthamiana leaves and then inoculated Pto DC3000-∆hopQ1 expressing EV or avrRps4 at 2 DPI. In response to Pto DC3000-∆hopQ1 (EV), only N. benthamiana leaves expressing WRKY54 showed enhanced resistance to bacteria, while others (WRKY46, WRKY53, and WRKY70) displayed similar bacterial growth to N. benthamiana leaves expressing EV (Figure S8B). These data indicated that WRKY54 acts as a positive regulator of plant defense.
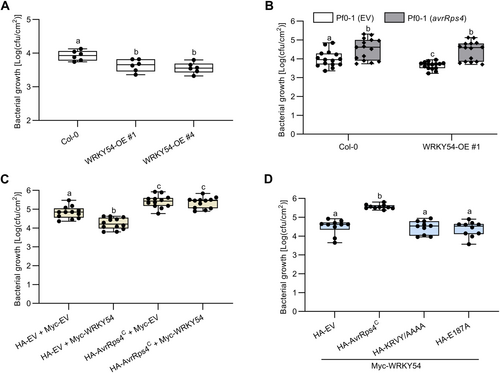
AvrRps4C compromises WRKY54 overexpression (OE)-enhanced resistance
(A) Growth curve assay of wild-type Col-0 and WRKY54-OE lines with the non-pathogenic bacterium Pf0-1 (empty vector: EV) at 23°C. Leaves were infiltrated with Pf0-1 at an optical density at 600 nm (OD600) of 0.1. Bacterial numbers were measured at 3 d post-inoculation (DPI). Values represent averages from 6 replicates (n = 6). Different letters indicate significant differences (P < 0.05). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. This graph represents data combined from two independent experiments. (B) Growth curve assay of wild-type Col-0 and WRKY54-OE with Pf0-1 (EV) and Pf0-1 (avrRps4) under 30°C. The leaves were infiltrated with Pf0-1 at an OD600 of 0.05. Bacterial numbers were measured at 4 DPI. Values represent averages from 15 replicates (n = 15). Different letters indicate significant differences (P < 0.05). Data were analyzed by Student's t-test. This graph represents data combined from five independent experiments. (C) Growth curve assay of N. benthamiana with DC3000-ΔhopQ1 (pVSP61-EV). Hemagglutinin (HA)-EV or HA-AvrRps4C and Myc-EV or Myc-WRKY54 were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration at an OD600 of 0.2 and 0.3, respectively. Two d later, DC3000-ΔhopQ1 (pVSP61-EV) was infiltrated in the leaves at an OD600 of 0.0001. Bacterial numbers were measured at 4 DPI. Values represent averages from 12 replicates (n = 12). Different letters indicate significant differences (P < 0.005). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments. (D) Growth curve assay of N. benthamiana with DC3000-ΔhopQ1 (pVSP61-EV). HA-EV or HA-AvrRps4C variants and Myc-WRKY54 were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration at an OD600 of 0.2 and 0.3, respectively. Two d later, DC3000-ΔhopQ1 (pVSP61-EV) was infiltrated in the leaves at an OD600 of 0.0001. Bacterial numbers were measured at 4 DPI. Values represent averages from 10 replicates (n = 10). Different letters indicate significant differences (P < 0.0001). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments.
Next, we tested the function of not only WRKY54 but also other WRKYs in plant immunity by using wrky T-DNA knockout lines (Figure S9A, B). In response to Pf0-1 (EV), the single wrky T-DNA mutants wrky46, wrky54-1, wrky54-2, and wrky70 did not show alteration of bacterial growth compared to Col-0 (Figure S9C). However, the wrky54-1 wrky70 double mutant displayed increased Pf0-1 growth (Figure S9D), suggesting that WRKYs function redundantly in plant immunity.
Furthermore, the effect of AvrRps4C on WRKY54-mediated enhanced resistance was characterized by growth curve assays in Arabidopsis and N. benthamiana. Compared to Pf0-1 (EV), Pf0-1 (avrRps4) showed enhanced bacterial growth in wild-type Col-0 (Figure 3B). Like in wild-type Col-0, the presence of AvrRps4C compromised the resistance phenotype of WRKY54-OE in response to Pf0-1. The same pattern was observed in the N. benthamiana system. Indeed, the transient expression of WRKY54 increased the resistance of N. benthamiana to Pto DC3000-∆hopQ1 (Figure 3C). In contrast, overexpression of AvrRps4C enhanced susceptibility to the bacteria. Interestingly, AvrRps4C also compromised the enhanced resistance phenotype induced by transient overexpression of WRKY54 (Figure 3C). To confirm whether this defense suppression is specific, we performed a set of growth curve assays with Pto DC3000-∆hopQ1 in N. benthamiana transiently expressing WRKY54 together with AvrRps4C variants. Consistent with previous data, only AvrRps4C, not its mutants, promoted the virulence of Pto DC3000-∆hopQ1 in N. benthamiana (Figure 3D). In addition, in ROS assays (Figure S10), WRKY54 boosted the ROS burst in response to flg22. For unknown reasons, the single expression of AvrRps4C also increased the ROS burst. However, AvrRps4C slightly attenuated the ROS burst caused by WRKY54 when these two proteins were co-expressed. Taken together, our data suggested that AvrRps4C compromises WRKY54-mediated resistance in plants.
AvrRps4C inhibits the transcriptional activity of WRKY54 and WRKY53 in planta
Since AvrRps4C targeted the WRKY54 transcription factor and compromised WRKY54-regulated resistance in plants, we then tested the effect of AvrRps4C on the transcriptional activity of WRKY54. We generated constructs in which WRKYs were fused with a Gal4 binding domain (Gal4BD) driven by the 35 S-promoter and employed a luciferase (LUC) reporter construct with a Gal4-DNA-binding site (GalBS) for Gal4BD (Figure 4A). Using this system, we could investigate the transcriptional activity of WRKY proteins through the binding activity of Gal4BD to Gal4BS. A construct containing the LUC reporter also harbors Renilla (REN) driven by the 35 S-promoter, which was used as an internal control. The transcriptional activity of WRKYs was tested in the presence or absence of AvrRps4C or its mutants. The designed constructs were transiently expressed in N. benthamiana, as this species does not trigger an HR to AvrRps4C. As shown in Figure S11A, WRKY46, WRKY53, WRKY54, and WRKY70 showed high relative LUC/REN activity, indicating strong transcriptional activity. In the presence of AvrRps4C, the transcriptional activity of WRKY46 and WRKY70 were not altered (Figures S11B, D), showing that AvrRps4C did not affect the function of these two WRKYs. Interestingly, the transcriptional activity of WRKY54 and WRKY53 was dramatically reduced when AvrRps4C was expressed in N. benthamiana (Figures 4B, C, S11C, D). However, the reduction of relative LUC/REN activity of WRKY54 by AvrRps4C was compromised in the presence of the E187A mutation, but not with the KRVY/AAAA mutation (Figure 4B). In the case of WRKY53 transcriptional activity assay, AvrRps4C, but not its mutants, suppressed WRKY53 transcriptional activity (Figure 4C). From this result, we propose that WRKY54 and WRKY53 function as strong transcriptional activators, and effector AvrRps4C suppresses the transcriptional activity of WRKY54 and WRKY53. Moreover, the suppression of WRKY54 transcriptional activity by AvrRps4C is interaction-dependent since the WRKY interaction-deficient E187A mutant failed to reduce the LUC/REN ratio.
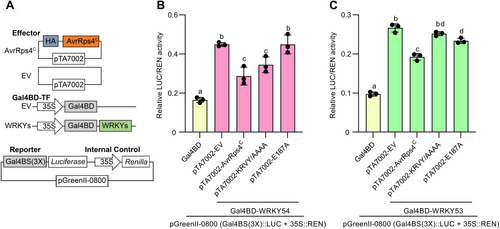
AvrRps4C inhibits the transcriptional activity of WRKY54 and WRKY53 in planta
(A) Vector system used in this assay. The reporter vector pGreenII-0800 contains a Gal4 binding site in the promoter region to control luciferase (LUC) expression. 35 S::REN (Renilla) in the same pGreenII-0800 vector was an internal control for protein expression. To measure the transcriptional activity of WRKY proteins, Gal4BD or Gal4BD-WRKYs were cloned into the pEG100 vector, which contains the 35 S-promoter. Vectors pTA7002 expressing effectors (AvrRps4C and its mutants) were used in the assay to check the effect of effectors on WRKY's transcriptional activity. (B) pGreenII-0800 (Gal4BS(3X)::LUC + 35 S::REN), 35 S::Gal4BD or 35 S::Gal4BD-WRKY54, and dexamethasone (DEX)-inducible effector constructs were transiently expressed in Nicotiana benthamiana leaves through Agrobacterium infiltration. DEX (50 μmol/L) was applied to induce the expression of effectors at 32 h post-infiltration (HPI). Samples were collected from three biological replicates 1 d post-DEX treatment. For LUC/REN measurements, leaf protein was isolated and LUC/REN intensity was measured. Values represent averages from three replicates (n = 3). Different letters indicate significant differences (P < 0.05). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Error bars denote SD. This experiment was repeated twice with similar patterns. (C) pGreenII-0800 (Gal4BS(3X)::LUC + 35 S::REN), 35 S::Gal4BD or 35 S::Gal4BD-WRKY53, and DEX-inducible effector constructs were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration. DEX (50 μmol/L) was applied to induce the expression of effectors at 32 HPI. Samples were collected from three biological replicates 1 d post-DEX treatment. For LUC/REN measurements, leaf protein was isolated and LUC/REN intensity was measured. Values represent averages from three replicates (n = 3). Different letters indicate significant differences (P < 0.01). Data were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. Error bars denote SD.
AvrRps4C reduces the binding of WRKY54 to the W-box sequence
WRKY transcription factors bind to W-box sequences in upstream regions of genes and control gene expression (Ulker and Somssich, 2004). For example, WRKY41 and the WRKY domain of RRS1 were reported to bind to the W-box sequence (Mukhi et al., 2021). Therefore, in this study, to know whether WRKY54 can target W-box-containing genes, electrophoresis mobility shift assay (EMSA) was employed. The recombinant fragment of WRKY54 (aa133-224) was purified according to the report by Hsin et al. (2022). In this assay, to determine the optimal concentration of WRKY54 protein, we tested the combination of recombinant WRKY54 with probes. As a result, the binding complex between WRKY54 and the W-box was displayed as a shifted band in the membrane. Interestingly, the binding activity was enhanced as recombinant WRKY54 protein concentration increased with enhanced density of the detected bands (Figure S12). From this data, 500 µmol/L WRKY54 was used in subsequent experiments. To investigate the effect of AvrRps4C on the binding of WRKY54 to W-box probes, an EMSA assay was performed with AvrRps4C. As expected, a probe band shift in the presence of recombinant WRKY54 protein was observed (Figure 5A). Meanwhile, in the absence of WRKY54, no shifted band was obtained, revealing that WRKY54 solely bound to the W-box sequence. Further, the combination of recombinant WRKY54 with AvrRps4C showed decreased band intensity with increased amounts of AvrRps4C. However, the complex of WRKY54 and W-box was still detected even in the presence of high concentrations of AvrRps4C. These results suggest that WRKY54 strongly binds to the W-box sequence in vitro, and this binding is suppressed in the presence of the effector AvrRps4C.
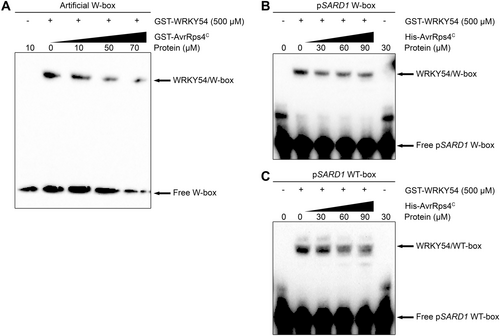
AvrRps4C inhibits the binding of WRKY54 with artificial W-box, pSARD1 W-box, and pSARD1 WT-box
Electrophoresis mobility shift assay of artificial W-box (A), pSARD1 W-box (B), and pSARD1 WT-box (C) DNA binding by WRKY54 (500 μmol/L) following preincubation of increasing concentrations of AvrRps4C. Probes without WRKY54 were used as a negative control for protein-DNA binding.
AvrRps4C represses the binding activity of WRKY54 to the W-box and WT-box on the SARD1 promoter
Previous studies showed that the W-box and WT-box on the SARD1 promoter were targeted by WRKY70 (Zhou et al., 2018; Liu et al., 2021). In this study, we characterized the binding of WRKY54 with SARD1 W-box and WT-box. For this purpose, EMSA assay was performed between purified WRKY54 (133-224) and 3ʹ-Biotin attached W-box/WT-box probes of the SARD1 promoter, with increasing concentrations of purified AvrRps4C. As shown in Figure 5B, C, WRKY54 binds to the W-box and WT-box of the SARD1 promoter. Interestingly, in the presence of AvrRps4C, the binding between WRKY54 and probes decreased as indicated by the reduction of shifted band intensity. This pattern was similar to the binding of WRKY54 to the artificial W-box oligonucleotide in the previous result. From this result, we speculate that WRKY54 may bind to the promoter region of SARD1, particularly its W-box and WT-box, to regulate SARD1 during the immune response. However, AvrRps4C suppresses the DNA binding of WRKY54 to the SARD1 promoter to promote its virulence.
AvrRps4C enhances group III WRKY–WRKY interactions in planta
As shown above, we proposed that WRKY54 may regulate the expression of the target gene SARD1. However, in terms of protein–protein interaction, components associated with WRKY54 in WRKY54-regulated signaling are still unknown. We also observed that AvrRps4C interacts with multiple WRKYs in vitro and in planta (Figure 2). Therefore, we tested whether WRKY54 interacts with other WRKYs in group III by Y2H assay. We failed to assess the interaction of WRKY46, WRKY54, and WRKY70 due to their self-activation activity (Figure S13). Indeed, yeast cells containing BD-WRKY46/WRKY54/WRKY70 with AD-EV still grew in selection media for the interaction. Among four WRKYs as bait, only WRKY53 showed interpretable results. In our experiments (Figure S13), WRKY53 interacted with WRKY46 and WRKY54 in yeast.
Next, to investigate whether one WRKY interacts with another in planta and how AvrRps4C affects the interaction, Co-IP experiments were performed. In WRKY54 Co-IP (Figure 6A), WRKY54 was associated with itself and WRKY53, respectively. Interestingly, their interactions were enhanced when AvrRps4C was expressed. We speculated that WRKY54 bound extremely weakly to WRKY46 and WRKY70. However, the interactions also increased in the presence of AvrRps4C. In WRKY70 Co-IP (Figure 6B), WRKY70 interacted with the other four WRKYs. Like WRKY54 Co-IP, AvrRps4C promoted these interactions. Moreover, not only WRKY54 and WRKY70 but also all other AvrRps4C-interacting WRKYs presented a similar enhancement of binding (Figure S14). Indeed, AvrRps4 enhanced the interaction of WRKY46 or WRKY53 with other WRKYs in group III. These data demonstrated that group III WRKYs can make complexes with one another, and AvrRps4C boosts their interactions.
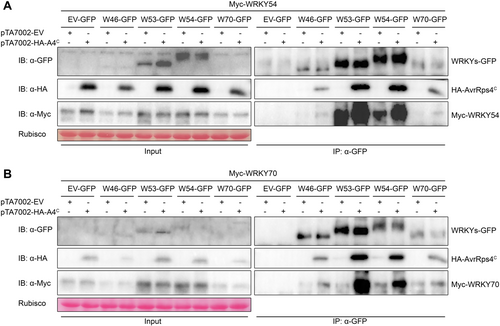
AvrRps4C enhances the interaction of WRKY54 and WRKY70 with other WRKYs in planta
Co-immunoprecipitation (Co-IP) assay of WRKY54 (A) and WRKY 70 (B) with AvrRps4C and other WRKYs. Myc-tagged WRKY54/WRKY70 and green fluorescent protein (GFP)-tagged WRKYs controlled by 35 S-promoter and hemagglutinin (HA)-tagged AvrRps4C controlled by dexamethasone (DEX)-inducible promoter were transiently expressed in Nicotiana benthamiana through Agrobacterium infiltration. Two d post-infiltration, DEX (50 μmol/L) was sprayed on the leaves to induce AvrRps4C induction. Leaves were then sampled 3 h post-DEX treatment, and total protein was extracted for Co-IP assay.
AvrRps4C translocates a portion of group III WRKYs to cytoplasm
Since our data showed that the positive immune regulator WRKY54 was targeted by AvrRps4C, we hypothesized that the localization of the WRKY54-AvrRps4C interaction plays an important role in the suppression of WRKY54's function. Therefore, a bimolecular fluorescence complementation (BiFC) assay was conducted. Our BiFC data indicated that AvrRps4C, but not AvrRps4N, interacted with WRKY46, WRKY53, and WRKY70, and their interactions were observed in the nucleus (Figure 7A). Interestingly, only WRKY54 showed a distinct pattern compared to the other three WRKYs. Indeed, yellow fluorescent protein (YFP) fluorescence resulting from an interaction between AvrRps4C and WRKY54 was localized in the cytoplasm and the nuclear periphery. Our previous Co-IP data showed that WRKY54 formed complexes with other WRKYs in group III (Figure 6). From these data, we questioned the localization of other WRKYs with or without AvrRps4C. To check this, we first determined that all four WRKYs mainly localized to the nucleus in N. benthamiana (Figure S15). Next, we co-expressed WRKY54 or WRKY70 with AvrRps4C or its mutant E187A to observe the green fluorescent protein (GFP) signal of WRKY54 and WRKY70. As shown in Figure 7B, WRKY54 was detected in the nucleus in planta. In combination with mCherry-HA, WRKY54 localized mainly to the nucleus and very weakly was detected in the cytoplasm. Interestingly, cytoplasmic GFP signals of WRKY54 were clearly visualized when wild-type AvrRps4C, not E187A, was co-expressed. In the case of WRKY70, its localization did not alter when WRKY70 was co-expressed with HA or E187A. Similar to WRKY54, cytoplasmic GFP signals of WRKY70 were obtained in the presence of AvrRps4C (Figure 7C). In addition, the interaction of WRKY70 and WRKY54, which was not detected by BiFC when only these two proteins were expressed (Figure 7D), was observed when AvrRps4C was co-expressed. Indeed, in the presence of AvrRps4C, WRKY54 and WRKY70 interacted with each other in the nucleus and cytoplasm (Figure 7D). From these results, we speculate that AvrRps4C translocates a portion of WRKY54 and WRKY70 to the cytoplasm by inducing WRKY54-WRKY70 complexes. However, overexpression of AvrRps4C without WRKY54 induced a cytoplasmic localization of WRKY70 (Figure 7C). We speculated that cytoplasmic localization of WRKY70 by AvrRps4C might be facilitated by native N. benthamiana orthologous WRKY(s) of Arabidopsis WRKY54. Moreover, in the presence of AvrRps4C, we also obtained nuclear and cytoplasmic signals representing the interactions of WRKY54-WRKY54 and WRKY54-WRKY53 (Figure S16). Taken together, AvrRps4C enhances cytoplasmic localization of WRKY54/WRKY70, thus inhibiting their role as transcription factors, which requires nuclear localization.
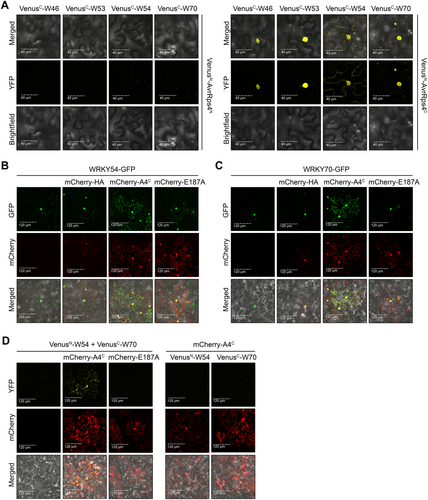
AvrRps4C translocates a portion of group III WRKYs to the cytoplasm
(A) Bimolecular fluorescence complementation (BiFC) assay of AvrRps4C and WRKYs. BiFC constructs were transiently expressed in Nicotiana benthamiana by Agrobacterium infiltration at an optical density at 600 nm (OD600) of 0.2. Two d post-bacterial infiltration, fluorescence signals in N. benthamiana leaves were observed under a confocal microscope. This experiment was repeated once with similar pattern. (B) Green fluorescent protein (GFP)-tagged WRKY54 and mCherry-tagged hemagglutinin (HA), AvrRps4C, and E187A controlled by 35 S-promoter were transiently expressed in N. benthamiana through Agrobacterium infiltration. Two d post-infiltration, fluorescence signals were observed under a confocal microscope. (C) GFP-tagged WRKY70 and mCherry-tagged HA, AvrRps4C, and E187A controlled by 35 S-promoter were transiently expressed in N. benthamiana through Agrobacterium infiltration. Two d post-infiltration, fluorescence signals were observed under a confocal microscope. (D) BiFC assay of WRKY70 and WRKY54. BiFC constructs were transiently expressed in N. benthamiana by agrobacteria at an OD600 of 0.3. Constructs of mCherry-tagged effectors were co-expressed with BiFC constructs at an OD600 of 0.2. Two d post-bacterial infiltration, fluorescence signals in N. benthamiana leaves were observed under a confocal microscope.
In the presence of AvrRps4C, the localization of WRKYs and WRKY–WRKY interactions depend on the localization of AvrRps4C
To gain a greater insight into the effect of AvrRps4C on the localization of WRKYs and WRKY–WRKY interactions, we generated AvrRps4C with a nuclear export signal (NES) and a nuclear localization signal (NLS). In our hands, AvrRps4C-NES, although showing strong cytoplasmic localization, was not completely excluded from the nucleus (Figure S17). Further, AvrRps4C-NLS was localized predominantly to the nucleus and extremely weakly in the cytoplasm (Figure S17). Consistent with previous results, with AvrRps4C, both nuclear and cytoplasmic localization of WRKY54 and WRKY70 could be obtained. In the presence of AvrRps4C-NLS, WRKY54 and WRKY70 were predominantly localized in the nucleus; however, these WRKYs were relocalized to the cytoplasm with AvrRps4C-NES (Figure 8A, B). In the presence of AvrRps4C-NES and AvrRps4C-NLS, the nuclear signals of WRKY54 and WRKY70 were reduced and enhanced, respectively. To further confirm that AvrRps4C is able to guide WRKYs, fractionation of WRKY54 in the presence of AvrRps4C, AvrRps4C-NES, and AvrRps4C-NLS was performed. As shown in Figure S18, WRKY54 was in the cytoplasmic fraction when co-expressed with AvrRps4C, but not with AvrRps4C-NLS. Interestingly, the cytoplasmic WRKY54 expression level was elevated in the presence of AvrRps4C-NES, which forced the majority of AvrRps4C to the cytoplasm. In the combination of WRKY54 and AvrRps4C-NES, WRKY54 weakly remained in the nuclear fraction, most likely because a minor fraction of AvrRps4C-NES was retained in the nucleus (Figure S17). These data suggested that the localization of AvrRps4C altered the localization of WRKYs.
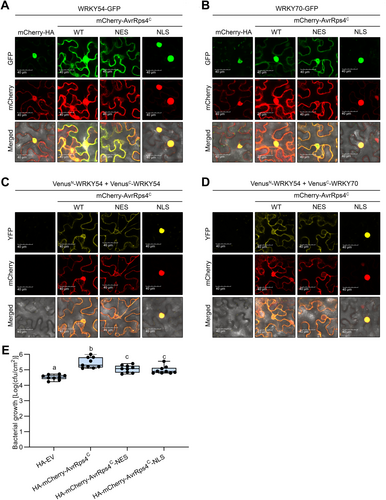
In the presence of AvrRps4C, localization of WRKYs and WRKY–WRKY interactions depend on localization of AvrRps4C
(A) Green fluorescent protein (GFP)-tagged WRKY54 and mCherry-tagged hemagglutinin (HA), AvrRps4C, AvrRps4C-NES (nuclear export signal), and AvrRps4C-NLS (nuclear localization signal) controlled by the 35 S-promoter were transiently expressed in Nicotiana benthamiana through Agrobacterium infiltration. Two d post-infiltration, fluorescence signals were observed under a confocal microscope. (B) GFP-tagged WRKY70 and mCherry-tagged HA, AvrRps4C, AvrRps4C-NES, and AvrRps4C-NLS controlled by the 35 S-promoter were transiently expressed in N. benthamiana through Agrobacterium infiltration. Two d post-infiltration, fluorescence signals were observed under a confocal microscope. (C) Bimolecular fluorescence complementation (BiFC) assay of WRKY54 and WRKY54. BiFC constructs were transiently expressed in N. benthamiana by Agrobacterium at an optical density at 600 nm (OD600) of 0.3. Constructs of mCherry-tagged effectors were co-expressed with BiFC constructs at an OD600 of 0.2. Two d post-bacterial infiltration, fluorescence signals in N. benthamiana leaves were observed under a confocal microscope. (D) BiFC assay of WRKY70 and WRKY54. BiFC constructs were transiently expressed in N. benthamiana by agrobacteria at an OD600 of 0.3. Constructs of mCherry-tagged effectors were co-expressed with BiFC constructs at an OD600 of 0.2. Two d post-bacterial infiltration, fluorescence signals in N. benthamiana leaves were observed under a confocal microscope. (E) Growth curve assay of N. benthamiana with DC3000-ΔhopQ1 (pVSP61-EV (empty vector)). HA-EV or HA-mCherry-AvrRps4C variants were transiently expressed in N. benthamiana leaves through Agrobacterium infiltration at an OD600 of 0.3. Two d later, DC3000-ΔhopQ1 (pVSP61-EV) was infiltrated into the leaves at an OD600 of 0.0001. Bacterial numbers were measured at 4 d post-inoculation. Values represent averages from 9 replicates (N = 9). Different letters indicate significant differences (P < 0.05). Data were analyzed by one-way analysis of variance followed by Tukey's multiple comparison test. This graph represents data combined from three independent experiments.
We also expected the same pattern to occur with the WRKY–WRKY interaction. As shown in Figure 8C, D, WRKY54 homodimerization and WRKY54-WRKY70 heterodimerization in the nucleus were significantly reduced when AvrRps4C was strongly excluded from the nucleus. In contrast, once AvrRps4C predominantly localized to the nucleus, the nuclear signals of those complexes were intensely concentrated. Based on these observations, we tested the impact of AvrRps4C localization on effector virulence function. Growth curve assays with DC3000-∆hopQ1 (pVSP61-EV) were conducted in N. benthamiana expressing EV, wild-type AvrRps4C, AvrRps4C-NES, and AvrRps4C-NLS. Interestingly, leaves expressing AvrRps4C-NES and AvrRps4C-NLS showed higher bacterial growth than those expressing EV (Figure 8E). However, this increase in bacterial growth was significantly smaller than with AvrRps4C, suggesting that proper AvrRps4C localization in the nucleus and cytoplasm is required for the full virulence function of AvrRps4C. Taken together, when AvrRps4C is expressed in plants, both its nuclear and cytoplasmic localization are required for AvrRps4C's virulence by affecting WRKY protein localizations and WRKY–WRKY interactions.
DISCUSSION
To date, AvrRps4-mediated resistance in Arabidopsis through its C-terminus has been well investigated (Sohn et al., 2012; Sarris et al., 2015). Further, Agrobacterium tumefaciens-mediated transient OE provided evidence showing turnip also recognizes AvrRps4C to trigger HR (Sohn et al., 2009). In Arabidopsis, AvrRps4, particularly its C-terminus, interacts with the WRKY domain of RRS1, leading to the activation of the resistance protein RPS4. Even though the avirulence function of AvrRps4 was known, its innate virulence function remained unclear. The contribution to virulence by full-length AvrRps4 was previously reported (Sohn et al., 2009). However, the study did not provide the determinant of the virulence function since AvrRps4 is processed in planta. Additionally, there was no WRKY-related downstream mechanism proposed yet. Our study provides insight into how AvrRps4 induces virulence to suppress plant immunity through its C-terminus. In detail, AvrRps4C targeted members of group III WRKYs, including WRKY46, WRKY53, WRKY54, and WRKY70, and suppressed WRKY54-mediated resistance in Arabidopsis and N. benthamiana.
Since WRKY transcription factors are highly conserved in their WRKY domain, effectors such as AvrRps4 and PopP2 can target multiple WRKYs (Le Roux et al., 2015; Sarris et al., 2015). Indeed, large numbers of WRKYs are acetylated by PopP2 (Le Roux et al., 2015). Further, in planta, AvrRps4 interacted with several WRKYs, such as WRKY33, WRKY41, WRKY60, and WRKY70 (Sarris et al., 2015). Here, we show several experiments proving that AvrRps4C physically interacted with four WRKYs (WRKY46/53/54/70) belonging to group III (Figures 2, S5). Several previous studies demonstrated or showed clues that the four WRKY interactors are involved in plant immunity, as described in the Introduction. However, under our experimental conditions, we obtained the results that only WRKY54 positively regulated disease resistance to bacteria, and its function was inhibited by C-terminal AvrRps4. Indeed, in growth curve assays with Pf0-1 in WRKY-transgenic plants, only WRKY54-OE plants showed enhanced resistance (Figure S8A). Similarly, when we transiently expressed WRKYs in N. benthamiana leaves, only OE of WRKY54 displayed resistance phenotypes to the bacterial pathogen DC3000-∆hopQ1 (Figure S8B). However, WRKY54-mediated defense was compromised in both Arabidopsis and N. benthamiana by AvrRps4C (Figure 3). In addition, as shown in the LUC/REN assay, AvrRps4C inhibited the transcriptional activity of WRKY54 (Figure 4), while it did not alter WRKY46's and WRKY70's activity (Figure S11). Interestingly, in BiFC results (Figure 7), only WRKY54-AvrRps4C showed interaction in both the nucleus and cytoplasm, but other combinations only displayed the fluorescence signal in the nucleus. The cytoplasmic signal of the interaction between WRKY54 and AvrRps4C is exclusive and may reflect a molecular mechanism for the suppression of WRKY54's function by AvrRps4C as a possible virulence activity.
Moreover, we also investigated how WRKY54 contributes to plant defense responses to bacteria and how AvrRps4C disrupts downstream regulation by WRKY54. We found that, like other WRKYs, WRKY54 strongly binds to the W-box sequence (Figure S12), suggesting that WRKY54 can target W-box-containing promoters of downstream genes. WRKY54's closest homolog, WRKY70, was reported to target a plant immunity gene called SARD1 (Zhou et al., 2018). In our data, through EMSA with purified WRKY54 (133-224), we demonstrated that the SARD1 promoter with its W-box and WT-box were also targeted by WRKY54 in vitro. It is possible that when AvrRps4 is delivered from bacteria to plant cells, the DNA-binding activity of WRKY54 to SARD1 is reduced. It is reasonable that AvrRps4C inhibits the DNA-binding activity of WRKY54 (Figure 5), thus preventing WRKY54's transcriptional function. Therefore, in this study, only WRKY54's function in plant immune regulation was affected by AvrRps4C even though AvrRps4C targeted closely related WRKYs in addition to WRKY54.
The KRVY motif in AvrRps4 was characterized to contribute to effector virulence. KRVY/AAAA mutation compromised the virulence of AvrRps4 in N. benthamiana (Sohn et al., 2009). In our data, like wild-type processed C-terminal AvrRps4, the KRVY/AAAA mutant still retained the interaction with four WRKYs in yeast and N. benthamiana (Figures 2D, E, S6). Our results are consistent with the previous study of RRS1/RPS4-mediated resistance (Mukhi et al., 2021). In detail, Mukhi and colleagues showed that KRVY/AAAA interacted with the WRKY domain of RRS1. Further, in Figure 4, the KRVY/AAAA mutant suppressed WRKY54's transcriptional activity as the wild-type AvrRps4C did. However, in growth curve assays in N. benthamiana (Figure 3D), KRVY/AAAA behaved like E187A and failed to induce susceptibility in contrast to wild-type AvrRps4C.
The residue E187 in AvrRps4 has been shown to play a function in AvrRps4's avirulence activity (Sohn et al., 2012). Indeed, plants failed to recognize the E187A mutant (Sohn et al., 2012). Our study showed that E187 also contributed to AvrRps4C's virulence. First, the E187A mutant failed to associate with four WRKYs in yeast and N. benthamiana, maybe due to the instability of AvrRps4C (E187A) (Figures 2E, S6). Second, the E187A mutant failed to inhibit WRKY54's transcriptional activity, suggesting that E187 is required for AvrRps4C's virulence in regulating WRKY54. Hence, further investigation is needed to elucidate a more detailed mechanism of the virulence function of AvrRps4, either through the KRVY motif or E187A.
WRKYs are known to homo- and heterodimerize. For instance, WRKY30 interacts with WRKY54 and WRKY70 (Besseau et al., 2012). In this study, we also investigated whether AvrRps4C-interacting WRKYs in group III can homo- and heterodimerize. Our Co-IP results suggested that these WRKYs interact with each other to varying degrees. Furthermore, the interaction was enhanced by AvrRps4C (Figures 6, S14). Based on this result and previous characterization of AvrRps4C's effects on WRKY54's function, we propose a working model of how AvrRps4C induces virulence by regulating WRKY54, as shown in Figure 9. In PTI or basal defense against bacterial pathogens, members of group III WRKY positively function together in defense gene regulation. Specifically, from our data, we expect that WRKY54 targets the promoter region of SARD1 for SARD1-mediated plant immunity because WRKY70 in the same group has previously been reported to regulate SARD1 expression (Zhou et al., 2018; Liu et al., 2021). In addition, WRKY54 and WRKY70 together positively regulate SARD1 expression (Chen et al., 2021). In the presence of AvrRps4 in bacteria, the effector is delivered through the type III secretion system to plant cells to possess virulence function. AvrRps4C interacts with WRKY46, WRKY53, WRKY70, and in particular WRKY54 to interfere with WRKY54's role in plant immunity. AvrRps4C may isolate WRKY54/70 from the SARD1 promoter by promoting complexes of WRKY54 with WRKY70 in the cytoplasm. Similarly, AvrRps4C also may sequester WRKY46 or WRKY53 from their target genes through complexes with WRKY54. Although AvrRps4C specifically interacts with four mentioned WRKYs in this study (Figure 2B, C) and with several WRKYs (Sarris et al., 2015), there is still the possibility that one or more WRKY(s) is/are the target for AvrRps4C virulence function. We propose that the role of WRKY hetero/homotypic protein complexes in plant immunity needs to be investigated further.
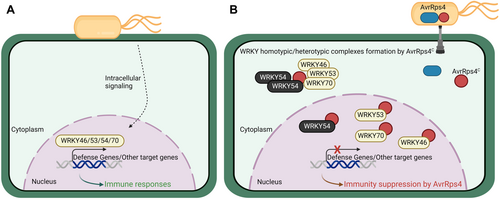
Proposed model of AvrRps4C targeting WRKY54 and suppression of plant immune responses
(A) In response to bacterial pathogens, Group III WRKYs, including WRKY46, WRKY53, WRKY54, and WRKY70, contribute to plant defenses. (B) AvrRps4C delivered by full-length AvrRps4 interacts with WRKYs, induces complex formation among WRKYs, and suppresses WRKY54 activity and its function in WRKY54-mediated defense response. The figure was created with BioRender.com.
Analyzing the effects of AvrRps4C on WRKY hetero/homotypic protein complexes, our data showed that increased cytoplasmic signals for WRKYs and WRKY–WRKY associations were obtained in the presence of AvrRps4C (Figure 7). Moreover, AvrRps4C controlled the localization of WRKYs and their interaction when this protein was expressed (Figure 8). In the study by Heidrich et al. (2011), nuclear localization of AvrRps4C was required for its avirulence function. Indeed, AvrRps4C-NES showed compromised ETI in resistant ecotype Ws. Surprisingly, in our manuscript, both nuclear and cytoplasmic localization of AvrRps4C are necessary for the effector's virulence function. However, our data are not in conflict with previous results. Indeed, for the innate virulence function, cytoplasmic AvrRps4C translocates WRKYs to the cytoplasm through effector-WRKY or WRKY–WRKY interactions as one partial function. Further, AvrRps4C may need to be in the nucleus for some suggested virulence activity, such as suppression of WRKY binding to DNA and of general WRKY transcriptional activity. In turn, plants developed a resistance signaling pathway to recognize AvrRps4C in the nucleus to trigger ETI responses.
To date, there have been numerous studies regarding how AvrRps4 triggers RRS1/RPS4-mediated resistance in Arabidopsis. Given the arms race between plants and pathogens, it is important to consider that the initial function of an effector is to suppress plant immunity or manipulate the host cell in other ways and not to induce ETI in plants containing corresponding resistance proteins. In plants lacking resistance proteins for effector recognition, the effectors retain their pathogenic activity. Therefore, it is still important to understand how an effector generates conditions favorable for the pathogen in order to devise strategies to engineer crop plants with improved resilience and resistance.
Recently, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) proteins have arisen as a novel tool for genome editing. Engineering plant disease resistance is also one of its applications (Borrelli et al., 2018; Schenke and Cai, 2020; Vuong et al., 2023). Until now, we have failed to find any application of CRISPR/Cas on resistance proteins in terms of the effector's avirulence. This suggests that it remains challenging to edit resistance proteins to gain a new effector recognition system. In plant immunity engineering, genome editing objects can be the host target protein of the pathogen effector (Vuong et al., 2023). Effector targets that play a role in basal defense responses or PTI can be positive regulators of basal defenses as well as components of effector-triggered susceptibility. Now, CRISPR/Cas provides a powerful way to precisely edit genes within a short DNA fragment. Ideally, the goal is to edit plant host proteins in such a way that they escape effector targeting but still maintain their functions in plant basal defenses and PTI. As a salient and well-studied example, further experiments on WRKY54 and AvrRps4C are required for an in-depth understanding of the virulence mechanism of the effector on WRKY54. It will be fascinating if WRKY54 can be engineered to escape the targeting by AvrRps4 but still retain its function in plant immunity.
MATERIALS AND METHODS
Plasmid constructs
The coding regions of effector AvrRps4C were re-amplified by polymerase chain reaction (PCR) using the entry construct containing a sequence of full-length AvrRps4 as the template and sub-cloned into pDONR201/207 or pDONRII entry vectors using BP reactions (Invitrogen). For all destination vectors of AvrRps4C except DEX-inducible vector pTA7002 and pEG100, the pDONR201/207-AvrRps4C were recombined into GATEWAY-compatible destination vectors using the LR reaction (Invitrogen). For the pTA7002 and pEG100 constructs containing AvrRps4C, pDONRII-AvrRps4C were recombined with pDONRI-3HA and pTA7002-EV or pDONRI-mCherry and pEG100-EV in a multiple-GATEWAY LR reaction (Invitrogen). All mutant variations of AvrRps4C mentioned in the study were made by site-directed mutagenesis using the entry clones as templates. AvrRps4C-NES and AvrRps4C-NLS were generated from AvrRps4C with additional NES or NLS sequences by PCR. In dual LUC/REN assay, Gal4BD or Gal4BD-WRKYs were cloned into pDONR207 through BP reaction and then cloned into pEG100 through LR reaction, while flanked KpnI-Gal4BS(3X)-BamHI was amplified from Gal4BS(3X)::LUC vector (Go et al., 2014) and cloned into pGreenII-0800 vector. Primer sequences used for cloning and site-directed mutagenesis are listed in Table S1.
The coding regions of WRKY30 were amplified by PCR using complementary DNA (cDNA) from Arabidopsis wild-type Col-0 and sub-cloned into the pDONR201/207 entry vector using BP reactions (Invitrogen). The ones of WRKY46/53/54/70 from the pDEST22 destination clone were cloned into pDONR201/207 using back BP reactions. The entry clones were recombined into GATEWAY-compatible destination vectors using the LR reaction (Invitrogen). Primer sequences used for cloning are listed in Table S1.
Plant growth conditions, generation of transgenic plants, and selection of T-DNA knockout lines
Arabidopsis plants were grown in a growth chamber under a 10 h light/14 h dark cycle at 23°C, 70%–80% relative humidity, and a light intensity of 160–200 µmol photons/m2/s. To generate the transgenic plants harboring pBA-HA-WRKYs or pTA7002-AvrRps4C constructs, the destination plasmids were electroporation-transformed into C58C1 (for WRKYs) and GV3101 (for AvrRps4C) and then transformed into wild-type Col-0 by flower dipping method. Plants were screened by spraying BASTA (1:2000) (for WRKYs) and by spreading seeds on Murashige and Skoog (MS) medium containing hygromycin (30 µg/mL) (for AvrRps4C).
T-DNA knockout lines wrky46 (SALK_134310), wrky54-1 (SALK_017254), wrky54-2 (SALK_111964), and wrky70 (SALK_025198) were obtained from the Arabidopsis Biological Resource Center. Primers for T-DNA confirmation, listed in Table S1, were used to select T-DNA homozygous lines. The wrky54-1 wrky70 double mutant line was generated by crossing wrky54-1 with wrky70.
Reverse transcription PCR (RT-PCR)
Arabidopsis Col-0 and T-DNA knockout lines were grown on 0.5X MS plates under long-day conditions (16 h light/8 h dark) at 23°C. Fourteen-d-old seedlings were sampled for RNA extraction. Total RNA was extracted using RNeasy® Plant Mini Kit (QIAGEN). cDNA was synthesized using ReverTra Ace™ (TOYOBO). PCR using cDNA as template and primers listed in Table S1 was performed to confirm absence of wild-type messenger RNA.
Yeast two-hybrid screening and assay
The prey library contained over 61 WRKY transcriptional regulators from Arabidopsis with full-length cDNAs in the GATEWAY-compatible pDEST22 prey vector (Invitrogen). The bait pDEST32-AvrRps4C and individual prey from the WRKY library were co-transformed into yeast strain PJ69-4a by a standard yeast transformation procedure (Clontech, www.clontech.com), and the transformation mixture was plated on synthetic defined (SD) drop-out media lacking Trp and Leu, or lacking Trp, Leu and His. Plates were kept at 30°C and examined 4 d post-spotting yeast cells. The combination of SALT TOLERANCE (STO) and ELONGATED HYPOCOTYL5 (HY5) (Jiang et al., 2012) was used as a positive control for the interaction, and pDEST32-AvrRps4C with empty vector pDEST22-EV (Invitrogen) as a negative control. Further, pairs of potential interactors were directly co-transformed into PJ69-4a and screened on selective media.
In planta bacterial growth curve assay
Pseudomonas syringae pv. tomato strain DC3000-∆hopQ1 expressing EV were generated by electroporating the pVSP61 constructs. Pseudomonas syringae pv. tomato strain DC3000 (pVSP61-EV), DC3000-∆hopQ1, and Pseudomonas fluorescens Pf0-1 expressing indicated effectors were grown on Pseudomonas Agar F. Bacterial growth assays in N. benthamiana were performed by syringe infiltration of leaves of 4-week-old plants with DC3000-∆hopQ1 expressing pVSP61-EV suspensions at optical density at 600 nm (OD600) of 0.0001 2 d post-agro-infiltration at 23°C. Recovered bacteria were plated and counted on Pseudomonas Agar F containing rifampicin and kanamycin selecting for DC3000-∆hopQ1 (pVSP61-EV), which eliminated Agrobacterium strains harboring expression vectors for transient expression (HA-pBA or Myc-pBA) with spectinomycin resistance. Pseudomonas fluorescens Pf0-1 growth curve assays in Arabidopsis were conducted by syringe infiltration of leaves of 4-week-old plants, which were placed in 30°C 1-d pre-bacterial infiltration, with bacteria suspensions at OD600 of 0.05 at 30°C. For the Pf0-1 growth curve assay in AvrRps4C transgenic plants, bacteria were co-infiltrated at OD600 of 0.05 with 50 μmol/L dexamethasone at 30°C. Three d post-bacteria inoculation, leaf discs with a total area of 1 cm2 per sample were ground in 10 mmol/L MgCl2, and solutions were plated in serial dilutions on selective media in triplicate or quadruplicate.
Pattern-trigger immunity suppression assay
The PTI suppression assays in N. benthamiana were performed as described (Le Roux et al., 2015). For suppression of PTI elicited by Pseudomonas fluorescens in N. benthamiana, bacterial suspensions of Pf0-1 (EV), Pf0-1 (avrRps4), and Pf0-1 (avrRps4N) at OD600 of 0.2 in 10 mmol/L MgCl2 were infiltrated into fully expanded leaves of N. benthamiana. PTI was allowed to develop for 7 h. Then, Pseudomonas syringae pv. tomato DC3000, which induces ETI response in N. benthamiana, with a suspension at OD600 of 0.02, was infiltrated into leaves of N. benthamiana to make overlapping areas. Collapsed tissue in the overlapping area was observed at 2 d post-infiltration.
Agrobacterium-mediated transient expression
Transient expression constructs were transformed into A. tumefaciens strain GV3101 by electroporation method. Bacteria cultured in liquid Luria–Bertani medium overnight were harvested by centrifugation and resuspended in 10 mmol/L MgCl2 with 200 μmol/L acetosyringone (Sigma-Aldrich, www.sigmaaldrich.com) to an OD600 of 0.1~0.4 depending on constructs and experiments. The agrobacteria were incubated for 2 h at room temperature and infiltrated into N. benthamiana leaves using a 1 mL needleless syringe. N. benthamiana plants were placed in a growth chamber under a 15 h light/9 h dark cycle at 23°C, 70% relative humidity. Tissues were collected 2 d after infiltration for protein extraction for western blotting, immunoprecipitation, and signal observation through confocal microscopy.
Reactive oxygen species measurement
Hemagglutinin (HA)- or Myc-tagged constructs were transiently expressed in tobacco leaves through Agrobacterium infiltration at an OD600 of 0.2 and 0.3, respectively. Two days post-infiltration, leaf discs were sampled and washed twice with 100 μL distilled water. Leaf discs were submerged in 100 μL distilled water and kept in the same condition as the experimented plants overnight. On the next day, the solution was replaced with 100 μL distilled water containing luminol (30 μg/mL), peroxidase (20 μg/mL), and flg22 (100 nmol/L) for ROS measurement.
Bimolecular fluorescence complementation, protein localization, and confocal fluorescence microscopy
Bimolecular fluorescence complementation was conducted using Agrobacterium-mediated transient expression in N. benthamiana. Using the linear form of entry vector pDONR201 clones by enzyme cutting, the sequences were cloned into the nVenus/cVenus tag binary vectors by LR reaction. Agroinfiltration was performed as described above. For co-infiltrations, each strain was adjusted to an OD600 of 0.3. mCherry-tagged proteins were expressed by agroinfiltration at an OD600 of 0.2. Two d post-infiltration, plant tissues were viewed under an FV3000 (OLYMPUS) confocal microscope to detect the fluorescence signal.
Protein localization was conducted using Agrobacterium-mediated transient expression in N. benthamiana. Agrobacteria harbored pMDC83 constructs to express GFP-tagged WRKY proteins and pEG100 constructs to express mCherry-tagged effector proteins. Agroinfiltration was performed as described above. Agrobacteria harboring pMDC83-WRKYs and pEG100-mCherry-effectors were adjusted to an OD600 of 0.3 and 0.2, respectively. Two d post-infiltration, 4ʹ,6-diamidino-2-phenylindole (1 μg/mL) staining was performed 30 min before using a confocal microscope to visualize the nucleus signal. Plant tissues were viewed under an FV3000 (OLYMPUS) confocal microscope to detect the fluorescence signal.
Western blot and Co-IP analysis
Agrobacterium strains C58C1 or GV3101 harboring designated constructs, were infiltrated into N. benthamiana leaves at OD600 of 0.2~0.4 depending on constructs. GV3101 bacteria containing P19 was co-infiltrated with each combination at OD600 of 0.2. For the experiment without DEX-inducible constructs, leaves were sampled 2 d post-agro-infiltration. For the experiment with DEX-inducible constructs, at 36 h post-agro-infiltration, N. benthamiana leaves were sprayed with DEX (50 μmol/L). After 4 h, tissues were collected. The leaves samples were ground in IP buffer (0.1 mol/L Tris-HCl pH = 7.5, 0.15 mol/L NaCl, 1 mM ethylenediaminetetraacetic acid, 0.5% NP40, 3 mmol/L dithiothreitol (DTT), 1 × Sigma protease inhibitor cocktail). The lysate was centrifuged twice at 21,200 × g for 10 min. The supernatant containing tagged proteins can be used for western blotting. For the Co-IP experiment, the supernatant was then collected and incubated at 4°C for 3 h with Myc-conjugated beads (Sigma) or GFP manually conjugated beads. Beads were washed five times with IP buffer. Beads were then boiled with a loading buffer for 10 min. Immunoblot was analyzed by treating α–HA-Peroxidase (1:5000) (Roche, 12013819001); α–Myc (1:5000) (Santa Cruz, sc-40 HRP); α–GFP (1:10000) (Abcam, ab290) overnight at 4°C. The blots were rinsed four times with Tris-buffered saline with Tween-20 (TBST). For the GFP blot, the secondary antibody, α–Rabbit (1:15000) (Abcam, ab150079), was applied at room temperature for 2 h. The blot was rinsed four times with TBST. The antigen protein was detected by chemiluminescence ECL reagent (BioRad).
Dual LUC/REN transcriptional activity assay
Constructs pEG100-Gal4BD or pEG100-Gal4BD-WRKYs were transformed into GV3101, while pGreenII-0800 (Gal4BS::LUC + 35 S::REN) was transformed into GV3101 (pSOUP). Agrobacteria harboring pEG100 and pGreenII constructs, with or without DEX-inducible constructs expressing effectors, were infiltrated into N. benthamiana leaves at OD600 of 0.2, 0.3, and 0.2, respectively. Then, infiltrated leaves were sprayed with DEX (50 μmol/L) at 32 h post-infiltration. One d post-DEX treatment, leaves were sampled for protein extraction using the IP buffer described in the previous section. Proteins were mixed with Luciferase Assay Reagent II (Promega), kept in the dark for 2 min, and then measured for LUC activity. After LUC measurements, Stop & Glo® Reagent (Promega) was added to the mixtures for REN measurements after being kept in the dark for 2 min. Both LUC and REN intensities were measured with a Promega GloMax machine using the luminescence method.
Electrophoretic mobility shift assay
For the recombinant protein in EMSA, WRKY54 (133-224) and AvrRps4C were cloned into pDONR207 in the Gateway system and then were incorporated into the pDEST15/pDEST17 vector. The constructs were transformed into Escherichia coli strain BL21-AI. At the OD600 of 0.5 in the large culture, protein production was induced by 0.02% Arabinose. Four h post-induction at 30°C, cells were harvested and resuspended in 1 × phosphate-buffered saline. Cells were then sonicated, and glutathione S-transferase-tagged and His-tagged proteins were purified with Glutathione Affinity Resin Beads (Abcam) and Ni-NTA Agarose (QIAGEN), respectively. For the probes in EMSA, nucleotide sequences with 3ʹ-Biotin attachment were synthesized (Bioneer). A primer pair with biotin labels at their 3ʹ-end was designed for amplification of a double-stranded artificial W-box probe following Mukhi et al. (2021). EMSA assay was performed, followed by LightShift™ Chemiluminescent EMSA Kit (Thermo Scientific™).
Protein fractionation assay
Agrobacterium strains harboring designated constructs were combined and infiltrated into N. benthamiana leaves at OD600 of 0.2 or 0.4. Two d post-infiltration, 2 g of leaf tissue were sampled for each combination. Fractionation assays were performed using CelLytic™ PN Isolation/Extraction Kit (Sigma-Aldrich). Tissues were ground in liquid nitrogen into a fine powder to which nuclear isolation buffer (NIB) was added. The suspension was passed gradually through a filter mesh. The passthrough was centrifuged, and the supernatant representing the cytoplasmic fraction was collected. The pellet was resuspended in NIBA buffer (NIB, 1 × Sigma protease inhibitor cocktail, 0.3% TRITON™X-100) and processed using the “Semi-pure Preparation of Nuclei” protocol according to the manufacturer's instructions. The lysate of pellet suspension was layered on top of 2.3 mol/L sucrose and then centrifuged at top speed in a tabletop microfuge. The green phase and sucrose were discarded, and the pellet was resuspended in NIBA for washing. The mixture was centrifuged. The pellet was resuspended in extraction buffer (5 mmol/L DTT, 1 × Sigma protease inhibitor cocktail) and thoroughly vortexed. The suspended pellet was used as the nuclear fraction. Protein blots were probed with α–Myc (1:5000) (Santa Cruz, sc-40 horseradish peroxidase) and α-mCherry (1:5000) (Cell Signaling, #43590) antibodies for the detection of proteins of interest. Immunoblotting with α–phosphoenolpyruvate carboxylase (PEPC) (1:5,000) (Agrisera, AS09 458) and α–H3 (1:5,000) (Agrisera, AS10 710) antibodies was conducted as a control for the degree of fractionation of cytoplasmic and nuclear proteins, respectively. A secondary α–Rabbit (1:10,000) (Abcam, ab150079) antibody was used with the antibodies against mCherry, PEPC, and H3.
The Arabidopsis Information Resource accession numbers
The Arabidopsis Information Resource accession numbers for the sequences referred to in this paper are AT2G46400 (WRKY46), AT4G23810 (WRKY53), AT2G40750 (WRKY54), AT3G56400 (WRKY70), AT5G24110 (WRKY30), and AT1G73805 (SARD1).
ACKNOWLEDGEMENTS
We thank Dr. Jong Chan Hong (Division of Applied Life Science, Gyeongsang National University, Korea) for providing the WRKY library for the Y2H screen. This research was supported by Basic Science Research Program and LAMP Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R1I1A3054417, 2022R1I1A1A01063394, RS-2023-00301974), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021M3A9I5023695, 2022R1A5A1031361), and grants from the New Breeding Technologies Development Program (RS-2024-00322125), Rural Development Administration, Republic of Korea.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
S.H.K. conceived the project. Q.M.N. and S.H.K. designed the experiments. Q.M.N., A.B.B.I., H.K., J.M., K.A.T.P., and G.H.S. performed experiments. Q.M.N. and S.H.K. analyzed data and wrote the manuscript. E.C., M.C.S., W.G., and S.H.K. edited the manuscript. S.H.K. supervised the project. All authors read and approved the contents of this paper.






