Bioactive food components and their inhibitory actions in multiple platelet pathways
Abstract
In addition to hemostasis and thrombosis, blood platelets are involved in various processes such as inflammation, infection, immunobiology, cancer metastasis, wound repair and angiogenesis. Platelets' hemostatic and non-hemostatic functions are mediated by the expression of various membrane receptors and the release of proteins, ions and other mediators. Therefore, specific activities of platelets responsible for the non-hemostatic disease are to be inhibited while leaving the platelet's hemostatic function unaffected. Platelets' anti-aggregatory property has been used as a primary criterion for antiplatelet drugs/bioactives; however, their non-hemostatic activities are not well known. This review describes the hemostatic and non-hemostatic function of human blood platelets and the modulatory effects of bioactive food components.
Practical applications
In this review, we have discussed the antiplatelet effects of several food components. These bioactive compounds inhibit both hemostatic and non-hemostatic pathways involving blood platelet. Platelets have emerged as critical biological factors of normal and pathologic vascular healing and other diseases such as cancers and inflammatory and immune disorders. The challenge for therapeutic intervention in these disorders will be to find drugs and bioactive compounds that preferentially block specific sites implicated in emerging roles of platelets' complicated contribution to inflammation, tumour growth, or other disorders while leaving at least some of their hemostatic function intact.
Abbreviations
-
- ARA
-
- arachidonic acid, 20:4n-6
-
- COX
-
- cyclooxygenase
-
- CVD
-
- cardiovascular disease
-
- CXCR4
-
- chemokine receptor 4
-
- ENA-78
-
- epithelial-neutrophil activating peptide
-
- GP
-
- glycoprotein
-
- ICAM
-
- intercellular adhesion molecule
-
- LCPUFA
-
- Long-chain polyunsaturated fatty acids
-
- PAMP
-
- pathogen associated molecular pattern
-
- PD-ECGF
-
- platelet-derived endothelial cell growth factor
-
- PDI
-
- protein disulfide isomerase
-
- PDGF
-
- Platelet-derived growth factor
-
- PMPs
-
- platelet microbicidal proteins
-
- PSGL1
-
- P-selectin glycoprotein ligand-1
-
- PTK2
-
- proline-rich tyrosine kinase-2
-
- RANTES
-
- regulated on activation, normal T cell expressed and secreted
-
- SFKs
-
- Src-family kinases
-
- TLRs
-
- toll-like receptors
-
- TxA2
-
- thromboxane A2
-
- VCAM
-
- vascular cell adhesion molecules
-
- VEGF
-
- vascular endothelial growth factor
-
- VSMC
-
- vascular smooth muscle cells
-
- vWF
-
- von Willebrand factor
1 INTRODUCTION
Platelets are the essential components of human blood that play a critical role in penultimate acute thrombotic events (O'Kennedy, Raederstorff, et al., 2017). During normal conditions, platelets reside quiescently. But platelets form a plug whenever any disruption happens in the vascular endothelial cell layer (Davi & Patrono, 2007; Ho-Tin-Noe et al., 2018). At the vascular injury site, adhesion of platelets occurs, followed by platelet activation and aggregation, which is a crucial step in forming the platelet plug (Jackson, 2007). In addition to their involvement in hemostasis and thrombosis, platelets also develop diseases involving processes such as inflammation, immunity, angiogenesis, atherosclerosis, and cancers (Hamzeh-Cognasse et al., 2015; Jain et al., 2010; Sonmez & Sonmez, 2017; Walsh et al., 2015).
Furthermore, these activated platelets activate the complement system, which is also involved in the coagulation cascade (Del Conde et al., 2005). Activated platelets produce the potent platelet agonist known as thrombin, adenosine diphosphate (ADP), collagen and thromboxane (Tx)A2, fibrinogen and factor V, and several others (Andrews & Berndt, 2008). Maintaining physiological platelet activity is crucial for overall hemostasis and other physiological activities (Ho-Tin-Noe et al., 2018). Inefficient activation of platelets compromises hemostasis and can result in excessive clinical bleeding whereas excessive platelet activation is associated with thrombotic complications such as myocardial infarction and stroke. Therefore, blood platelets are a primary target for preventing recurrent cardiovascular events.
Platelets showed increased sensitivity at baseline for platelet plug formation in persons with diabetes, obesity, insulin resistance, hypertension, sedentary lifestyle, regular smoking, and so on (O'Kennedy, Raederstorff, et al., 2017). Platelet hyperactivity is associated with the release of various active components and the shedding of membrane particles. Platelet membrane particles play essential roles in developing atherosclerosis, endothelium dysfunction, blood flow, inflammation, cancer metastasis, and hypertension. Hyperactive platelets engage with the secretion of various sticky growth factors and the release of macro-particles and inflammatory agents, reducing blood flow within the vessel and creating a prothrombic condition (Gawaz et al., 2008). Platelet microparticles are also involved in the atherosclerosis process; thrombus and foam cell formation, cancer metastasis, and inflammation. Platelet membrane glycoprotein (GP), GPIbα, GPV, GPVI, amyloid βA4, TLT-1 (TREM-like transcript-1), P-selectin (CD40L), amyloid-like protein 2, and semaphorin 4D are the most abundantly shed platelet proteins (Jurk & Kehrel, 2005). This eventually leads to platelet hyperactivity and endothelial dysfunction (Kaplan & Jackson, 2011; Massberg et al., 2002; Natarajan et al., 2008; O'Kennedy, Raederstorff, et al., 2017). Also, platelets have several mediators that modulate the functions of platelets and different neighboring cells in the circulation and vascular bed endothelium (Dutta-Roy, 2002; Kaplan & Jackson, 2011).
Apart from hemostasis, platelets play several roles in inflammatory processes (Franco et al., 2015), wound healing processes (von Hundelshausen et al., 2007), host defence mechanism against microbes (Klinger & Jelkmann, 2002), generation of innate immune responses (Semple et al., 2011), tumour growth and malignancies (Franco et al., 2015), angiogenesis (Italiano et al., 2008), development of lymphatic vessel (Hess et al., 2014), and also atherosclerosis processes. Blood platelets can also influence innate and adaptive immune responses (Clemetson et al., 2000; Cognasse et al., 2015; von Hundelshausen et al., 2007). A complex hemostatic mechanism participates in the pathophysiology of various diseases, including cardiovascular disorders, diabetes, hypertension, inflammation, and infections. Among these factors, platelet dysregulation is associated with atherosclerosis and mainly involves the aggregation of platelets and a reduction in blood flow in the vascular endothelium. Blood platelets also act through multiple time-dependent activities, including signal-dependent pre-mRNA splicing and constitutively expressed mRNA translation, in addition to producing instantaneous messengers.
Aspirin, the key inhibitor of COX-1 mediated platelet synthesis of TxA2, has several side effects, which make it inappropriate for primary CVD prevention (Cai et al., 2016; Hennekens & Dalen, 2014). In addition, 25%–30% of people are aspirin resistant (Cai et al., 2016). However, aspirin has multifactorial or pleiotropic effects, including inhibition of uncoupling of oxidative phosphorylation, inhibition of activation of nuclear factor binding kappa-B (NFκ-B), inhibition or stimulation of mitogen-activated protein kinases (MAPK), inhibition of nitric oxide (NO) synthetase activity, decrease ATP level and increase the level of adenosine in the extracellular medium thereby it causes suppression of platelet aggregation in the prevention of cardiovascular events (Amin et al., 1999).
There is rising interest in searching for naturally occurring antiplatelet bioactive compounds that may not have the same adverse effects as many antiplatelet drugs (Dutta-Roy, 2002; Vilahur & Badimon, 2013). Many studies have reported the antiplatelet compounds present in fruits and vegetables (Dizdarevic et al., 2014; Dutta-Roy, 2002; Dutta-Roy et al., 2001; Duttaroy & Jorgensen, 2004; O'Kennedy, Crosbie, Song, et al., 2006; O'Kennedy, Crosbie, van Lieshout, et al., 2006; O'Kennedy, Crosbie, Whelan, et al., 2006; O'Kennedy, Raederstorff, et al., 2017). However, their actions on non-hemostatic functions of platelet are not well investigated. Achieving a better understanding of the roles and mechanisms of activity of these bioactive compounds on platelet functions in non-hemostatic processes may prevent several diseases, including cardiovascular disease (CVD). Since the role of platelets in the development and severity of a variety of diseases other than thrombosis is now well known, the roles of bioactive compounds in these processes deserve more attention (Franco et al., 2015). However, the hemostatic and inflammatory functions of platelets overlap; therefore, antiplatelet agents may also impact platelets' non-hemostatic activities depending on their mechanism of action. This review describes the non-hemostatic functions of human blood platelets and the roles of antiplatelet bioactive compounds in these processes.
2 NON-HEMOSTATIC PROPERTIES OF HUMAN BLOOD PLATELETS
Human blood platelets preserve many functions of the primitive hemocyte. Platelets have broader physiological roles and are involved in many pathological conditions other than thrombosis. Platelets are active participants in several non-hemostatic processes, such as an immune function to tumour biology, atherosclerosis, infection, and diabetes. The challenge for therapeutic intervention in these diseases is to identify bioactive compounds/drugs that preferentially block or prevent specific targets involved in the complex contribution of platelets to these pathological processes while leaving hemostatic functions unaffected. Figure 1 describes the various roles of blood platelets in multiple diseases.
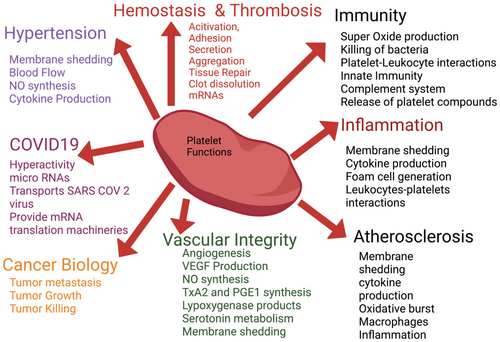
2.1 Human blood platelets and vessel walls interactions
Endothelial dysfunction is a major feature in metabolic syndrome, atherosclerosis, hypertension, diabetes, hyperlipidemia and CVD (Feletou & Vanhoutte, 2006; Guajardo et al., 2018). The critical importance of platelets for maintaining vascular integrity was shown first by Gimbrone and Garcia-Cardena (2016). The molecular mechanisms of forming a hemostatic plug formation are relatively well understood. When the subendothelial matrix proteins are exposed, platelets either interact directly with matrix proteins and integrin α2β1 to subendothelial collagen or bind to von Willebrand factor (vWF) at the site of injury (Chauhan et al., 2007). Expressed GPIIa-IIIb receptors on the platelet surface involve in the aggregation of platelets. Thrombin, TxA2, and ADP generate an activation signal and promote thrombus propagation via G protein-coupled receptors (GPRs) (Del Conde et al., 2005; Kerrigan et al., 2019).
On the other hand, the vascular endothelium uses three different pathways for controlling of reactivity of platelets, (a) arachidonic acid, 20:4n-6 (ARA)–prostacyclin (PGI2) pathway, (b) the L-arginine–NO pathway, and (c), the endothelial ecto-adenosine diphosphatase (ecto-ADPase) pathway (Dutta-Roy et al., 1991; Jin & Loscalzo, 2010; Jurk & Kehrel, 2005). PGI2 is derived from ARA in endothelial cells by COXs and PGI2 synthase (Dutta-Roy & Sinha, 1987; Hechtman et al., 1991). PGI2 inhibits platelet aggregation by increasing cyclic AMP (cAMP) levels in the platelets (Dutta-Roy et al., 1989, 1991; Dutta-Roy & Sinha, 1987). Increased cAMP levels in platelets cause phosphorylation of proteins that restrict GPIIb/IIIa activation from the inside out by inhibiting cytoskeletal re-arrangement (Floyd & Ferro, 2012).
Endothelial nitric oxide synthase (eNOS) produces NO that mediates several antiplatelet effects. NO is diffused into blood platelets to inhibit their adhesion, activation, and aggregation via cGMP-mediated pathways (Dangel et al., 2010; Gkaliagkousi & Ferro, 2011). Ecto-ADPase of the endothelial cell also inhibits platelet aggregation by metabolizing ADP released from activated platelets. By this mechanism, ecto-ADPase regulates the plasma levels of ADP and ATP (Marcus et al., 1997). GPVI and C-type lectin-like 2 (CLEC-2) are immunoreceptor tyrosine-based activation motif (ITAM) receptors of platelets membrane protect vascular integrity at the site of inflammation (Lee & Bergmeier, 2016).
The pathogenesis of atherosclerosis is believed to be initiated by endothelial dysfunction. Endothelium produces angiotensin II (Ang II), endothelin-1 (ET-1), prostaglandin (PG)H2, NO, PGI2, Bradykinin, and so on, and thus contributes to vasoconstriction and vasodilation of the blood vessel (Lai & Kan, 2015). In the absence of a functional endothelium, atherosclerosis and thrombosis are initiated by leukocyte adhesion, activation of platelets, pro-oxidation of mitogens, dysregulation of the production of PGI2, NO, and endothelium-derived hyperpolarizing factor (EDHF), as well as other vasoconstriction factors such as Ang II and PGH2 (Sena et al., 2013). Vasoconstrictors (TxA2, PGH2, ET-1) and vasodilator (NO, EDHF, PGI2) determine vascular tone. Atherosclerosis is also influenced by endothelium expression of adhesion molecules such as selectins, intracellular adhesive molecules (ICAMs), and vascular adhesive molecules (VCAM-1). In addition, trans-endothelial cell migration is promoted by platelet lysophosphatidic acid, including invasion by tumour cells (Ward et al., 2018). Figure 2 describes the impacts of antiplatelet bioactive and aspirin targeting platelet-vessel wall interactions.

2.2 Human blood platelets and atherosclerosis process
Physiological vascular integrity, vascular repair, and pathological derangements of the cardiovascular system depend on the cellular determinants of blood platelets. Blood platelet activation accelerates atherosclerotic processes by forming a link between various metabolic and hemodynamic abnormalities. Platelets become activated at the sites of atherosclerotic lesions and promote angiogenesis by releasing modulators to endothelial progenitors and monocytes (Gawaz et al., 2005). Platelet hyperactivity is associated with various physio-pathological conditions, including hypertension, diabetes mellitus, insulin resistance, smoking, obesity, hypercholesterolemia, and a sedentary lifestyle (El Haouari & Rosado, 2016) and plays a crucial role in the progression of atherosclerosis and thereby promotes CVD (O'Kennedy, Raederstorff, et al., 2017; Tran & Anand, 2004).
Blood platelets of diabetic and hypertensive patients show spontaneous aggregation (Gabbianelli et al., 2003). Hyperactive platelets are involved in inflammatory and atherosclerosis processes by secreting several inflammatory and agonists. Indeed, compounds present in the granules of platelet contribute to the cross-talk between platelets and inflammatory cells during vascular inflammation. Activated platelets produce platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), thus promoting atherosclerosis by increased proliferation of vascular smooth muscle cells (VSMCs) (Andrae et al., 2008). Activated platelets also produce serotonin, ADP, ATP, and lysophosphatidic acid that raise the intracellular Ca2+ concentration in VSMCs, thus enhancing vasoconstriction of a blood vessel and catecholamine response. Isoprenaline and Ang II are the two important agonists of β–adrenoceptors that raise [Ca2+] levels and stimulate VSMC contraction, platelet activation, and aggregation.
Furthermore, Ang II can also increase pH in platelets in hypertensive patients, possibly contributing to platelet hyperactivity. Platelets also have increased intracellular Ca2+ levels and release more β-thrombomodulin and P-selectin. Hypertensive individuals have increased reactive oxygen species (ROS) levels in their platelets, which promote platelet activity by inhibiting NO bioavailability and increasing [Ca2+], among many other cellular effects.
Obesity is associated with increased platelet activation, elevated surface expression of P-selectin, and increased shedding of platelet microparticles. Activated platelets modulate the chemotactic characteristics of endothelial cells by stimulating the production of monocyte chemoattractant protein. Activated platelet α-granules also produce TGF-β, facilitating type-1 plasminogen activator inhibitor secretion from adipose tissue. Activated platelets, adipose tissue, and the endothelium of blood vessels form a feedback loop, leading to an atherothrombotic vascular environment.
vWF is a determinant of atherosclerotic plaque development and mediates platelet recruitment at the site of vascular damage. As endothelial cells release inflammatory stimuli, platelets can be drawn to the area when interacting with vWF. By rolling on endothelial cells, platelets release vWF, modulating the blood flow. In addition, activated platelets express P-selectin involved in platelet–endothelium interactions and thus influence plaque development. P-selectin induces monocytes and macrophages to create chemoattractants and growth factors. In apoE-/- mice, activated platelets aggravate atherosclerosis in a P-selectin–dependent way (Huo et al., 2003).
It is necessary to find new approaches to bridge the gap between the large body of evidence supporting blood platelet involvement in atherogenesis and the relatively modest number of diseases associated with the condition. Several platelet inhibitors from dietary sources or functional foods may represent an effective primary prevention solution, given that CVD is becoming more prevalent due to obesity, diabetes, sedentary lifestyles, smoking, bad diets, and pollution.
2.3 Human blood platelets and the immune system
Human blood platelets act similarly to classic immune cells like macrophages and mast cells, engulf bacteria, secrete chemokines, and remove pathogens from circulation. Many of the underlying mechanisms platelets employ in their immunological response are analogous to their hemostatic activities. Blood platelets contribute to pathological lesions in inflammatory disease and tumour growth due to incorrect reactions at the wrong location and time.
Platelets internalize pathogens, microbes, bacteria, and viruses and kill various internalized pathogens to promote their clearance from the bloodstream and tissues (Cognasse et al., 2015; Li et al., 2020). Platelets exhibit different immunoreceptor attributes and antimicrobial properties against microbial pathogens (Ali et al., 2015; Li et al., 2020; Yeaman, 2010). Apart from the primary agonist, chemokines of platelets play a crucial role in the activation of platelets. The low agonist level induces the secretion of various chemokines from the alpha granule of platelets (Gear & Camerini, 2003). After interacting with chemokine receptor 4, such as CXCR4, chemokines such as CCL4 and CCL17 bind to chemokine receptor 4 (CXCR4/CCR4) and cause platelet aggregation. The chemokines PF4 and SDF-1 also cause platelet aggregation, whereas CCL17 and TARC cause plaque adhesion through CXCR4/CCR4 (Gleissner et al., 2008; Xiao et al., 2008). Several other chemokines are also released from alpha granules that help attract the leukocytes and activate platelets at the injury site. These chemokines are CCL3, also known as MIP1α, CCL5, also known as RANTES, CCL7, initially called MCP3, CXCL1, originally known as GRO-α, CXCL5, also known as ENA-78 and CXCL8 (IL-8), etc (Gleissner et al., 2008; Maurer & von Stebut, 2004). Platelets release the chemokines from alpha granules to combat microbes by trapping and engulfing them (Caudrillier et al., 2012; Klinger & Jelkmann, 2002; Mezger et al., 2019; Morrell et al., 2014; Ribeiro et al., 2019; Sonmez & Sonmez, 2017; Stocker et al., 2017). Chemokines and cytokines released by immune cells or activated platelets regulate inflammatory processes. CCL2/MCP-1, CCL3/MIP-1, and CCL4/MIP-1 recruit monocytes, which are essential in the innate immune response. CCL5/RANTES and CXCL10/IP-10 chemo-attract activated T cells, implicated in the adaptive immune response. CXCL8/IL-8 chemokine attracts neutrophils (Baggiolini, 1998). Platelets release various molecules, including chemokines, and express functional immunoreceptor, which modulates the immune system (Ribeiro et al., 2019; Sonmez & Sonmez, 2017). Platelets also influence the development and activation of neutrophils, macrophages, and dendritic cells by expressing immunological receptors and modulating innate and adaptive immune responses (Banchereau et al., 2000; Morrell et al., 2014).
Interactions between platelets and bacteria induce platelet adhesion, degranulation, shape change, and aggregation. The platelet-bacteria interaction is a complex process in which bacteria use a wide range of receptors, including complement receptors like Fc gamma receptor type 2a (FcγRIIa), GPIIb-IIIa, GPIb and pattern recognition receptors (PRRs), including toll-like receptors (TLRs), nod-like receptors (NLRs), and C-type lectin receptors (CLRs) families to interact with platelets (Hamzeh-Cognasse et al., 2015). TLRs regulate the release of various cytokines from platelets in response to bacterial lipopolysaccharide (Cognasse et al., 2015; Shiraki et al., 2004; Vijay, 2018). TLRs cause inflammation when they come into contact with microbial or inflammatory tissue products. TLRs on platelets may be responsible for the direct engulfment of pathogens similar to leukocytes. PAMPs of various cellular compartments, like plasma membrane, endosomes, endolysosomes, and lysosomes, are recognized by TLRs expressed on platelets (Hally et al., 2020). There are multiple signalling pathways through which TLRs affect platelet function, including ERK1/2, PI3K/AKT, and NF-kB (Marin Oyarzun et al., 2020).
Human blood platelets are also involved in innate immune response with the help of TLRs on the platelet membrane against invaded microbial pathogens (Clark et al., 2007; Cognasse et al., 2015; Semple et al., 2011). TLR1, TLR2, and TLR6 are the predominantly expressed TLRs on platelet surfaces (Ebermeyer et al., 2021). Human blood platelets also express TLR4, TLR8, and TLR9, but TLR2 and TLR9 are highly expressed on platelets at the time of activation (Cognasse et al., 2005; Ebermeyer et al., 2021; Shiraki et al., 2004). Different biological reactions are triggered when TLRs are activated on platelets (Cognasse et al., 2015; Foley & Conway, 2016), leading to the release of TNFα (Aslam et al., 2006) and interleukin (IL)-1 (Blair et al., 2009). Platelets also recognize different isoforms of LPS, resulting in a varied reaction and the release of certain chemokine and cytokine types (Berthet et al., 2012).
Human platelets release platelet microbicidal proteins (PMPs), or platelet kinocidins and PF4, which have antimicrobial activity. Plasma PF4 showed antimicrobial activity against various pathogenic bacteria and fungi, and its levels significantly increased in Plasmodium falciparum-induced malarial disease conditions. Platelets also release CXCL4, CXCL7, and CCL5, defensins, human β defensin 2, thymosin β4, some derivatives, thrombocidins, and fibrinopeptide A and B (Scheuerer et al., 2000; Yeaman, 2014). These chemokines and kinocidins ensure the process of platelet recruitment and accumulation at the infection site (Clemetson et al., 2000; Yeaman, 2014). Moreover, platelets can also modulate other immune cells to release chemokines, kinocidins, and RANTES (CCL5) (Yeaman, 2014). Platelet-derived RANTES modulates immune function by increasing the cytotoxic ability of T cells and the production of cytokines. In addition, platelet-derived PF4 can kill erythrocytes infected with parasites. Platelets also play a role in innate immunity because thrombocidins contain antibacterial and antifungal characteristics. Even non-specifically, the constituents of platelet lysosomal granules, such as cathepsin, degrade the microbes (Sonmez & Sonmez, 2017; von Hundelshausen & Weber, 2007). Indeed, platelets and neutrophils can combine to form a neutrophil extracellular trap (NET) (Kraemer et al., 2011). Neutrophils use a NET to trap and destroy pathogens. The development of NET is a crucial mechanism of neutrophil death; inhibiting this process can lead to a rise in infections (Kraemer et al., 2011). A small antimicrobial cationic peptide β-defensin released from the platelet also can create a NET. The case a gram-positive bacterial infection causes the activation of platelets. These activated platelets then surrounded and trapped them and limited their proliferation using β-defensin 1.
P-selectin is an essential component of the alternative pathway complement system that acts as a C3b binding protein on the platelet surface to induce complement activation (Del Conde et al., 2005). Leukocytes can roll on a template of adhering platelets, firmly adhere, and subsequently transmigrate through the adherent platelets, similar to how they interact with inflammatory endothelium (Ley et al., 2007; Wagner & Frenette, 2008). Adhesive receptors, cellular shape, and, most importantly, shear forces created inside moving blood regulate leukocyte rolling and adherence to platelets or endothelial cells (Hammer & Apte, 1992). The selectin family of sticky receptors mediates the first stage of cellular rolling.
Endothelial cells expressed both P-selectin and E-selectin, which have a role in endothelial cell–leukocyte interactions. P-selectin glycoprotein ligand-1 (PSGL1) is the best-characterized leukocyte ligand for P-selectin; it can bind directly with all subtypes of selectin molecules. Furthermore, PSGL1, with the help of its cytoplasmic domain, causes the activation of leukocyte 2 integrins (Miner et al., 2008). Firm leukocyte adhesion and arrest require both immobilized and released chemokines. Immobilized chemokines induce leukocytes to be arrested in reconstituted systems [113,114], primarily due to the activation of GPRs. Platelet-generated chemokines such as PF4/CXCL4, CCL5/, and CXCL1/GRO- can modulate leukocyte activity and platelet–leukocyte interactions (Clemetson et al., 2000; Koenen et al., 2009).
The PSGL-1 and GPRs direct leukocytes to produce transcription factors, cytokines, and chemokines (Lindemann et al., 2005; Weyrich et al., 1996). Integrin αMβ2 (Mac-1) found on neutrophils plays a key role in integrin adherence by binding to GPIb and/or other ligands; notably, fibrinogen, which also interacts with integrin αIIbβ3 of platelets proline-rich tyrosine kinase-2 is a type of tyrosine kinase that occurs in the phosphorylated condition in leukocytes when they attach to platelets. Pyk2 played an essential role in maintaining the adhesion of platelet–neutrophils in murine and human cells, which could be a critical downstream modulator of Src-family kinase-dependent signalling (Evangelista et al., 2007).
Platelets promote wound healing by secreting thrombin and a range of growth factors, cytokines, and chemokines (Dutta-Roy et al., 1986; Nurden, 2007). Thrombin also acts as a chemoattractant, bringing macrophages, stromal cells, and endothelial cells as a growth factor for mitogenic activity and as an angiogenesis promoter (Tsopanoglou et al., 1993). Platelet CD154 has a role in modulating adaptive immunity and modulating innate immunity. In a murine model, platelet CD154 induces CD8+ T-cell responses to regulate adaptive immunity. It is necessary for producing different antibodies such as IgG, IgM, and IgA by interacting with CD40 found on antigen-presenting cells (Elzey et al., 2003). Furthermore, alloantibodies can activate complement pathways, causing endothelial damage and the subsequent recruitment of platelets (Yamakuchi et al., 2007).
2.4 Human blood platelets and inflammation
Platelets play a larger role in vascular inflammation (Bain et al., 2014). Platelets contribute to the progression of a chronic inflammatory disease such as atherosclerosis by inducing chemokine deposition, aggregation of inflammatory cells, and recruitment of leukocytes at the vascular wall (Gawaz et al., 2005). Activated platelet causes the alteration of chemotactic, adhesive, and proteolytic properties of endothelial cells by releasing inflammatory mediators, mitogenic molecules, and the local circulatory microenvironment (Koenen et al., 2009; von Hundelshausen & Schmitt, 2014). As a result, these altered endothelial cells help support the chemotaxis, adhesion, and transmigration of monocytes to the site of inflammation (Aoki et al., 2006). This sequence encourages monocyte migration and adherence to the location, which further leads to an increase in the formation of atherosclerotic plaques (Badimon et al., 2012). The involvement of platelet in inflammation is evident in antiplatelet drugs' effectiveness in inflammatory conditions (Muller et al., 2015; Pitchford, 2007).
Human platelets store, express, and release various inflammatory mediators, predominantly stored in α-granules (Sonmez & Sonmez, 2017). Eicosanoids, serotonin, fibrinogen, vWF, PDGF, platelet-derived angiogenesis mediators such as VEGF, FGF, and other plasma proteins protease inhibitors (Coppinger et al., 2004; Koupenova et al., 2018). Platelets play a role in inflammation by allowing these mediators to adhere to other cells via immunoreceptors or by enabling them to produce chemokines. Platelets interact with leukocytes, neutrophils, monocytes, endothelial cells, lymphocytes, dendritic cells, erythrocytes, and cancer cells (Franco et al., 2015; Ghasemzadeh & Hosseini, 2013; Koupenova et al., 2018).
PF4 also contributes to atherosclerosis by stimulating atherogenesis and causing vascular inflammation (Duchene & von Hundelshausen, 2015). PF4 prevents the degradation of LDL by inhibiting the interaction between LDL and its receptors (Gawaz et al., 2008). PF4 promotes neutrophil granule release and endothelial cell adhesion, inhibits monocyte apoptosis, and increases monocyte differentiation into macrophages and ROS generation (Scheuerer et al., 2000). Platelet-derived CD154 (CD40L) produces inflammatory reactions in the vascular endothelium layer by associating with CD40 expressed in vascular endothelium (Badimon et al., 2012). Platelet CD40L, in turn, can cause endothelial E-selectin, VCAM1, and ICAM1 expression, as well as MCP1 and IL-8 secretion from endothelial cells (Badimon et al., 2012). In addition, endothelial cells can be induced to create ROS, adhesion molecules, chemokines, and tissue factors by platelet-derived CD40L, all of which contribute to inflammatory and atherosclerotic processes (Badimon et al., 2012). In LDL-receptor-/- mice, blocking the CD40-CD40L signalling pathway dramatically reduces atherosclerotic plaque formation and arterial lipid deposition (Mach et al., 1998). Furthermore, in various types of CVD, cigarette smoking and type 2 diabetes mellitus act as significant risk factors by releasing the increased amount of CD40L (Jinchuan et al., 2004).
IL-1 causes active platelets to produce more chemokines and increases neutrophils adhering to the endothelium. IL-1 increases platelet binding to collagen and fibrinogen and promotes platelet aggregation (Beaulieu et al., 2014). The P-selectin expression on activated endothelial cells or platelets encourages the migration of platelet microparticles harbouring the PSGL-1 and tissue factor to regions of vascular damage (Zaldivia et al., 2017).
Platelet microparticles account for 60 per cent to 90 per cent of all microparticles in circulation. Platelet microparticles are similar to exosomes and, apoptotic bodies, extracellular vesicles. Platelet microparticles contain GPIIb/IIIa, P-selectin, and vWf, all of which are involved in primary homeostasis. Since platelet microparticles include procoagulant factors, nucleic acid, mitochondria, chemokines, and cytokines, they can influence the inflammatory pathway, atherosclerotic plaque development, and thrombosis (Zaldivia et al., 2017).
Platelet microparticles increase COX-2-mediated prostaglandins synthesis in monocytes and endothelial cells (Vajen et al., 2015). Chemokines of platelet microparticles act as a chemoattractant for monocytes or encourage them to differentiate into macrophages. PF4 causes endothelial cells to produce E-selectin (Yu et al., 2005) and release matrix metalloproteinases-2 and -9, which cause atherosclerosis by degrading the extracellular matrix component (Javaid et al., 2017).
O2 is a critical NO scavenger that regulates redox-sensitive ectonucleotidases on platelet and endothelial cell membranes (Davi & Patrono, 2007). ROS prevents NO-mediated late disaggregation of thrombus as it scavenges NO. The COX-1 enzyme involves forming ROS by active platelets by metabolizing ARA. The different isoforms of NADPH oxidase in platelets are activated by agonists that promote platelet activity. The synthesis of O2- by platelets via the route dependent on these oxidases aids in the recruitment of platelets to a developing thrombus.
Furthermore, NO can be removed more quickly by reacting with COX-1 products. ROS causes the peroxidation of membrane phospholipids and LDL, resulting in enhanced production of F2-isoprostanes. F2-isoprostanes can modify the adhesive responses and platelet activity. The synthesis of F2-isoprostanes and thromboxane has a continuous link, suggesting that thromboxane-dependent platelet activation may be triggered by a low-grade inflammatory state and associated metabolic diseases.
Thrombocytopenia is a condition in which platelet count decreases, which can reduce inflammation-induced permeability of endothelial and extravasation of leukocytes without producing haemorrhage; platelet inflammatory functions are more responsive to platelet count reductions than inflammation-associated hemostasis ("Coronary heart disease in seven countries. Summary," 1970). Diverse platelet concentration threshold requirements for different platelet functions during inflammation suggest that it might have a role in targeting separately to discover innovative therapeutic strategies for platelet-dependent diseases. ADP, TxA2, or thrombin allow more platelets to be attracted and aggregated by activating GPIIb-IIIa on the platelet surface. In an animal model, platelet GPRs were shown to have a role in inflammation-related hemostasis (Ho-Tin-Noe et al., 2018).
In inflammation-related scenarios, platelets can prevent bleeding without GPIIb/IIIa-mediated aggregation (Ho-Tin-Noe et al., 2018). Platelets GPVI and CLEC-2 are one type of CLR, used ITAM for signal transduction which has a role in antigen and Fc receptors mediated signalling to their signalling on the mouse and human platelets, may be necessary for platelets' functions in tumour vascular protection (Ho-Tin-Noe et al., 2018). The ability of platelets to prevent bleeding without aggregation has gotten a lot of attention in recent years because it explains why selective can effectively prevent the formation of occlusive thrombus without a significant risk of bleeding in homeostasis (Lebas et al., 2019).
2.5 Human blood platelets and cancers
Human blood platelets have a role in cancer metastasis and angiogenesis in various malignancies, including colon cancer, breast cancer, lung cancer, ovarian carcinomas, and melanoma. In cancer patients, hypercoagulable states are associated with hemostatic and platelet abnormalities (Varki, 2007). Tumours express several membrane receptors that bind and activate platelets (Palumbo et al., 2005), thus, inducing platelet activation or aggregation. The activated platelets mediated coagulation system helps in the pathophysiology of cancer by promoting tumour development and progression. Adhesion, activation, and aggregation of blood platelets influence cancer patients' coagulation cascade and thrombus formation. Platelet membrane proteins such as integrins, glycoproteins, and many other signalling receptors are involved in these processes. Within the circulatory system, platelets help spread cancer via angiogenesis and help in immune evasion by providing physical and mechanical support, promoting their attachments to the endothelium, and helping in extravasations to the secondary organ to generate secondary lesions. Because of such contributions, platelets to tumour cell survival and spread are now recognized as a new target for therapy (Gay & Felding-Habermann, 2011). Different types of cells present in bloodstreams, such as endothelial cells, platelets, lymphocytes, macrophages, mast cells, fibroblasts, bone marrow-derived progenitor cells, and hypercoagulable state forming components, also help in metastasis processes.
Diverse set receptors of platelet surface are implicated in cancer and cancer cells (Monzavi-Karbassi et al., 2007). Platelets have a vast range of pro-and antiangiogenic substances that have a role in physiological and pathological angiogenesis and tumour formation (Battinelli et al., 2011; Walsh et al., 2015). Platelets contain antiangiogenic inhibitors such as endostatin, TSP-1, PF4, plasminogen activation inhibitor-1, and TGFβ proteins, as well as pro-angiogenic activators such as VEGF, PDGF, FGF-2, and MMP-9 (Basak et al., 2013; Battinelli et al., 2011; Johnson & Wilgus, 2014). In cancer patients, the amount of VEGF generated by platelets and released into the blood is high, and it is an essential prognosis for numerous malignancies. Platelet-derived endothelial cell growth factor (PD-ECGF) is another pro-angiogenic factor found in platelets. Platelet activation and the coagulation system are essential in cancer metastatic development. The GPIb-IX-V knockout mice had a 15-fold reduced melanoma cell metastasis (Jain et al., 2007). Other platelet membrane receptors, such as GPVI and GPI-Ib-IIIa, have a role in cancer progression (Jain et al., 2009). PDGF signalling pathway is involved in the growth of ovarian cancer, prostate cancer, and glioma cell lines. In addition, PDGF is involved in activating cancer cells (Pietras et al., 2001). Since platelets play pro-tumorigenic roles predominantly in various cancers, these are a possible target in anticancer therapy research. Designing optimum anticancer therapy in patients with active tumour malignancy is extremely challenging. As a result, before these drugs are administered, future trials, including antiplatelet medications in standard anticancer therapy, must carefully analyze various parameters, including cancer type, degree of malignancy, sex, age, bleeding profile, and other risk factors for patients.
3 EFFECTS OF ANTIPLATELET COMPOUNDS ON THE NON-HEMOSTATIC FUNCTION OF HUMAN BLOOD PLATELETS
Even though hyperactive platelets have a role in the pathophysiology of CVD and other disorders, the processes by which platelets become hyperactive are not entirely understood. Many bioactive compounds may affect blood platelet function in various ways (Bachmair et al., 2014; Dutta-Roy, 2002). New dietary regimens for a long-term strategy to modify human blood platelet activity favourably are being developed (Bachmair et al., 2014; Dizdarevic et al., 2014; Dutta-Roy, 2002; Naemura et al., 2005; O'Kennedy, Raederstorff, et al., 2017).
Dietary long-chain polyunsaturated fatty acids (LCPUFAs) are beneficial in CVD (Dutta-Roy, 2002; Innes & Calder, 2020). These LCPUFAs modulate CVD risk factors, inflammation, cancers, and platelet hyperactivity (Adili et al., 2018;Bachmair et al., 2014; Cohen et al., 2011; Dutta-Roy, 2002). N-3 PUFAs inhibit platelet aggregation by decreasing the platelet TXA2 and increasing NO production in vascular endothelial cells (Mallick & Duttaroy, 2022). Several lines of evidence suggest that polyphenols reduce CVD by different mechanisms, including suppressing platelet activity (Adili et al., 2018; Bachmair et al., 2014; Cohen et al., 2011; Dutta-Roy, 2002). In addition, tomato, garlic, ginkgo biloba, zinc, and other bioactive compounds inhibit platelet aggregation (Dutta-Roy, 2002; Dutta-Roy et al., 1999). Curcumin, quercetin, capsaicin, piperine, eugenol, and allyl sulfide have antiplatelet activity (Duttaroy, 2018). The phenolic compounds, which include flavonoids and non-flavonoids, also affect blood vessels. Natural phytochemicals have complementary and overlapping functions, such as antioxidant properties, immune system activation, and anti-inflammatory reactions. Studies have shown that polyphenols reduce atherosclerosis by inducing antiplatelet activity via modulation of GPVI–collagen, COX-1–thromboxane, protease-activated receptor 1 (PAR1)–thrombin, and P2Y1/P2Y12–ADP mediated pathway of platelet activation (Ed Nignpense et al., 2019).
Aqueous extracts of various fruits have antiaggregatory effects on human blood platelets (Dizdarevic et al., 2014; Dutta-Roy et al., 2001; Duttaroy & Jorgensen, 2004; O'Kennedy, Crosbie, Whelan, et al., 2006). The water-soluble and heat-stable tomato extract, later named Fruitflow®, is the most studied antiplatelet agent used worldwide (Lazarus & Garg, 2004; O'Kennedy, Raederstorff, et al., 2017). Fruitflow® can inhibit ADP-induced platelet activation via several mechanisms, including inhibiting protein disulfide isomerase (PDI) and lowering the P-selectin level (O'Kennedy, Crosbie, van Lieshout, et al., 2006; O'Kennedy, Raederstorff, et al., 2017). Kiwifruit extract (KFE) also showed antiplatelet activity in vitro and ex vivo (Duttaroy & Jorgensen, 2004). Fruitflow®, KFE, and polyphenols from other sources also lower blood pressure by inhibiting angiotensin-converting enzymes and increasing vasodilation (Biswas et al., 2014; Dizdarevic et al., 2014; Duttaroy, 2018). Table-1
3.1 Effects of antiplatelet compounds on platelet-vessel wall interactions
As previously stated, platelet-vessel wall interaction is critical in atherosclerosis's early and late phases, atherothrombotic events, hemostasis, and vascular healing. Numerous medicines not initially intended for antiplatelet treatment change platelet–vessel wall interactions. Aspirin and receptor antagonists of the P2Y12 and GPIIb-IIIa integrins influence platelet-vessel wall interaction. Aspirin has other pharmacological effects besides inhibiting platelet aggregation (O'Kennedy et al., 2021). Inhibitors of platelet synthesis of TXA2 may also affect the cross-talk between platelets and vascular endothelium as it influences vessel wall constriction and vascular cell proliferation (Zucker et al., 1997). Aspirin reduces the development of atherosclerosis in mice lacking the thromboxane receptor, suggesting that it may act as an anti-atherosclerotic agent (Kobayashi et al., 2004). In addition, aspirin's antitumor activity is mediated via platelet-vessel wall interaction (Tesfamariam, 2016).
The N-3 and n-6 LCPUFAs and their eicosanoid derivatives play various physiologic roles in cell growth and development, inflammation, and the cardiovascular system (Mallick & Duttaroy, 2022). The eicosanoid-mediated depends on the ratio of n-6 and n-3 fatty acids-derived eicosanoids. PGE2 and TxA2 are essential in maintaining vascular homeostasis (Moncada, 1982). PGI2 is a vasodilator and a platelet inhibitor (Dutta-Roy et al., 1989), whereas TxA2 is a vasoconstrictor and activator/aggregator of platelets (Dutta-Roy et al., 1986, 1996). Therefore, an imbalance in PGI2 and TxA2 production is implicated in many cardiovascular diseases (Moncada, 1982; Oates et al., 1988). NO affects vasorelaxation and platelet inhibition by cGMP levels via activating intracellular guanylyl cyclase (Moncada & Higgs, 2006). The vaso-protective function of endothelial cells is associated many factors including the biosynthesis and release of NO, PGI2, PGE2, and tissue plasminogen activator (tPA) (Mallick & Duttaroy, 2022). Platelet activation is inhibited by PGI2 and PGE2, produced from ARA from the endothelium by various vasoactive agents, including thrombin. NO produced by eNOS enhance the effect of PGI2. The endogenous fibrinolytic system, responsible for the dissolution of the plaques, is regulated by the endothelium-derived tissue plasminogen activator (tPA) and its inhibitor, plasminogen activator inhibitor type-1 (PAI-1) (Oliver et al., 2005). Certain fatty acids can regulate PAI-1 and tPA activity; however, further work is required for definitive confirmation (Byberg et al., 2001; Hunter et al., 1997, 2001). These endothelium-derived compounds can inhibit the activation of blood platelets and leukocytes and enhance the fibrinolytic process. They also protect vascular wall integrity against acute damage and chronic remodelling. Dysfunction of endothelial is caused by a deficit in the release of these compounds but also by the secretion of deleterious compounds such as PGH2, PGG2, TxA2, superoxide anions (O2-, peroxynitrite (ONOO-)), and PAI-1.
Circulating non-activated blood platelets do not interact with the negatively charged membrane surface of the endothelium. However, activated platelets bind GpIbα to either P-selectin or vWF of the endothelium indirectly via a fibrin bridge joining GpIIb/IIIa and ICAM-1 molecules. In contrast, NO reduces the intracellular level of Ca2+, the transformation of the GPIIb/IIIa receptors (fibrinogen receptors), and suppresses the integrin's binding to fibrinogen (Jin et al., 2005; Moncada, 2006). The ecto-ADPase (CD-39) of endothelial cells hydrolyzes both ATP and ADP to generate AMP, thereby decreasing platelet aggregation/activation (Dutta-Roy et al., 1989; Jin et al., 2005). TxA2 activates platelets and induces expressesion of adhesive co-factors for platelets, such as vWF, fibronectin, and factor V (Dutta-Roy et al., 1986; FitzGerald, 1991).
3.2 Immune modulatory activities of antiplatelet compounds
Lymphocytes, monocytes/macrophages, and neutrophils modulate immune response via different mechanisms. Circulating monocytes respond to activation cues (such as PAMPs and TLRs) by enhanced production of multiple cytokines and chemokines. Monocytes move via ligands in the endothelial cell layers to develop into tissue-resident macrophages. Antiplatelet Fruitflow® reduces CCL4/MIP-1 production in vitro while increasing CXCL8/IL-8 production (Hunter & Jones, 2015). Fruitflow® affects cell trafficking, activation of the classical or alternative pathway, and cellular differentiation in diverse immune system compartments by modulating various cytokines, chemokines, and adhesion molecules. However, the modulatory roles of Fruitflow® in platelets' immune and inflammation responses are yet to be know. (Table 1).
| Anti-platelet bioactive compounds | Actions | Platelet and study types | References |
|---|---|---|---|
| Scopoletin | Inhibits platelet aggregation via Ca2+ mobilization | Rabbit platelets, in vitro studies | (Okada et al., 1995) |
| Doctrine | Inhibits TxA2 formation and increases the cyclic AMP level in rabbit platelets | Rabbit platelets, in vitro studies | (Yu et al., 1992). |
| Rutaecarpine alkaloid | Inhibits collagen and thrombin-induced platelet aggregation via reduced TXA2 levels and intracellular free Ca2+ | Human platelets, in vitro studies | (Sheu et al., 1998) |
| Prenylflavonoids | Inhibit ARA, collagen, and platelet-activating factor (PAF)–induced platelet aggregation | Whole blood, in vitro studies | (Jantan et al., 2010) |
| Ruta graveolens | Inhibit platelet aggregation | Rabbit platelets, in vitro studies | (Tripathi et al., 1993) |
| Isorhamnetin flavonol | Significantly inhibits collagen-induced platelet aggregation, possibly via mitochondrial regulation | Human platelets, in vitro studies | (Ostertag et al., 2011) |
| Coffea arabica | Inhibits platelet aggregation induced by ADP, collagen, epinephrine, and ARA. Inhibitor of COXs | Human platelets, in vitro studies | (Hutachok et al., 2020). |
| Phenolic compounds | Inhibits human blood platelet activation and aggregation | Human platelets, ex vivo and in vitro studies | (Singh et al., 2008) |
| Acid amides | Dose-dependently inhibits rabbit platelet aggregation induced by collagen, ARA, and PAF | Rabbit platelets, in vitro studies | (Park et al., 2007) |
| Piperlongumine | It inhibits collagen-induced aggregation in rabbit platelets and acts via TXA2 receptor antagonist | Rabbit platelets, in vitro studies | (Bezerra et al., 2013) |
| Piperine | Inhibits platelet aggregation by reducing cPLA2 and TXA2 synthase | Rabbit platelets, in vitro studies | (Son et al., 2014) |
| Veratroylgermine alkaloid | Strongly inhibits platelet aggregation induced by ARA | Rabbit platelets, in vitro studies | (Tang et al., 2010) |
| Spiramine C1 alkaloid | Diterpene alkaloids inhibit PAF-induced platelet aggregation | Rabbit platelets, in vitro studies | (Li et al., 2002) |
| Beta-carboline alkaloid | Inhibition of platelet aggregation is mediated by inhibiting PLCγ2 and suppressing Ca2+ mobilization and ARA release | Rabbit platelets, in vitro studies | (Im et al., 2009) |
| N-methoxycarbonyl aporphine alkaloid | Inhibits collagen, ARA, and PAF-induced platelet aggregation. | Rat platelets, in vitro studies | (Kuo et al., 2001) |
| Leonurine pseudo-alkaloid | Inhibits platelet aggregation induced by thrombin, ARA, and collagen | Rat platelets, ex vivo studies | (Jin et al., 1991) |
| Ajmaline alkaloids | Inhibit PAF-induced platelet aggregation in vitro and in vivo studies | Human platelets, ex vivo and in vitro studies | (Rahman et al., 1991) |
| Coumarin (polyphenolic compounds) | Inhibit platelet aggregation by inhibiting COX activity | Human platelets, in vitro studies | (Zaragoza et al., 2021) |
| Mango extract | Inhibits ADP-induced platelet aggregation | Human platelets, in vitro studies | (Alanon et al., 2019) |
| Pumpkin seed extract | Inhibits platelet aggregation induced by ADP, TRAP-6, and collagen. In addition, it inhibits P-selectin secretion and glycoprotein IIb/IIIa activation | Human platelets, in vitro studies | (Sanzana et al., 2021). |
| Asteraceae family | Inhibit ADP-mediated platelet aggregation TXA2 formation, reduction of intracellular Ca2+ mobilization, and phosphoinositide breakdown | Human Platelets, In Vitro Studies | (Rolnik et al., 2022) |
| Olive oil | Reducing platelet sensitivity to aggregation reduces vWF and plasma levels of TXA2 | Human platelets, ex vivo studies | (Lopez-Miranda et al., 2007). |
| Onion | Reduces platelet aggregation and blood pressure | Rat studies, ex vivo study | (Chen et al., 2000) |
| Garlic | Inhibits platelet aggregation in vitro and ex vivo | Human platelets, ex vivo studies | (Kiesewetter et al., 1991) |
| Pomegranate (Punica granatum) products, | Inhibits collagen and ARA-mediated platelet aggregation via Ca2+ mobilization and TXA2 production | Human platelets, in vitro studies | (Mattiello et al., 2009) |
| Hawthorn leaf extract (terpenoid, flavones) | Inhibit ADP-induced platelet aggregation. | Rat platelets, in vitro studies | (Gao et al., 2019) |
| Leuzea carthamoides (Flavonoid) | Inhibits platelet aggregation induced by collagen and ADP | Human platelets, in vitro studies | (Koleckar et al., 2008) |
| Aristotelia chilensis (leaves and unripe fruits) (phenolic and anthocyanin) | Inhibits platelet aggregation and platelet granule secretion by reducing the platelet membrane exposure of P-selectin and CD63 | Human platelets, in vitro studies | (Rodriguez et al., 2021) |
| Citrus aurantifolia leave extract | Inhibits ADP and epinephrine-induced platelet aggregation | Human platelets, in vitro studies | (Piccinelli et al., 2008) |
| Acanthopanax sessiliflorus (fruit extract) | Inhibits ADP- induced platelet aggregation | Rat platelets, in vitro studies | (Jin et al., 2004) |
| Varthemia iphionoides | Inhibits ADP and collagen-induced platelet aggregation | Human platelets, in vitro studies | (Afifi & Aburjai, 2004) |
| Extract lemon balm (Melissa officinalis) | Inhibits ADP-induced platelet aggregation | Human Platelets, in vitro Studies | (Males et al., 2017) |
| Dandelion root component (Taraxacum officinale L.) | Inhibits platelet action in an in vitro study | Human platelets, in vitro studies | (Lis et al., 2020) |
| Lavender extracts (Lavandula hybrid) | Inhibits platelet aggregation induced by ARA, U46619, collagen, and ADP | Guinea pig platelets, in vitro studies | (Ballabeni et al., 2004) |
Fruitflow® inhibited platelet activation by lowering phosphorylation of Akt, which is a downstream molecule of the PI3K signalling pathway; GSK3; ERK, JNK, and p38 MAPK, which are downstream molecules of the MAPK signalling pathway; and Hsp27 (Jeong et al., 2016). Fruitflow® also affected various platelet proteins (O'Kennedy, Crosbie, et al., 2017). PDI is highly expressed on the activated platelet membrane and isomerizes disulfide bonds on the platelet membrane to help in the aggregation of platelets, secretion from platelet granules, and fibrinogen binding GPIIb-IIIa. PDI do all these functions by targeting thiol-containing protein expressed on platelet membrane, and all these functions will be inhibited if PDI is blocked using inhibitory antibodies (Cho, 2013; Manickam et al., 2008). Blocking cell-surface thiol isomerases inhibited platelet function (Jordan et al., 2005). In addition, PDI interacts with quercetin-related glycosides, of which several are present in Fruitflow® (Jasuja et al., 2012; Sheu et al., 2004). The interaction of polyphenols with PDI revealed a mechanism through which Fruitflow® components may suppress platelet aggregation in various pathways.
Aspirin can inhibit experimental vascular inflammation with a reduction in inflammatory molecules (CRP, M-CSF, MCP-1), as well as pro-inflammatory factors (TXA2, S1P, sICAM-1, IL-6) (Hohlfeld & Schror, 2015). Eicosanoids synthesized from n-6 LCPUFAs promote inflammatory responses (Yui et al., 2015), whereas n-3 LCPUFAs produce anti-inflammatory or neutral eicosanoids. N-6 eicosanoids modulate cellular growth, inflammation, blood coagulation, and vascular integrity (Glatzel et al., 2018; Mitchell et al., 2014). Also, n-6 eicosanoids increase the synthesis of cytokines and adipokines, which are involved in metabolism and inflammation (Makki et al., 2013). Linoleic acid, 18:2 (LA) initiates inflammation by raising the levels of TNF-α, MCP-1, VCAM-1, and ICAM-1 through the activation of NF-κB and activator protein 1 (Toborek et al., 2002).
Fruitflow® lowered the inflammatory responses by inhibiting the production of NO, PGE2, TNFα, IL1β, IL6, and IL12 from macrophages involved in chronic inflammatory processes (O'Kennedy, Raederstorff, et al., 2017). In addition, fruitflow® increases cellular awareness and senses altered immunological homeostasis in the vascular-endothelial compartment during acute inflammation, according to these in vitro findings. Furthermore, Fruitflow® influenced the production and expression of different inflammatory mediators by modulating the transcription factors of the NF-κB signalling pathway (Schwager et al., 2016).
3.3 Effects of fatty acids on blood-vessel-platelet interactions
PUFAs influence the adhesion molecules of endothelial cells and modulate leukocyte-endothelial adhesion (Baker et al., 2018). Endothelial cells express adhesion molecules such as selectins and immunoglobulin superfamily members such as ICAM-1, ICAM-2, and ICAM-3 and vascular cell adhesion molecules (VCAM) 1 and 2. In addition, several adhesion molecules, including integrins and selectins (e.g., P-selectin glycoprotein-1 (PSGL-1), which bind to E, L, and P-selectin), are expressed on leukocytes to function as counter-receptors to those expressed on endothelial cells (Granger & Senchenkova, 2010).
N-3 LCPUFAs can reduce the ARA content of platelet cell membrane phospholipids, hence lowering the synthesis of ARA-derived eicosanoids. By integrating n-3 fatty acids into membrane phospholipids, dietary n-3 fatty acids can affect any cell's membrane structure and function. The ARA content of platelet cell membranes is reduced due to N-3 LCPUFA consumption, resulting in lower n-6 eicosanoid synthesis. In addition to their antiplatelet effects, n-3 fatty acids have the ability to suppress the production of pro-inflammatory cytokines (Duttaroy, 2018).
3.4 Fruit extract and platelet-vessel interactions
Several fruits extract, including tomato and kiwifruits, have been found in numerous studies to reduce platelet aggregation both in vitro and ex vivo (Dizdarevic et al., 2014; Dutta-Roy et al., 2001; O'Kennedy, Crosbie, et al., 2017; O'Kennedy, Crosbie, van Lieshout, et al., 2006). Furthermore, antiplatelet components of tomato, strawberry, and kiwifruit inhibit the angiotensin-converting enzyme (ACE), as well as relax the endothelium, which protects the blood vessels (Biswas et al., 2014; Duttaroy, 2018). Fruitflow® also reduced endothelial dysfunction-associated expression of adhesion molecules such as ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells, thereby enhancing blood flow (O'Kennedy, Raederstorff, et al., 2017).
Both Fruitflow® and aspirin modulate platelet membrane proteins, platelet secretion, fibrinogen beta chain 5, Ras-related proteins, redox system proteins, and HSP70s (O'Kennedy, Crosbie, et al., 2017). Fruitflow® and aspirin affected 11 of the 26 proteins with changed expression following intervention aspirin alone affected 14, and Fruitflow® alone affected one (O'Kennedy, Crosbie, et al., 2017). Consuming antiplatelets flavonoids present in fruits may lower blood pressure (Biswas et al., 2014; Dizdarevic et al., 2014). As the IC50 values of flavonoids were higher than those of the prescribed drugs for hypertension, flavonoids could be used as preventative nutraceuticals against hypertension rather than as a therapeutic drug.
3.5 Antiplatelet compounds and the role of platelets in cancer metastasis
Platelets are essential in almost every step of tumour growth and metastasis (Sharma et al., 2014). Several compounds from platelet are released due to direct interactions between platelets and cancer and stromal cells (Ballerini et al., 2018). Several tumours can activate platelets, causing them to release various growth factors such as VEGF, PDGF, and fibroblast growth factors. These growth factors are associated with tumour progression, angiogenesis, metastasis, and poor prognosis (Li et al., 2017).
Green tea contains an antiplatelet and anti-inflammatory factor, epigallocatechin gallate (EGCG), which strongly prevents cancers (Fujiki et al., 1998). Furthermore, the ginseng compound enhanced EGCG's impacts on suppressing colon tumour cells, showing that green tea may function as a successful chemopreventive agent when used along with an anticancer agent.
Antiplatelet curcumin inhibited the expression of PDGFR and increased the proliferation of human hepatic myofibroblasts (Duttaroy, 2018; Zheng & Chen, 2006). Furthermore, curcumin inhibits the activity of ERK, JNK, and PI3/AKT and slows cell proliferation, and induces apoptosis in a dose-dependent manner (Kunnumakkara et al., 2008). Additionally, curcumin alleviates the inhibitory effect of PDGF signalling on cell growth by lowering PPARγ genes' expression (Zhou et al., 2007).
On the other hand, antiplatelet resveratrol inhibits smooth muscle cells' migration and monocytes' adhesion induced by TNF-α (Duttaroy, 2018; Venkatesan et al., 2009). Finally, ellagic acid (EA), a platelet inhibitor, is an antimutagenic and anticarcinogenic compound. EA inhibits angiogenesis by repressing PDGF-R movement and phosphorylation of its substrate (Labrecque et al., 2005).
4 ANTIPLATELET BIOACTIVE COMPOUNDS IMPACT THE ATHEROSCLEROSIS PROCESS
The antiatherosclerotic effects of bioactive compounds have been demonstrated both in vitro and in vivo (Amirullah et al., 2018). Consuming n-3 fatty acids modulate the cardiovascular system, including platelet function, fibrinolytic system, and coagulation cascade, thus reducing atherosclerosis risk (Dutta-Roy, 2002; Fung et al., 2009). Increased consumption of marine fish oil or fish products is associated with a lower incidence of CVD (Innes & Calder, 2020). Polyphenolic substances also have antioxidant effects and increase eNOS activity. The expression of eNOS is a powerful biomarker for vascular tone and blood pressure regulation. Consumption of vegetables containing inorganic nitrates, which form nitrite intermediates, raises vascular NO levels. Nitrate is taken into the body via a mechanism that is not dependent on endothelium and depends on oral bacteria converting nitrate to nitrite and then forming NO in blood vessels. Strawberries lower blood pressure, increase HDL cholesterol, which results in favourable changes in platelet function, and reduce CVD risks (Erlund et al., 2008).
4.1 Diabetes mellitus, hypertension, and antiplatelet compounds
Diabetes mellitus (DM) is associated with an increased risk of atherogenesis and atherothrombotic complications (Katakami, 2018). DM patients have a prothrombotic state due to various mechanisms, including platelet hyperactivity and atherosclerosis, indicating the importance of antiplatelet therapy for secondary prevention in these patients. After a CVD episode, patients undergoing percutaneous coronary interventions (PCI), dual antiplatelet therapy (DAPT) with aspirin, and the P2Y12 inhibitor clopidogrel has been the mainstay of treatment. Though DAPT reduces atherothrombotic recurrences in patients with DM, these rates remain high, highlighting the need for more effective treatments. In addition, inhibitors of platelet P2Y12 receptor with enhanced potencies, such as prasugrel and ticagrelor, and antiplatelet therapies, such as vorapaxar inhibit the thrombin-mediated platelet signalling pathway be used to treat patients with DM.
Quercetin enhances cell membrane fluidity and transmembrane potential while inhibiting inflammation in immunological and endothelial cells, notably useful in late-stage diabetes (Margina et al., 2013). In diabetes, quercetin's antiplatelet action slows thrombus development and serves as an antioxidant by decreasing the synthesis of lipid hydroperoxides and enhancing glutathione peroxidase activity. Furthermore, it modulates NF-kB signalling and the mitochondrial pathway, avoiding cell death.
4.2 Obesity and human blood platelet activity
Obese people have increased platelet sensitivity to aggregation, increased sCD40L levels, and mean platelet volume (Unek et al., 2010). Adipose tissue produces leptin, adiponectin, TNF-α, IL-6, and resistin, affecting platelet function directly or indirectly. Platelet leptin receptors increase platelet aggregation in response to agonists, implying a possible link between obesity and CVD. Platelets suffer functional changes due to obesity, which may compromise normal vascular function and poorly respond to antiplatelet medication. Obese people are resistant to antiplatelet medicines. The poor response to the platelet P2Y12 antagonist, such as clopidogrel, leads to increased comorbidities, diabetes, renal impairment, and obesity (Angiolillo et al., 2007).
5 HUMAN BLOOD PLATELET FUNCTIONS AND SEVERITY OF COVID-19
Platelet hyperactivity plays a vital role in the pathology of COVID-19 from its onset, and platelets may play a vital role as COVID-19 progress. In COVID-19, severe disseminated intravascular coagulation and platelet hyperactivity cases are associated with poor prognosis and a higher mortality rate due to the hyperactivation of blood platelets and activation of the coagulation system (O'Kennedy et al., 2021).
Platelet hyperactivity is part of the general viral infection-mediated thrombosis process, but its effect on COVID-19 infection may be greater than expected. As blood platelets are found in abundance at sites near the cells that the SARS-CoV-2 virus initially targets, they could well be the first blood cells to interact in large numbers with the virus. Consequently, they may be able to internalize SARS-CoV-2 and play a significant role in initiating the first wave of response (Koupenova & Freedman, 2020).
The importance of Zn2+ in hemostasis was recognized in 1982, as Zn2+-deficient men had bleeding and clotting abnormalities (Gordon et al., 1982). Zinc has a potential association with CVD risk as it can modulate platelet function. Zinc deficiency is associated with platelet-related bleeding disorders in humans and rodents (Badimon et al., 2012; Emery et al., 1990). Zn2+ participates in blood clotting by modulating platelet aggregation, coagulation, and fibrinolysis (Little et al., 2010; Taylor & Pugh, 2016). At the injury site, Zn2+ released from activated platelets stimulates coagulation and reduces fibrinolysis. Though zinc is an essential and physiologically relevant cofactor in hemostasis, the role of zinc in platelet activation and pathophysiological thrombus formation has not been well investigated.
The demand for nutritional supplements and nutraceuticals has increased during the COVID-19 pandemic due to their perceived immune-boosting effects. Both in vitro and in vivo studies have shown that nutraceuticals containing phycocyanobilin, N-acetylcysteine, glucosamine, selenium, or phase 2 inductive nutraceuticals (e.g., lipoic acid, ferulic acid) can inhibit and modulate RNA virus infections through an increase in mitochondrial antiviral-signalling protein activation and TLR7 activation (McCarty & DiNicolantonio, 2020).
5.1 Effects of N-3 polyunsaturated fatty acids on platelets and COVID-19
Antiplatelet compounds such as n-3 PUFAs have been studied to combat various viral infections (Zhang & Liu, 2020). Blood samples from 100 patients with COVID-19 had higher n-3 indexes, a measure of red blood cells' eocosapentaenoic acid (EPA) and docosahexaenoic acid, 22:6n-3 (DHA) content, and a lower risk of death (Asher et al., 2021). In addition, several studies have established a link between n-3 PUFAs and clinical benefits in COVID-19 patients, as thrombotic complications, such as arterial and venous thrombosis, are common in some COVID-19 patients (Becker, 2020).
5.2 Antiplatelet polyphenols and COVID19
The severity of COVID-19 infection may be lessened by consuming tomato extract, especially before or during the early stages. It may be particularly beneficial for people at risk for endothelial dysfunction and platelet dysfunction. Fruitflow® inhibits platelet granule secretion by suppressing the Src-PLCγ2- protein kinase C (PKC) mediated granule secretory pathway (O'Kennedy, Raederstorff, et al., 2017; Zhang et al., 2021). Platelet granules secretion occurs when collagen and thrombin bind to its receptor GPVI, and PARS causes the activation of the downstream signalling pathway. In this pathway, sarcoma, a tyrosine-protein kinase (Src) family member (Lyn, Fyn, and Src) is first activated by phosphorylation, activated Syk then phosphorylates and activates LAT, which in turn causes phosphorylation of an adaptor protein PLCγ2 in which PKC bind leading to the activation of downstream effector, which induces platelet granule secretion (Zhang et al., 2021). Fruitflow® modulates the generation of different interleukins such as IL-1β, IL-6, IL-10, and IL-12 and various chemokines such as CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, CXCL8/IL-8, CXCL10/IP-10 in peripheral blood leukocytes (Schwager et al., 2016). Figure 3 shows the effects of antiplatelet bioactive drugs on patients with COVID-19.
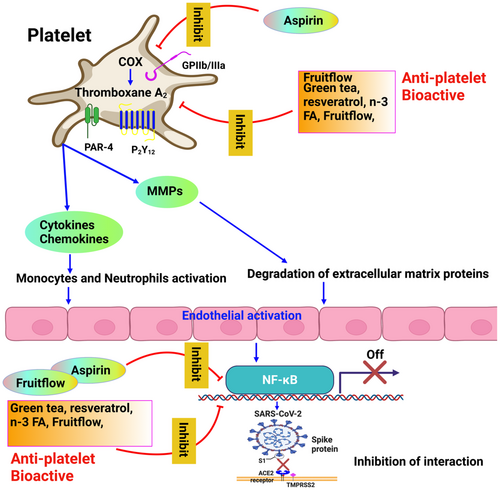
6 CONCLUSIONS
While the contributions of human blood platelets to hemostasis and thrombosis have been well established, it is increasingly evident that platelets have a more wider role in physiology. For example, platelets can bind bacteria, secretes chemokines, and clear invading pathogens from circulation, like traditional immune cells such as macrophages and mast cells. Platelets have emerged as critical biological factors of normal and pathologic vascular healing and other diseases such as cancers and inflammatory and immune disorders. They work not just by releasing various lipid and protein mediators but also by triggering previously undiscovered time-dependent activities, including signal-dependent pre-mRNA splicing and constitutively expressed mRNA translation. New approaches are required to bridge the gap between the massive body of evidence supporting the role of platelets in the onset and course of experimental atherogenesis and the comparatively limited evidence for the roles of platelets in human atherogenesis. Given the evidence of persistent platelet activation in obese women who are otherwise healthy and relatively young, this could provide a suitable group for obesity and platelet studies. This sort of research may also shed further light on the processes that link inflammatory mediators to platelet activation. The key to maintaining hemostasis and proper blood flow is regular platelet activity. Many of the fundamental mechanisms of platelet use in their immune response are similar to or extensions of hemostatic processes. The challenge for therapeutic intervention in these disorders will be to find drugs and bioactive compounds that preferentially block specific sites implicated in platelets' complicated contribution to non-hemostatic function while leaving at least some of their hemostatic function intact. This review describes the involvement of various food components and their inhibitory roles in the hemostatic and non-hemostatic process of blood platelets that will help develop new prevention and therapy for CVD.
6.1 Future perspectives
Functional foods are essential to improve and maintain the physiological function of blood platelets to encounter CVD worldwide. Recent progress has shown that bioactive compounds have different antiplatelet mechanisms of action, increased efficacy, and low toxicity. Several in vitro, animal, and human studies have provided evidence of bioactive compounds as an alternative to present therapies. Although the platelet activation pathway may be an appealing therapeutic target for various inflammatory disorders, it is unclear whether inhibiting platelet aggregation would be beneficial. Molecular research is needed to determine the precise mechanisms that govern platelet activation-induced inflammation and the risk of CVD. The importance of platelet activation and related signalling pathway research stems from its involvement in many inflammatory-related disorders. Future research will likely focus on platelet activation in concert with many other inflammatory mediators for potential multi-modal therapies. As a result, much more research is needed to completely comprehend the processes of antiplatelet bioactive substances on multiple platelet pathways in physiology and pathology. This review has highlighted the effects of bioactive compounds on platelets' hemostatic and non-hemostatic activities. However, further research targeting these pathways might develop effective antiplatelet strategies for maintaining hemostasis.
AUTHOR CONTRIBUTIONS
Asim K. Duttaroy drafted, conceptualized and critically edited the manuscript. Diptimayee Das and Subhamay Adhikary (DBT junior research fellow, DBT/2021-22/CARE/1592) wrote and edited the manuscript. Sujay Paul, Arun Kumar Radhakrishnan, Antara Banerjee, Surajit Pathak and Ranjit Kumar Das edited the manuscript. All authors have read and agreed to the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS DECLARATIONS
No animal and human data are involved.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




