Fructose promotes more than glucose the adipocytic differentiation of pig mesenchymal stem cells
Abstract
The goal of this study was to evaluate how glucose and fructose affected the adipose differentiation of pig newborn mesenchymal stem cells (MSCs). Cells were grown with or without inosine in 7.5 mM glucose (substituted with 1.5 or 6 mM fructose). MSCs displayed adipose morphology after 70 days of differentiation. Fructose stimulated the highest levels of PPARγ and C/EBPβ. Fructose at 6 mM, but not glucose at 7.5 mM or fructose at 1.5 mM, promotes differentiation of MSCs into adipocytes and increases 11-hydroxysteroid dehydrogenase (11β-HSD1) and NADPH oxidase 4 (NOX4) mRNA in the absence of hepatic effects (as simulated by the inosine). Fructose and glucose increased xanthine oxide-reductase (XOR) catalytic activity almost 10-fold and elevated their products: intracellular reactive oxygen species (ROS) pool, extracellular H2O2 pool by 4 orders of magnitude, and uric acid by a factor of 10. Therefore, in our experimental model, differentiation of MSCs into adipocytes occurs exclusively at the blood concentration of fructose detected after ingestion by people on a high fructose diet.
Practical applications
The results of this study provide new evidence for fructose's adipogenic potential in mesenchymal stem cells, a model in which its effects on XOR activity had not been studied. The increased expression of genes such as C/EBPβ, PPARγ, and NOX4, as well as the increased XOR activity and high production of ROS during the differentiation process in the presence of fructose, coincides in pointing to this hexose as an important factor in the development of adipogenesis in young animals, which could have a great impact on the development of future obesity.
1 INTRODUCTION
Obesity has become a global epidemic in the twenty-first century (Polyzos & Mantzoros, 2019). In recent decades, global fructose consumption has increased, which has been linked to a rise in obesity and other chronic diseases (Malik et al., 2013). It is known that moderate fructose consumption (50 g/d) appears acceptable and potentially beneficial, but the effects of higher doses on long-term quality of life in individuals with elevated dysglycemia or elevated dyslipidemia are unknown (Livesey & Taylor, 2008). Its metabolism causes disorders in the organs in which it is metabolized by depleting phosphate and increasing inosine, a nucleotide that is degraded to form uric acid (Van der Berghe et al., 1977).
According to a meta-analysis, fructose consumption is associated with an increased risk of diabetes, metabolic syndrome (MetS), as well as cardiovascular disease (Bray & Popkin, 2014). In comparison with glucose, fructose's ability to achieve MetS characteristics was unaffected by changes in energy intake (Roncal-Jimenez et al., 2011). In obese adults, fructose promotes “de novo” lipogenesis (DNL), dyslipidemia, decreased insulin sensitivity, and increased adiposity (Stanhope et al., 2009).
Fructose is absorbed and transported to the liver at a higher concentration than in other tissues (Softic et al., 2016). In humans, the liver metabolizes nearly 70% of the fructose consumed orally (Francey et al., 2019).
Additionally, the different responses of adipose cells and some of their precursors to glucose and fructose have been studied in cell culture experiments, 550 μM fructose plus 11 mM glucose increased GLUT-5 gene expression and promoted adipogenesis in cultured 3 T3-L1 cells (Du & Heaney, 2012). In comparison with glucose-containing media, cells cultured in 5.5 mM fructose-containing media (supplemented with isobutyl methylxanthine, dexamethasone, and insulin) increased the expression of 11-hydroxysteroid dehydrogenase (11-HSD1) as well as its activity and demonstrated increased lipolysis (Legeza et al., 2014). Finally, Sodhi et al. (2016) demonstrated that uric acid (5–10 mg/dL) plus 25 mM glucose and 500–1000 μM fructose increased adipogenesis via NADPH oxidase (NOX) expression.
However, except for one datum in the above-listed works (Du & Heaney, 2012), the fructose concentrations used to promote adipogenesis were up to 20 times greater than those detected in peripheral circulations between meals or during fasting conditions in a variety of mammalian species, including humans (Francey et al., 2019; Hannou et al., 2018; Ishimoto et al., 2012; Patel et al., 2015).
Additionally, there was an increase in the quantity and adipogenic potential of visceral adipose tissue precursor cells. Furthermore, the direct “in vitro” addition of fructose to isolated adipocyte precursor cells (obtained from rats given 10 percent fructose in their drinking water for 8 weeks) revealed an all-around adipogenic capacity (Zubiría et al., 2016).
Two reviews have been published on the subject, one establishing the role of fructose in adipocytes in activating the glucocorticoid action mediated by 11-HSD1 by increasing the availability of its cofactor NADPH, thereby promoting adipogenesis (Legeza et al., 2017). The other is a comprehensive review of the subject (Hernández-Díazcouder et al., 2019), which covers a variety of topics, including the synthesis of reactive oxygen species (ROS) as a mechanism for eventually activating the adipogenic process.
The monosaccharide concentrations in the cultures were carefully designed in this study; 7.5 mM glucose was used, which was replaced with 1.5 mM or 6 mM fructose. We calculated the circulating peripheral blood fructose concentration to be 300 μM using blood fructose values previously reported with normal fructose diets. This value was multiplied by 5 and 20 to obtain fructose concentrations of 1.5 mM and 6 mM, respectively, to replace an equivalent amount of glucose. A higher fructose concentration was used as a positive control to ensure that the results were comparable with those previously reported in the literature for humans (Francey et al., 2019; Hannou et al., 2018; Ishimoto et al., 2012; Patel et al., 2015). The lower fructose concentration was included in an attempt to obtain new results using fructose concentrations that were lower but still supraphysiological. Inosine was added because it is generated in the liver when high doses of fructose are administered orally (Van der Berghe et al., 1977) and we tried to simulate the substance that reaches the cells after high fructose is consumed. We used MSCs isolated from neonatal porcine bone marrow (PBM) that were capable of differentiation into adipose or muscle cells in cell culture conditions (Pérez-Serrano et al., 2017). On MSCs in the presence of adipocyte markers C/EBP and PPAR, the role of glucose alone in the blood versus fructose replacing equimolecular glucose concentrations was examined.
2 MATERIALS AND METHODS
2.1 Animals
The Internal Committee for the Use and Care of Experimental Animals (CICUAE-UNAM, NOM-062-ZOO-1999 4.2.2, #065) approved all procedures involving animals.
2.2 Isolation and cell culture
As previously described (Pérez-Serrano et al., 2017), adipogenic differentiation from porcine bone marrow-derived mesenchymal stem cells (PBM-MSCs) was performed. PBM-MSCs were isolated from the femur and tibia aspirates of four 0–2 day old piglets and pelleted at 450 × g for 5 min at room temperature The pellets obtained were washed three times with an isolation medium and re-suspended in Dulbecco's modified Eagle medium [DMEM, 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin and 25 μg/ml amphotericin)]. Three 3.5 cm culture dishes (104 cells/dish, Corning Inc., Corning, NY, USA) were seeded with cells for each animal. Every 4 days, stationary subcultures were collected until the fourth passage, at which point adipogenic cultures were performed (Pérez-Serrano et al., 2017). Daily observations of cells were made with an epifluorescence microscope (Olympus, Melville, New York, USA) equipped with a digital color camera and Motic Image Plus software 2.0 (Motic Asia, Hong Kong).
2.3 Expression of MSCs markers
The methodology proposed by Pérez-Serrano et al. (2017) was used to characterize PBM-MSCs. The genotype was determined using PBM-MSCs seeded at a density of 1x104 in 3.5-cm culture dishes. PCR was used to determine the mRNA expression of MSCs marker genes in passage 4. CD90 (thy-1 cell surface antigen), CD44 (hyaluronate receptor), and CD105 (endoglin) were the genes involved. Table 1 contains the primer sequences.
| Gene | Primer sequence (forward/reverse) | Product size (bp) | Annealing temperature (°C) | Reference sequence accession No. |
|---|---|---|---|---|
| CD44 | F 5′CGGACCTGCCCAATGCCTTTGA | 212 | 62 | NM_174013 |
| R 5′TGCACAGTTGGGAGGTGCGT | ||||
| CD90 | F 5′TGCCGATTGTGCGGGAAGCA | 198 | 60 | NM_001034765 |
| R'AGGTCCGAGTTCTGGAAGCGC | ||||
| CD105 | F 5′CCATCAAAAGCCTGACCTTCGG | 142 | 60 | NM_001076397.1 |
| R 5′GCCTCTGAAATCTGTCGTTG | ||||
| YWHAZ | F 5′CGGACACAGAACATCCAGT | 243 | 60 | NM_174814.2 |
| R 5′TTTTCTCAGCACCTTCCGTCT | ||||
| PPIA | F 5′AGCACTGGGGAGAAAGGATT | 248 | 58 | NM_021130 |
| R 5′AGCCACTCAGTCTTGGCAGT | ||||
| RPL13a | F 5′CTGCCCCACAAGACCAAGC | 188 | 60 | NM_001076998.1 |
| R 5′TGGTACTTCCAGCCAACCTCA | ||||
| 18 s | F5′AACCAGACAAATCGCTCCACC | 263 | 63 | NR_002170.3 |
| R 5′CGGCGTTATTCCCATGACCC | ||||
| C/EBPβ | F 5′GCCGCCTGCCTTTAAATCCAT | 159 | 55 | NM_001199889.1 |
| R 5′CGTGGTCACCGATGCTACCC | ||||
| NOX4 | F 5′GGAGCAATAAACCAGTCACC | 200 | 55 | XM_021062623.1 |
| R 5′GTGGACCCCAAATGTTGCTT | ||||
| PPARγ | F 5′GTTCGAGTTTGCTGTGAAGT | 224 | 60 | NM_214379 |
| R 5′AGGTCTGTCATTTTCTGGAG | ||||
| 11β-HSD1 | F 5′GCGAATCCACTTGTTGCT | 213 | 55 | NM_214248.3 |
| R 5′GATATTTCCAGGGCGCATT |
- Abbreviations: C/EBPβ, CCAAT enhancer binding protein b; CD44, hyaluronate receptor; CD90, thy-1 cell surface antigen; CD105, endoglin; NOX4, NADPH oxidase 4; PPARγ, peroxisome proliferator activated receptor gamma; PPIA, peptidylprolyl isomerase A; RPL13A, ribosomal protein L13a; YWHAZ, tyrosine 3-monooxygenase/tryptophane 5-monooxygenase activation protein, zeta; 11β-HDS1, 11b-hydroxysteroid dehydrogenase type 1; 18 s, 18 s ribosomal.
2.4 Adipose induction and treatments
Previously, it was reported that when adipogenesis was induced in PBM-MSCs, passage 4 showed the highest expression of PPARγ (Pérez-Serrano et al., 2017). As a result, we performed various treatments at this stage of culture in this work. Before adipogenic induction, the medium was changed every other day; cells were maintained in the basal medium for 5 days until they reached 60% pre-confluence. The adipogenic protocol was divided into two phases: phase 1 consisted of a 24-h incubation in DMEM supplemented with 0.5% of 3-isobutyl-1-methylxanthine (IBMX, Invitrogen), 100 nM dexamethasone (Sigma-Aldrich, Saint Louis, MO, USA), and 10 μg/ml insulin (Invitrogen). During phase 2, cells were maintained in glucose-free DMEM (Sigma-Aldrich) supplemented with 15 mg/L phenol-red (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 3.7 g/L sodium bicarbonate (JT Baker, Waltham, MA, USA), 4 mM glutaMAX (Thermo Fisher, Waltham, MA, USA), and 5% FBS, 10 μg/ml insulin, and antibiotics, until they gained adipose morphology (Pérez-Serrano et al., 2017).
In phase 2, the following glucose (D-glucose, Sigma-Aldrich) and fructose (D-fructose, Sigma-Aldrich) concentrations were used: HGF0 (glucose 7.5 mM, no fructose), HGLF (glucose 6 mM + fructose 1.5 mM), LGHF (glucose 1.5 mM + fructose 6 mM), HGLFI (6 mM glucose + 1.5 mM fructose + 30 μM inosine), LGHFI (glucose 1.5 mM + fructose 6 mM + 30 μM inosine).
PPARγ2 and C/EBPβ were used as positive markers of adipogenesis (see Table 1 for the primer sequences) and were measured by qRT-PCR.
2.5 RNA Isolation and RT-qPCR
RNA was extracted using Trizol according to the manufacturer's protocol (Life Technologies, Carlsbad, CA, USA). Using a NanoDrop 1000 spectrophotometer, samples were eluted in 30 μl of RNase-free water and quantified at 260 nm (Thermo Fisher Scientific Inc., Wilmington, DEL, USA). The ratios of the samples ranged from 260 to 280 nm in the range of 1.8 to 2.1, and their integrity was determined using 1% agarose gel electrophoresis. To eliminate contamination from genomic DNA, the samples were incubated for 15 min at room temperature and 5 min at 70°C with DNase-I (Roche, Indianapolis, IND, USA). To obtain cDNA, reverse transcription was performed according to the manufacturer's protocol using SuperScript II (Invitrogen) and oligo dT15.
The qPCR reaction was performed using the StepOne Real-Time PCR System with 1 mM MgCl, 1 μl Sybr Green (Roche Applied Science, Mannheim, Germany), and 0.25 μM of each gene-specific primer in a final reaction volume of 10 μl (Applied Biosystems, Foster City, CA, USA). The program began with a 10-min preincubation at 95°C, followed by 40 cycles of three steps: denaturation at 95°C for 1 min, alignment to the specified temperature (Table 1) for 10 s, and amplification at 72°C for 15 s, followed by a melting curve starting at 50°C with a 0.35°C/s ramp to verify the amplicons' specificity.
The qPCR experiments were conducted by the MIQE guidelines (Bustin et al., 2009). To determine gene expression, the 2−ΔΔCt method was used (Livak & Schmittgen, 2001). We calibrated the geometric mean of the best pair of housekeeping genes using the diluent control (Vandesompele et al., 2002). The ratio of gene expression to the control was calculated and expressed as the induction fold. To determine the best housekeeping genes, the following four were tested: tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (YWHAZ), ribosomal protein L13a (RPLP13a), peptidylprolyl isomerase A (PPIA), and 18S ribosomal protein (18 s) (refer to Table 1 for primer sequences). The NormFinder program (MDL, Aarhus, Denmark) was used to determine the stability of the housekeeping gene using data from a random sample with two replicates per treatment per animal (Andersen et al., 2004).
The Oligo 7 program was used to design gene-specific primers based on mRNA sequences published in the NCBI. The correct size of the amplified qPCR product was determined using agarose gel electrophoresis, and the qPCR products were sequenced using Big Dye version 3 (Applied Biosystems) in the 310 ABI Prism sequencer and compared with the target genes using BLAST (National Center for Biotechnology Information, Bethesda, MD, USA).
2.6 XOR activity
The activity of xanthine oxide-reductase (XOR) was determined exclusively using an assay kit (Sigma-Aldrich). One XOR unit is defined as the amount of enzyme required to produce 1 μmol of hydrogen peroxide per minute at a temperature of 25°C.
2.7 Intracellular ROS production
The determination of intracellular reactive oxygen species was performed using the fluorescent 2′,7′-dichlorofluorescein diacetate (DCFHDA) (Thermo Fisher), which penetrates the cell and, after dissociation of the acetate group by intracellular esterases, is oxidized, resulting in a highly fluorescent compound that is used as an indicator of reactive oxygen species. For 30 min at 37°C, cells were incubated with 10 μM DCFHDA. After removing excess DCFHDA, the cells were washed and then incubated for 4 h at 37°C with or without thymoquinone (2.5–10 μM). Intracellular ROS production was quantified using a microplate reader equipped with an excitation wavelength of 485 nm and an emission wavelength of 535 nm to detect dichlorofluorescein (DCF) fluorescence as the oxidized product of DCFHDA.
2.8 Extracellular hydrogen peroxide pool
Hydrogen peroxide production was determined using the Amplex® Red kit (Molecular Probes, Invitrogen), which contains a fluorescent probe that is oxidized to resorufin using horseradish peroxidase (HRP) as a catalyst and measured at 571 and 585 nm, respectively, for excitation and emission.
2.9 Uric acid pool
The uric acid concentration was determined using the MAK077 kit (Sigma-Aldrich). The technique is based on measuring the concentration of uric acid via an enzymatic reaction and then by fluorescence (LEX = 535/LEM = 587 nm).
2.10 Statistical analysis
Changes in gene expression data, XOR activity, ROS, H2O2, and uric acid concentration were analyzed using a completely randomized model with a 2 × 5 (days × treatment) factorial arrangement. A one-way ANOVA was obtained for each analysis using the general linear model procedure of the SAS statistical software. The least-square means ± SEM was used to analyze the differences between treatments. p values ≤ .05 were considered statistically significant.
3 RESULTS
3.1 Cell isolation and Expression of MSC markers
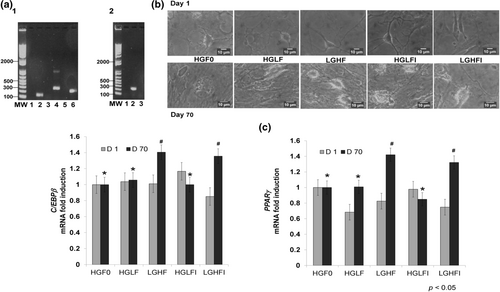
The cell isolation and extraction method were effective in obtaining MSCs from neonatal pigs. Our data demonstrated that the cultured cells expressed MSCs marker genes CD90, CD44, and CD105 (Figure 1a1).
3.2 Differentiation of adipose tissue
The cells reached adipose morphology on day 70 as shown in Figure 1b. Furthermore, at the molecular level, the differentiation potential of our isolated MSCs into mature lineages was evaluated by the expression of the adipocyte marker PPARγ (Figure 1a2). Figure 1c shows the changes in the expression of C/EBPβ and PPARγ due to the addition of all treatments. The results showed the effect of fructose (LGHF and LGHFI) over C/EBPβ and PPARγ expression (p < .05). The effects of fructose on both genes were similar, about 40% higher.
3.3 NOX4 and 11β-HSD1 expression
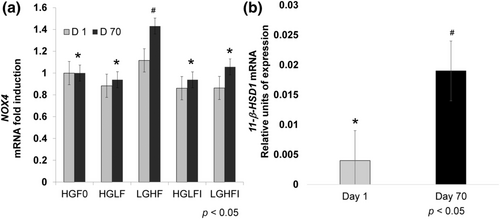
NADPH oxidase 4 (NOX4) expression was higher in LGHF treatment (p < .05, Figure 2a). 11β-HSD1 showed no differences between treatments by 2−ΔΔCt method (p > .05); however, we analyzed the Ct values by 2−ΔCt method, and the results showed that only the LGHFI treatment had differences between day 1 and 70, with a 2.98-fold increase on day 70 concerning day 1 (Figure 2b, p < .05).
3.4 XOR activity
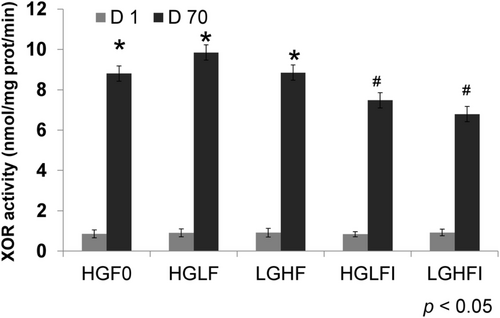
XOR activity has been considered a regulator of adipogenesis (Cheung et al., 2007). It showed no differences among treatments on day 1 (Figure 3, gray bars). Interestingly enough, adipose differentiation induced a very large, almost 10-fold increase in XOR activity (Figure 3, black bars); such increase was smaller when micromolar concentrations of inosine were added to the treatments, whether they were high in fructose or glucose (Figure 3 black bars, p < .05). On day 70, XOR activity was not that great in HGLFI and LGHFI compared with the other treatments, showing an inosine effect (Figure 3, p < .05).
3.5 Intracellular ROS production and extracellular hydrogen peroxide pool
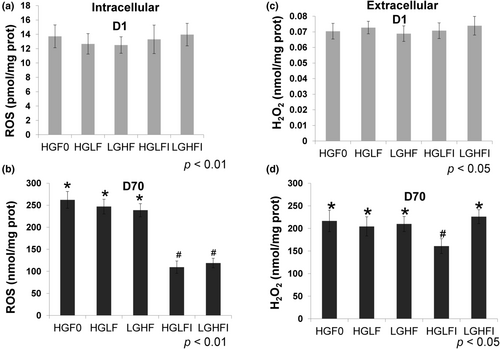
The compound DCFHDA was used to evaluate the intracellular ROS pool, and the results obtained on day 1 (Figure 4a) indicated a very low intracellular ROS pool; however, on day 70, the pool increased significantly (among the different treatments 7000 and more than 17,000 fold, Figure 4b). These results agree with a similar increase in the extracellular hydrogen peroxide pool, which was detected with Amplex Red reagent, and showed an increase between 2000 and 3000-fold among the various treatments (Figure 4c,d). Similar to the effect of inosine, which reduces the catalytic activity of XOR, inosine reduced the ROS pool (Figure 4b) and H2O2 pool, the latter only in HGLF (Figure 4d, p < .05), and not in LGHF media (Figure 4d). When the ROS concentration was compared at the end of the adipose differentiation, both media enriched with inosine (HGLFI and LGHFI), showed lower ROS values (p < .05) by approximately 60% (Figure 4b).
3.6 Uric acid pool
Regarding the concentration of uric acid in the cell culture media, there was no difference between the different treatments on day 1 ( 0.0104 ± 0.007 ng/μl). However, the uric acid pool on day 70 increased on average 12.4-fold as compared with values on day 1 ( 0.1292 ± 0.024 ng/μl).
4 DISCUSSION
4.1 Mesenchymal stem cells
Pérez-Serrano et al. (2017) observed that PBM-MSCs from porcine neonate exhibited high proliferation and were positive for MSCs markers CD44, CD90, CD105, and positive expression of stem cells genes (OCT4, NANOG). Subsequently, Nishimura et al. (2019) reported that newborn PBM-MSCs expressed positive MSCs markers CD29, CD44, and CD90 and differentiated into chondrocytes, osteocytes, and adipocytes. Consistent with these results in the present work, we observed the expression of CD44, CD90, and CD105 in PBM-MSCs by PCR (Figure 1a1).
Pérez-Serrano et al. (2017) observed adipose morphology on day 35 in PBM-MSCs, cultured in the presence of 25 mM glucose. Legeza et al. (2014) observed that 2-day post confluence, murine 3 T3-L1 was subjected to a differentiation medium that contained glucose of fructose (25 mM) as the only carbohydrate source, and adipose morphology was reached on day 8. In the present study, PBM-MSCs reached adipose morphology on day 70 (Figure 1b), but used lower concentrations of glucose and/or fructose.
4.2 Differentiation of adipose tissue
MSCs are typically cultured in a medium supplemented with IBMX, indomethacin, dexamethasone, and insulin for adipogenic differentiation (Chen et al., 2016; Legeza et al., 2014; Pittenger et al., 1999; Puri et al., 2012). The latter promotes glucose uptake and triglyceride synthesis in adipocytes (Chen et al., 2016). Additionally, heme-induced oxidative stress inhibited Sirtuin 1 activity and promoted adipogenesis in human MSCs and mouse preadipocytes 3 T3-L1 (Puri et al., 2012).
In this study, fructose (LGHF and LGHFI) induced a 40% increase in the expression of C/EBPβ and PPARγ. A study on 3 T3-L1 cells showed that fructose is an effective adipocyte differentiation agent by stimulating NADPH generation in the endoplasmic reticulum and the subsequent activation of glucocorticoids (Legeza et al., 2014). High fructose levels accelerated lipid metabolism by increasing the activity of hormone-sensitive lipase (HSL) and adipose triglyceride ligase (ATGL), which may be stimulated by the increased activation of glucocorticoids. Fructose was found to be superior to glucose in promoting 3 T3-L1 adipocyte differentiation.
Fructose produces alterations in cell signaling and inflammation cascades on insulin-sensitive organs, these signaling molecules include different nuclear factors (Rutledge & Adeli, 2007). The results obtained in this work are consistent with those obtained in other studies where fructose alone or mixed with glucose increased the expression of C/EBPβ and PPARγ, (Du & Heaney, 2012; Legeza et al., 2014; Legeza et al., 2017). However, it is relevant to mention that Du and Heaney (2012) and Legeza et al. (2014) used 3 T3-L1 cells; we considered it of greater interest to explore the response to fructose in a physiological process, i.e., the evolution of MSCs into adipocytes. On the other hand, the levels of glucose and fructose used by both research groups were higher than those used in this study. Du and Heaney (2012) used fructose levels of 0, 55, 550, 5500 μM plus constant glucose 11.1 mM for all treatments, and Legeza et al. (2014) used 0.55, 5.5, and 25 mM glucose or fructose, without mixing. In our experiment, a maximum dose of 7.5 mM of these monosaccharides was used alone or in mixtures, which is the physiological value of glucose in normal glycemic humans. Inosine was added because it is generated in the liver when high doses of fructose are administered orally (Van der Berghe et al., 1977) and we tried to simulate the substance that reaches the cells after high fructose is consumed.
4.3 NOX4 and 11β-HSD1 expression
NADPH oxidases are pro-oxidant enzymes whose primary function in the cell is the production of reactive oxygen species (ROS). NOX4 generates ROS on a constitutive basis, and its regulation occurs at the transcription and translational levels (Li et al., 2012). According to Mahadev et al. (2004), NOX4 is required for insulin-induced glucose uptake in adipocytes. Insulin is also a critical regulator of adipocyte differentiation in vitro and in vivo, where it regulates NOX4 expression (Li et al., 2012). NOX4 levels are elevated in the adipose tissue of obese rodent models and humans with severe insulin resistance (Park et al., 2005). On day 70, we observed NOX4 overexpression in cells treated with LGHF (Figure 2a, p 0.05), which is consistent with previous reports (Park et al., 2005). Consistent with this, it is well established that increased ROS production resulting from increased NOX4 activity is associated with insulin resistance and adipose tissue development (Atashi et al., 2015; Han, 2016).
Fructose metabolism in adipocytes results in the production of precursors for fatty acid synthesis and the induction of NADPH-generating enzymes. Fructose increases the expression of its transporter GLUT5 and 11-HSD1 in adipocytes' plasma membranes (Legeza et al., 2014), enhancing adipocytes' ability to produce active glucocorticoids (Senesi et al., 2010). We observed that 11-HSD1 mRNA expression levels increased almost three-fold after adipocyte differentiation (Figure 2b, p < .05). Interestingly, in previous studies, significantly higher 11β-HSD1 activity was also observed in adipocytes differentiated in the presence of fructose (Legeza et al., 2014).
4.4 Activity of XOR
XOR is the primary enzyme involved in the oxidation of purine metabolites to uric acid, a highly antioxidant compound found in mammals. Hyperuricemia is associated with an increase in adiposity, obesity, and metabolic syndrome (Pacher et al., 2006). XOR expression is consistently higher in adipose tissue than in other tissues, and in obese states, XOR expression, enzymatic activity, and urate products are all increased (Cheung et al., 2007). Thus, adipocyte-derived XOR activity may directly contribute to obesity-related hyperuricemia. Our results demonstrated that adipose differentiation of MSCs induced XOR activity almost 10-fold more than that observed on day 1 (Figure 3). Tsushima et al. (2013) demonstrated that adipose tissue is a major organ that has an abundant activity of XOR, similar to the small intestine or liver. On day 70, XOR activity increased in all cases, however the increase was lower in HGLFI and LGHFI (p < .05), showing an inosine effect (Figure 3, p < .05).
4.5 Intracellular ROS production and extracellular H2O2 production
There is accumulative evidence that shows that altered metabolic processes, such as mitochondrial metabolism, oxidative stress, and glucose uptake, affect MSCs differentiation. An increase in mitochondrial metabolism and ROS generation is a key characteristic of MSCs undergoing adipogenic differentiation (Chen et al., 2016; Puri et al., 2012). However, it is unknown whether these changes are a causal factor or a consequence of adipogenic differentiation. It has been shown that mitochondrially targeted antioxidants could decrease the adipogenic differentiation of MSCs, while exogenous hydrogen peroxide could restore it. Furthermore, ROS generated by mitochondrial complex III is essential for the activation of adipogenic transcription factors (Tormos et al., 2011). These results suggest that increased mitochondrial metabolism is an early cause of adipogenesis. Indeed, increased mitochondrial metabolism is a prerequisite for adipogenic differentiation displayed by specific blockage or mitochondrial respiratory pathways (Chen et al., 2016; Zhang et al., 2013).
The results of intracellular ROS production are shown in Figure 4; on day 70 (Figure 4b) the concentration increased greatly compared with day 1 (among the different treatments 7000 and more than 17,000 fold, Figure 4a). When treatments were compared at the end of adipose differentiation, those added with 30 μM inosine (HGLFI and LGHFI) showed a lower increase of ROS production (p < .05, Figure 4b).
These data are consistent with the increment in extracellular peroxide recorded; the effect of the treatments applied during the adipose differentiation of MSCs over the concentration of H2O2 is also shown. We could observe that between days 1 and 70 the extracellular concentration of H2O2 increased almost 3000 fold among the various treatments (Figure 4c,d).
High levels of peroxide may indicate that aquaglyceroporins such as AQP3, AQP7, and AQP9 promote an effective diffusion system. Nevertheless, studies have shown that insulin activates the PI3K/AkT/mTOR pathway in adipose tissue so that the presence of these channels would facilitate the release of glycerol (Chiadak et al., 2016). Other works have reported that adipocytes release large amounts of glycerol in the presence of fructose (Romero et al., 2015).
Finally, several authors reported the presence of ROS during the process of MSCs adipogenesis. Different studies agree that the higher ROS concentration favors differentiation in the adipose lineage (Mahadev et al., 2004; Romero et al., 2015). Reports which involve inosine nucleotide in the development of adipogenesis are scarce. Su et al. (2009) showed that the inhibition of inosine monophosphate dehydrogenase produced a reduction in adipogenic markers as well as the differentiation process. However, some reports mention that adenosine plays a role in inflammation, adipogenesis, and insulin resistance (Su et al., 2009).
4.6 Production and secretion of uric acid
As previously stated, XOR is involved in the differentiation and adipogenesis of pre-adipocytes. XOR and uric acid take part in cell transformation and proliferation as well as in progression and metastasis. The collected evidence confirms the contribution of XOR and uric acid in metabolic syndrome. However, in some cases, XOR and uric acid may have antioxidant protection. The dual effect of XOR and uric acid explains the contradictory results obtained with XOR inhibitors and caution is recommended in their therapeutic use (Pardo et al., 2017). Uric acid is the catabolic product of exogenous dietary compounds, as well as endogenous purines, and its serum levels are produced by the balance between its generation (mainly in the liver), and elimination (mostly through the kidney). We found no differences between treatments on day1 ( 0.0104 ± 0.007 ng/μl) or day 70 ( 0.1292 ± 0.024 ng/μl); however, on day 70 uric acid increased on average 12.4-fold with respect to day 1, that is, PBM-MSCs adipose differentiation induced the formation and secretion of uric acid.
Tsushima et al. (2013) showed that mature adipocytes and adipose tissue produced and secreted uric acid. It is known that the increase in serum uric acid after the intake of fructose in the diet depends on the phosphorylation of fructose by hepatic fructokinase, which is not limited by negative feedback, thus leading to ATP depletion and excessive purine degradation. The resulting hyperuricemia may cause changes that are characteristic of MetS as they are: (a) visceral obesity and hypertriglyceridemia; (b) inflammation, endothelial dysfunction, and hypertension; (c) insulin resistance, hyperinsulinemia, and hyperglycemia (Pacher et al., 2006; Pardo et al., 2017).
5 CONCLUSIONS
Based on the physiological similarities between humans and pigs, our porcine model might offer advantages over rodent models and can better understand human MetS installation at the molecular level. With the use of this model, after 70 days in culture in all five different incubation media (7.5 mM glucose, 6 mM glucose + 1.5 mM fructose with or without 30 μM inosine, and 6 mM fructose + 1.5 mM glucose, with or without 30 μM inosine) our MSCs achieved adipose morphology, a 10-fold increase in XOR catalytic activity and uric acid pool, and a several thousand-fold increase in intracellular ROS and extracellular H2O2. The above changes are repeatedly related to reports on the adipogenic process. However, an interesting controversy was observed: the induction of adipocyte genes (C/EBPβ and PPARγ), and higher expression of adipocyte enzyme (NOX4) appeared only in a fructose-rich medium at detected concentrations in the blood of excessive fructose consumers (6 mM fructose + 1.5 mM glucose vs. 7.5 mM glucose). In conclusion, for the first time our data showed an earlier differentiation of MSCs into adipocytes in a medium with higher fructose concentration, previously reported in human blood, and the micro-molar concentrations of inosine can prevent the accumulation of ROS, which requires further experimental analysis.
AUTHOR CONTRIBUTIONS
Francisco Campos-Maldonado: Methodology; Data curation. María L. González-Dávalos: Methodology; Data curation; Supervision; Formal analysis; Writing - review & editing; Project administration. Enrique Piña: Conceptualization; Validation; Writing - review & editing. Miriam Aracely Anaya-Loyola: Conceptualization; Formal analysis; Writing - original draft. Armando Shimada: Conceptualization; Writing - original draft; Formal analysis. Alfredo Varela-Echavarria: Conceptualization; Supervision; Data curation; Resources. Ofelia Mora: Conceptualization; Funding acquisition; Writing - original draft; Writing - review & editing; Supervision; Data curation; Resources; Formal analysis.
ACKNOWLEDGMENTS
We thank Martín García-Servín, Alejandra Castilla, and Adriana González-Gallardo [INB (Neurobiology Institute)-UNAM, Querétaro, México] for their technical assistance. This work is part of the master in sciences thesis FC-M. submitted to the Universidad Autónoma de Querétaro (UAQ). FC-M. thanks CONACYT for a scholarship at Facultad de Ciencias Naturales, UAQ.
FUNDING INFORMATION
This research was supported by grant 211559 from CONACYT (Consejo Nacional de Ciencia y Tecnología, México-City, México) to Ofelia Mora.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
ETHICS STATEMENT
All procedures involving animals were approved by the Internal Committee for the Use and Care of Experimental Animals (CICUAE-UNAM, NOM-062-ZOO-1999 4.2.2, #065).
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.




