Baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway
Abstract
Aberrant activation of the nuclear factor-kappa B (NF-κB) signaling pathway is closely implicated in colorectal cancer (CRC) growth, metastasis, and immune escape. In the present study, we reported natural derived compound of baicalin (BA), an efficient inhibitor of NF-κB, with good anti-tumor effect on CRC. CCK8 and colony formation assays showed that Baicalin significantly inhibit viability and proliferation in HCT-116 and CT26 cells. Additionally, Baicalin dramatically triggers mitochondria-mediated apoptosis in both HCT-116 and CT-26 cells, which is evidenced by loss of mitochondrial membrane potential and elevated cellular reactive oxygen species level. Treatment with Baicalin suppresses migration and invasion of CT26 cells by impairing TLR4/NF-κB signaling pathway. What's more, administration of Baicalin significantly retarded tumor growth rate in a subcutaneous xenograft tumor mouse model of CT26 cells. Treatment with Baicalin could ameliorate tumor immunosuppressive environment by downregulation of PD-L1 expression and proportion of myeloid-derived suppressor cells (MDSCs) and upregulation of percent of CD4+ and CD8+ T cells in CT26 tumors, thus improving anti-tumor immunity. In conclusion, our study demonstrated that baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway, suggesting it might serve as a potential candidate drug for the treatment of CRC.
Practical applications
In the present study, we reported natural derived compound of baicalin (BA), an efficient inhibitor of NF-κB, with good anti-tumor effect on CRC. We demonstrated that baicalin triggers mitochondria-mediated apoptosis, inhibits migration, and improves anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway.
1 INTRODUCTION
Colorectal cancer (CRC) is regarded as a malignant cancer with an increasing occurrence and a high rate of mortality (Arnold et al., 2017). Based on the global statistical report, cancer of colon or rectum has reached the fourth-most frequent cancer that leads to cancer mortality among males and females in the worldwide (Siegel et al., 2020). Metastasis from primary tumors to distant organs including liver, lung, bone, and brain is a main reason for high mortality of CRC (Li et al., 2020). Even though patients who receive chemotherapy and radiotherapy after resection operation obtain certain therapeutic effect, side effects result from chemotherapy and radiotherapy impair their life equality and patients usually suffer from recurrence and distant metastasis (Dekker et al., 2019; Kim, 2015). Furthermore, the low overall survival of CRC which has not improved well in decades has thrown attention of worldwide to seek for a new and better therapeutic treatment for CRC.
Nuclear factor-kappa B (NF-κB) which is a key regulator of cell proliferation, apoptosis, migration and invasion, and angiogenesis is usually quickly induced by DNA damage, infection, or oxidative stress (DiDonato et al., 2012; Sun, 2017). More and more investigators revealed that NF-κB is abnormally upregulated in various tumor cells, including in ovarian cancer, breast cancer, colon cancer, and plays a key role in tumor prognosis and metastasis (Perkins, 2012; Taniguchi & Karin, 2018). Activation of NF-κB can trigger metastatic dissemination via epithelial–mesenchymal transition (EMT) process and induce tumor cells migrate from the primary lesion to distant organs, including the lungs, bone, brain, and lymph nodes via the lymphatic vessels or blood (Awasthee et al., 2019). Additionally, NF-κB activation can promote migrative and invasive ability of cancer cells via induction of matrix metalloproteinases (MMPs) such as MMP-2 and MMP-9 (Ren et al., 2016). What's more, a growing number of studies revealed that NF-κB is closely participate in the tumor immune escape. It is reported that abnormal activation of NF-κB signaling pathway directly triggers the transcription of PD-L1 and proliferation of regulatory T cells (Treg; Maeda et al., 2018; Vanamee & Faustman, 2017). Inhibition or knockdown of key genes of NF-κB can significantly regulate the immunosuppressive microenvironment of tumors and enhance anti-tumor immunity (Song et al., 2020). That is to say, NF-κB signaling pathway might be a potential therapeutic target for efficient therapy of CRC. However, chemical drugs that can efficiently suppress activation of NF-κB are rarely found to application in clinic. It is urgent to seek for a potent NF-κB inhibitor and applied it into clinic.
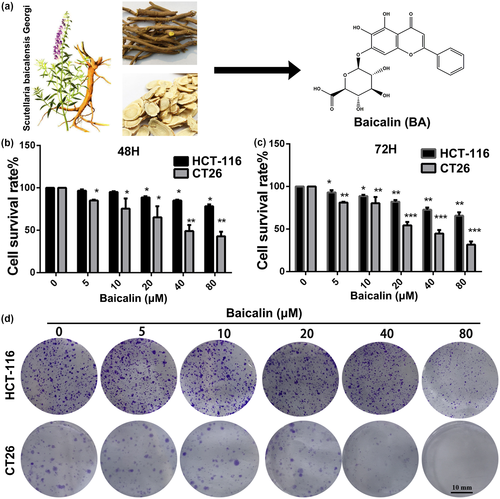
Plant-derived products which have been exploited to treat diseases for thousands of years are important and significant in the process of new drug research and development (Newman & Cragg, 2016; Rodrigues et al., 2016). As is known to all, most anti-tumor and anti-infection drugs in clinic are derived from natural plants (Fontana et al., 2020; Wohlleben et al., 2016). Natural chemical products exhibit various excellent properties, such as high efficiency, low toxicity, and economy (Xie et al., 2015). Therefore, it becomes a promising trend of new drug development to seek natural chemical agents from medicinal/natural plants to treat diseases. Baicalin (Figure 1a), which was extracted from the dry root of the herb Scutellaria baicalensis, shows a lot of biological functions, such as anticarcinogenic, antioxidant, antibacterial, and antitumor activities, and was proven to be non-toxic and safe for use in animals and humans (Liang et al., 2019; Miao et al., 2021; Zeng et al., 2020). Increasing studies displayed that Baicalin induces apoptosis and suppresses growth, invasion, and migration of breast and lung cancer cells (Du et al., 2010; Duan et al., 2019). Chung et al. demonstrated that NF-κB-mediated EMT of human breast epithelial cells can be significantly inhibited by treatment with Baicalin (Chung et al., 2015). Additional study also revealed that Baicalin ameliorated myocardial ischemia-reperfusion injury and attenuated lipopolysaccharide-induced inflammation by downregulation of the activity of NF-κB signaling pathway (Luan et al., 2019; Yang et al., 2019). Based on the important biological function of NF-κB in CRC, we suspected that BA, a potent inhibitor of the NF-kB signaling pathway, could serve as a promising drug for efficient therapy of CRC. Therefore, the anti-proliferation, anti-migration, and tumor immune regulation of BC were investigated in the present study.
2 MATERIALS AND METHODS
2.1 Reagents and antibodies
Baicalin (free of endotoxin) as a 98% purity that detected by HPLC was provided by Chengdu Biopurify Phytochemicals Ltd. In vitro experiments, Baicalin was initially prepared as stock solution (80 mM) dissolved in DMSO and kept at −20°C in dark place for further use. LPS was purchased from Beyotime Biotechnology. For animal studies, Baicalin was dissolved in 5.5% DMSO in corn oil, and 5.5% DMSO in corn oil was used as a vehicle control. The primary antibodies against Bax (#2772), Bcl-2 (#3498), caspase 3 (#9662), cleaved caspase 3 (#9664), MMP-2 (#87809), MMP-9 (#13667), TIMP2 (#5738), p-IκBα (#2859), IκBα (#4814), TLR4 (#14358), NF-κB p65 (#8242), PD-L1 (#60365), Ki-67 (#12202), and β-actin (#4970) were purchased from Cell Signaling Technology.
2.2 Cell viability assays
HCT-116 and CT26 cell lines were bought from Shanghai Cell Bank, the Chinese Academy of Sciences. RPMI1640 medium (Gibco) containing 10% fetal bovine serum (FBS, Gibco) and 1% antibiotics (streptomycin and penicillin) was exploited to culture CRC cells in atmosphere of 5% CO2 and 37°C.
Viability of Baicalin-treated CRC cells was measured by cck8 assay. Briefly, harvest HCT-116 and CT26 cells were planted in 96-well plates (3000–5000 cells per well) and incubated at 37°C for 6-8h. Subsequently, cells in plates were exposed to designated doses (0, 5, 10, 20, 40, and 80 μg/mL) of Baicalin for 48 and 72 hr. After different treatments, 10 μL of cck8 solution was added to each well, and cells were incubated for an additional 1–4 hr at 37°C. Finally, absorbance of each well at 450 nm was measured by a microplate reader. The experiments were performed at least in triplicates.
2.3 Colony formation assays
Single cell suspension of HCT-116 and CT26 was added to 6-well plate (200–500 cells/well), and incubate at 37°C for overnight. The next day, cells were exposed to determined concentrations of Baicalin (0, 5, 10, and 20 μM) for 10–14 days. Baicalin-contained culture medium was refreshed every 2 days. Experiment was stopped when the control group formed larger clones. The culture medium was discarded, and cells were washed with phosphate-buffered saline (PBS) and fixed with ethanol for 15–30 min. Then, cells were treated with crystal violet dye at dark for 20 min. After washed with PBS, cell colonies of different groups were photographed and countered with a light microscope (Olympus, Japan).
2.4 Cell apoptosis analysis
Apoptotic rate of CRC cells was evaluated by flow cytometry (FCM) analysis. HCT-116 and CT26 cells which were seeded in 6-well plate were exposed to different concentrations (0, 5, 10, 40 μM) of Baicalin at 37°C for 48 hr. Then, cells were harvested, washed with ice-cold PBS, and stained with apoptosis detection kits (4A Biotech Co., Ltd). Finally, stained cells were applied to flow cytometer (BD Biosciences). Cell apoptosis rate was displayed as sum of proportions of annexin V+/PI+ and annexin V+/PI-. Data were showed as means ± SD of at least three independent experiments.
2.5 Measurement of cellular reactive oxygen species and mitochondrial membrane potential (Ψm)
After treated with designed concentrations of Baicalin (0, 5, 10, 20 μM) for 48 hr, cells were harvested, washed with PBS, and treated with 10 μM Rh123 at 37°C for 25 min in the dark place. After washed twice with cold PBS, cells were subjected to FCM analysis. For intracellular reactive oxygen species (ROS) detection, cells treated with BA were harvest and incubated with 10 μM DCFH-DA dye for 30 min at 37°C. Stained cells were then harvest, washed twice with cold PBS, and detected by FCM. Data were displayed as means ± SD of at least three independent experiments.
2.6 Wound-healing migration assay
Wound-healing migration assay was exploited to evaluate the migrated ability of cells after treated with Baicalin. CT26 cells were seeded to 6-well plate and incubated for additional time. When cell confluence reached about 80%, a sterile 10 μL pipette tip was used to scrap on the cell monolayer. Then, cell culture medium was replaced by fresh medium containing determined concentrations (0, 5, 10, and 20 μM) of Baicalin. For further pathway activator study, cells were pre-treated with 50 ng/ml of LPS for 2 hr. After incubated for 48 hr, cells were washed, fixed, and imaged by a light microscope (Olympus, Japan). The percentage of migrated cells in control group was considered as 100%.
2.7 Transwell assays
For the cell migration experiment, 100 μL CT26 cells suspension (5 × 105) with no serum and containing different concentrations of Baicalin was seeded into the upper chamber, meanwhile, 600 μL of 10% FBS-containing medium was added to the lower chamber. After incubated for 48 hr at 37°C, the migrated cells in the lower side of membrane were washed, fixed with 95% ethanol, and stained with 0.5% crystal violet. Migrated cells were photographed and counted by using a light microscope. For cell invasion assays, matrigel (dilution: 1:3, 60 μL/well, BD Biosciences) was pre-coated in the upper chamber. The following operations were similar with that of cell migration assays. Migrated/Invasive cells were counted in six randomly selected fields by using a light microscope. Data are showed as migrated and invasive rate in comparison to the control group.
2.8 Western blot analysis
Proteins of Baicalin-treated CRC cells involved in signaling pathway were measured by Western blot experiment. Briefly, HCT-116 and CT26 cells which were exposed to various doses (0, 5, 10, and 20 μM) of Baicalin for 48 hr were harvest and washed twice with cold PBS for protein extraction. The concentrations of proteins in different groups were determined by the Lowry method. Proteins (equal amount) from each group were loaded and separated by sodium dodecyl sulfate-polyacryl amide gel electrophoresis (SDS-PAGE) gels and then transferred with polyvinylidene difluoride membranes (Amersham Bioscience, Piscataway, N.J.). Subsequently, the membranes containing target proteins were treated with 5% non-fat milk for 2 hr at 37°C and incubated with corresponding primary antibodies (dilution, 1:1,000) for overnight at 4°C. After incubated with corresponded stained secondary antibodies, targeted bands in the membrane were visualized by using an enhanced chemiluminescence kit (Amersham Bioscience.).
2.9 Anti-tumor evaluation of Baicalin in xenograft tumor mouse model
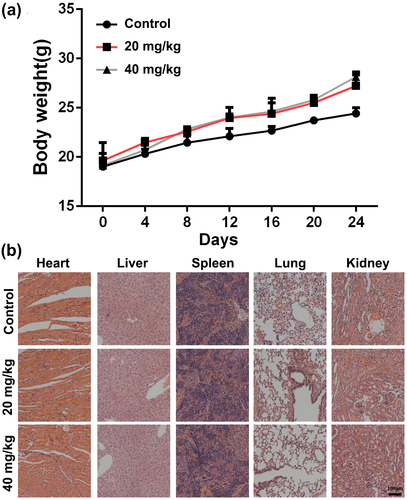
All experiments about animal in the present study were approved by the Institutional Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine. Eighteen female Balb/c mice (6 weeks old, 18–20 g) which were bought from Beijing HFK Bioscience Co, Ltd (Beijing, China) were adapted to the environment for a week prior to experiment. CT26 cells (1 × 106 cells per mouse) suspension was subcutaneously injected into right flank of each mouse. When tumor size grew to about 100 mm3, mice were randomly divided into three groups. Then, all mice in each group were intraperitoneally administered with different concentrations of Baicalin (Control, 20 mg/kg and 40 mg/kg) once daily for 24 days. Mice in the control group were treated with equal amount of corn oil. Tumor volume and body weight of mice in each group were measured and recorded every 3 days. Volume of tumors was calculated as follow: tumor volume (mm3) = 0.5 × length (mm) × width (mm)2. At the termination of animal experiment, all mice were euthanized, and tumors from each group were collected, photographed, and weighed.
Main organs (heart, liver, spleen, lung, and kidney) of mouse in each group were collected, fixed in 10% (v/v) neutral-buffered formalin, and sectioned (5 μm) for histopathological evaluation by hematoxylin & eosin assay.
2.10 Immunohistochemistry
At the endpoint of animal experiment, tumors were collected from each group, fixed with 10% (v/v) paraformaldehyde, embedded, and sectioned for immunohistochemistry analysis. Tumor sections were stained with Ki-67, p-IκBα, and PD-L1. Images of immunohistochemistry results were taken with a light microscope (Olympus).
2.11 Tumor immune microenvironment evaluation
After treatment with Baicalin, tumors of each group were isolated and digested to obtain single tumor cell suspension. Subsequently, tumor cells were incubated with CD3, CD4, CD8, Gr-1, and CD11b for 30 min, and subjected to FCM for detection of proportion of CD4+/CD8+ T cells and myeloid-derived suppressor cells (MDSCs) in tumors.
2.12 Statistical analysis
All experiments in this study were conducted at least in triplicate. Data are displayed as mean ± standard deviation. The software of SPSS 16.0 (SPSS) was exploited to perform statistical analysis. p-values for comparison of two groups were calculated by two-tailed Student's t-test. p-values for statistical significance were indicated as follows: *p < .05; **p < .01; ***p < .001.
3 RESULTS
3.1 Baicalin significantly inhibits viability and proliferation of CRC cells
In order to study the potential pharmacological effect of Baicalin on CRC cells, we firstly assessed the cytotoxicity of Baicalin in HCT-116 and CT26 cells. As displayed in Figure 1b,c, Baicalin significantly suppressed viabilities of HCT-116 and CT26 cells in a dose and treated time-dependent manner. Secondly, we used colony formation experiment to further study the anti-proliferation effect of Baicalin on HCT-116 and CT26 cells. As evidenced by Figure 1d, clonogenic abilities of both HCT-116 and CT26 cells were dramatically suppressed in a dose-dependent manner after treated with BA. Taken together, these results suggested that Baicalin exhibits efficient anti-proliferation activity in CRC cells in a concentration and time-dependent manner.
3.2 Baicalin promotes mitochondria-mediated apoptosis in CRC cells
For the purpose of investigating whether cytotoxicity of Baicalin in CRC cells resulted from apoptosis, Annexin V/PI dual-staining assay was used to detect CRC cells apoptotic level after treatment of Baicalin. As displayed in Figure 2a, the apoptosis induction effects were apparently observed after Baicalin treatment for 48 hr. Compared with control group (14.12 ± 1.26%), 20 μM Baicalin induced 35.83 ± 1.65% apoptosis in HCT-116 cell. Meanwhile, 56.38 ± 1.76% apoptosis was detected in 20 μM–treated CT26 cells in comparison to control group (5.48 ± 0.63%). Results above indicated that Baicalin significantly triggers apoptosis in CRC cells.

To further verify apoptotic induction of Baicalin in CRC cells, changes in apoptosis-associated proteins were evaluated by Western blot assay after exposure to Baicalin for 48 hr. As evidenced by Figure 2b,d, treatment with Baicalin dramatically upregulated expression of cleaved caspase3 in both HCT-116 and CT26 cells, which indicts apoptosis-induced effect of Baicalin. Furthermore, with an increase in BA-treated concentration, the expression of Bax/Bcl-2 ratio elevated distinctly (Figure 2c,e), implying a hypothesis that apoptosis triggered ability of Baicalin might result from mitochondrial destruction.
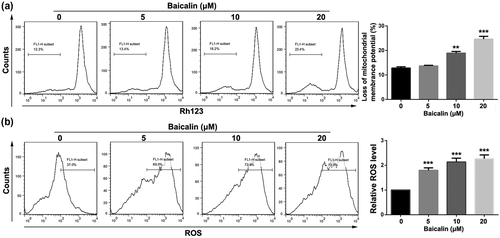
To verify this hypothesis, changes in the mitochondrial membrane potential was detected by Rh123 stain assay after Baicalin treatment. It can be clearly seen from Figure 3a that treatment of CT26 cells with 20 μM Baicalin leads to a dramatical loss of mitochondrial membrane potential in comparison to control group. In order to further test destruction effect of Baicalin in mitochondrial membrane, level of cellular ROS was measured by DCFH-DA staining after Baicalin treatment. As displayed in Figure 3b, the cellular level of ROS was distinctly upregulated in the dose-dependent manner in CT26 cells by treatment with Baicalin. Taken together, Baicalin triggers apoptosis via mitochondrion-related apoptosis pathway in CT26 cell.
3.3 Baicalin represses migration and invasion of CRC cells by suppressing TLR4/NF-κB signaling pathway
To investigate whether Baicalin can influence migration and invasion of CRC cells, wound-healing, transwell migration, and invasion assays were performed in CT26 cells after treated with determined dose of Baicalin for 48 hr. As shown in Figure 4a, Baicalin treatment can significantly suppress wound-healing ability of CT26 cells. Moreover, migration and invasion abilities of CT26 were dramatically inhibited in a concentration-dependent manner after treatment with different dose of Baicalin (Figure 4b,c).
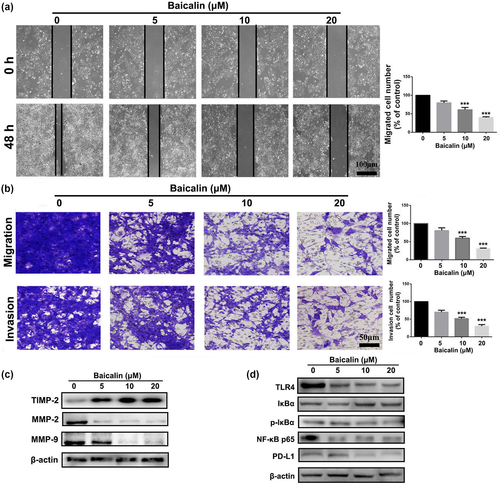
For the purpose of further investigating the suppressed effect of Baicalin on migration and invasion of CT26 cells, Western blot experiment was used to measure changes in expression of cell metastasis-associated proteins after treated with Baicalin. As displayed in Figure 4c, Baicalin-treated CT26 cells exhibited higher expression of TIMP-2, and lower expression of MMP-2 and MMP-9 in comparison to control group, indicating a good anti-migration effect. Furthermore, compared with control group, expression levels of TLR4, NF-κB p65, and p-IκBα in CT26 cells were also significantly downregulated by treatment with Baicalin, suggesting an impairment of TLR4/NF-κB signaling pathway in CT26 cells (Figure 4d). Because of important role of NF-κB signaling pathway in transcription of PD-L1, we also measured whether Baicalin inhibits PD-L1 expression in CRC cells. As displayed in Figure 4d, Baicalin treatment remarkably inhibited expression level of PD-L1 in CT26 cells, which suggests that inhibition of NF-κB signaling pathway by Baicalin might ameliorate tumor immunosuppressive environment in vivo. As shown in Figure S1, mobility of CT26 cells was suppressed by treatment of 20 μM of Baicalin. However, inhibited effect of Baicalin was attenuated by pre-treated with LPS, an activator of TLR4. Taken together, these data suggested that Baicalin suppressed CRC cells migration and invasion by suppressing TLR4/NF-κB signaling pathway.
3.4 Baicalin exerts efficient antitumor efficacy by enhancing antitumor immunity via NF-κB signaling pathway
Because of the good apoptosis-induced effect of Baicalin in CRC cells in vitro, subcutaneously xenograft tumor mouse model of CT26 cells was established to assess anti-tumor activity of Baicalin in vivo. We can find from Figure 5a that administration of Baicalin significantly retarded tumor growth rate of CT26 cells in comparison to control group. At the endpoint of in vivo experiment, tumors of mice in each group were collected, and tumor size and weight were found to be much lower in Baicalin-treated groups (Figure 5b,c) than that of control group. Immunohistochemistry analysis of tumor sections of mice in each group was conducted to investigate anti-tumor mechanism of Baicalin. As shown in Figure 5d, Ki-67 positively stained cells in Baicalin-treated groups were fewer than that in control group, suggesting Baicalin-inhibited proliferative ability of colorectal tumor cells. Moreover, expression level of TLR4 and p-IκBα, key proteins of NF-κB signaling pathway, was dramatically downregulated in tumor section of Baicalin-treated groups, indicating that activation of NF-κB was inhibited by treating with Baicalin.
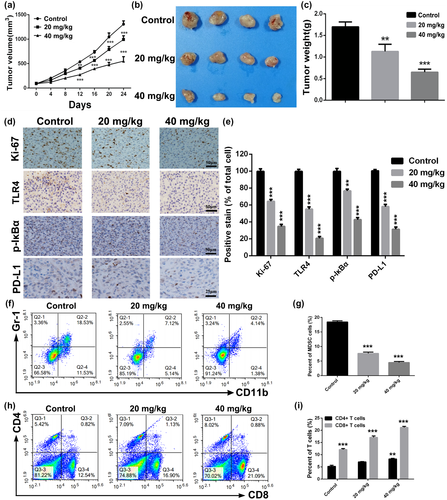
Inspired by the inhibited effect of Baicalin on expression of PD-L1 in CT26 cells in vivo, we further investigated whether Baicalin could suppress PD-L1 expression in vivo. As displayed in Figure 5d, significantly downregulated expression level of PD-L1 was observed in tumor section of tumors in 20 and 40 mg/kg treatment groups, implying remission of tumor immunosuppressive microenvironment. Therefore, we collected and digested isolated tumors of mice in each group to test variation of immune cells. As indicated in Figure 5f,g, proportion of myeloid-derived suppressor cells (MDSCs), which could negatively regulate system immunity, was remarkably downregulated in BA-treated groups. Furthermore, proportion of CD4+ and CD8+ T cells was significantly upregulated after Baicalin treatment (Figure 5e,h), indicating an improvement of anti-tumor immunity. Above all, our data suggested that Baicalin exerts efficient antitumor efficacy in colorectal cancer by improving antitumor immunity via NF-κB signaling pathway.
3.5 Safety evaluation of Baicalin in xenograft tumor mouse model
Systematic safety of Baicalin was evaluated in the treatment process in tumor mouse model of CT26. Compared with control group, the body weight of the mice in BA-treated groups did not display any abnormal variation during the therapy process (Figure 6a). All tumor-bearing mice were killed at the end of the experiment, and major organs (heart, liver, spleen, lung, and kidney) were collected and subjected to histopathological examination. No significant pathological changes were found following administration of Baicalin in comparison to control group (Figure 6b). Above all, Baicalin may be a safe agent for clinical therapy.
4 DISCUSSION
Colorectal cancer (CRC) is regarded as a malignant cancer with an increasing occurrence and a high rate of mortality (Dekker et al., 2019). Even though patients who receive chemotherapy and radiotherapy after resection operation obtain certain therapeutic effect, side effects resulting from chemotherapy and radiotherapy impair their life equality and patients usually suffer from recurrence and distant metastasis (Dekker et al., 2019; Kim, 2015). Furthermore, the low overall survival of CRC which has not improved well in decades has thrown attention of worldwide to seek for a new and better therapeutic treatment for CRC. Therefore, it is urgent for us to develop new effective anti-CRC drug.
Natural products from medical plants have good abilities of high efficiency and low toxicity (Kashyap et al., 2019). It is reported that abnormal activation of NF-κB signaling pathway is closely associated with the CRC progression and metastasis (Patel et al., 2018). Various studies demonstrated that Baicalin protects against ethanol-induced chronic gastritis and impairs epithelial-to-mesenchymal transition via inhibition of the NF-κB (Avila-Carrasco et al., 2019; Ji et al., 2019). As such, we hypothesized that as a potent inhibitor of NF-κB, Baicalin might exhibit good therapeutic activity for the CRC treatment.
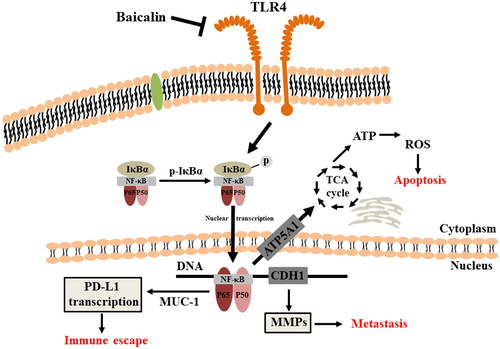
In the present study, anti-CRC activity of Baicalin was extensively investigated in vitro and in vivo. Firstly, CCK8 and colony formation experiments were conducted to demonstrate that Baicalin could significantly inhibit viability and proliferation of HCT-116 and CT26 cells. Moreover, FCM analysis showed that Baicalin inhibited CRC cells proliferation by induction of apoptosis. Upregulated expression level of Bax/Bcl-2 revealed that apoptosis induction of Baicalin might result from destruction of mitochondrial membrane. It is well known that mitochondrial membrane structural integrity accounts for the accumulation of cellular ROS level. Loss of potential of mitochondrial membrane and elevated cellular ROS level in CT26 cell after treatment with Baicalin verified that Baicalin efficiently triggers mitochondria-mediated apoptosis. Existed study have shown that Baicalin triggers mitochondria-mediated apoptosis in MCF-7 cells by generation of intracellular ROS level, which is consistent to the result of our study (Liu et al., 2019). It is reported that a synthesized NF-κB subunit c-Rel inhibitor (IT-901) could trigger a dose-dependent mitochondrial ROS production and a distinct decrease in ATP production in chronic lymphocytic leukemia cell lines, resulting from downregulated expression of NF-κB-regulated gene (ATP5A1), involved in scavenging processes or tricarboxylic acid cycle (Capece et al., 2020; Vaisitti et al., 2017). Based on this, we hypothesized that baicalin might inhibit tricarboxylic acid cycle to induce cellular ROS level and then trigger mitochondria-dependent apoptosis in CRC cells by impairing NF-κB pathway.
For the purpose of studying the effect of Baicalin on the CRC cells’ metastasis ability, we performed wound-healing assay and transwell migration and invasion experiments. Our results demonstrated that migration and invasion ability of CT26 cells were distinctly suppressed by treatment of Baicalin. Western blot analysis showed that Baicalin treatment downregulates expressions of MMP-2 and MMP-9 and upregulates TIMP-2 expression. Furthermore, treatment with Baicalin dramatically downregulated NF-κB-associated proteins (TLR4, p-IκBα, and NF-κB p65) in CT26 cells. It demonstrated that Baicalin inhibits CRC cells migration and invasion by inhibiting activity of TLR4/NF-κB signaling pathway.
Additionally, results of in vivo experiment displayed that Baicalin significantly delays tumor growth rate of CT26 cells in mouse model. Administration of Baicalin significantly suppressed proliferation of CRC cells, and inhibited activation of NF-κB. It has been well demonstrated that NF-κB, a key transcription factor of immunity and inflammation, is emerging as an important positive regulator of PD-L1 expression in cancer (Antonangeli et al., 2020). Therefore, we found that treatment of Baicalin distinctly ameliorated tumor immunosuppressive environment, which is evidenced by downregulation of PD-L1 expression and proportion of MDSCs and upregulation of percent of CD4+ and CD8+ T cells in CT26 tumors.
5 CONCLUSION
All our results demonstrated that Baicalin inhibited viability and proliferation of CRC cells by triggering a mitochondrion-mediated apoptosis. Additionally, Baicalin suppressed migration and invasion of CRC cells by suppressing TLR4/NF-κB signaling pathway (Figure 7). Furthermore, administration of Baicalin exhibited efficient anti-tumor effect on subcutaneous xenograft tumor mouse model of CT26 cells by improving anti-tumor immunity. Therefore, Baicalin might be a promising and effective candidate for CRC therapy in clinic.
ETHICS APPROVAL AND CONSENT TO PARTICPATE
All the animal experiments in this study were performed according to the National Institutes of Health (Bethesda, MD, USA) guidelines and were approved by the Ethical Committee of Chengdu University of Traditional Chinese Medicine (Chengdu, China).
ACKNOWLEDGEMENTS
This work was supported by the China Postdoctoral Science Foundation (2019M653833XB); Foundation of Science and Technology Department of Sichuan Province (2020YJ0147, 2021JDZH0040); and Foundation of “apricot grove scholar” of Chengdu University of Traditional Chinese Medicine (2019yky09, 2020yky02).
CONFLICT OF INTEREST
Authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
Linjiang Song: Conceptualization; Funding acquisition; Investigation; Validation; Writing-original draft. Shaomi Zhu: Investigation; Software. Chi Liu: Formal analysis; Investigation; Writing-review & editing. Qinxiu Zhang: Supervision; Writing-review & editing. Xin Liang: Conceptualization; Funding acquisition; Supervision; Writing-review & editing.




