Successful implementation of handheld reflectance confocal microscopy as the standard of care in the (surgical) management of lentigo maligna (melanoma)
Linked article: C. Sinz et al. J Eur Acad Dermatol Venereol. 2025;39:459–460. https://doi.org/10.1111/jdv.20549.
Abstract
Background
Reflectance confocal microscopy (RCM) has shown promise in predicting surgical outcomes by non-invasively detecting subclinical lentigo maligna (melanoma) (LM/LMM).
Objectives
To assess the effects of presurgical mapping using handheld RCM (HH-RCM) on surgical treatment, follow-up outcomes and management decisions.
Methods
A total of 117 consecutive LM/LMM cases (2015–2023) were included. The diagnostic accuracy of HH-RCM in detecting subclinical LM and invasive components was evaluated. The primary endpoints included histological margin status and changes in management based on the outcomes of the HH-RCM mapping procedure. Margin and follow-up outcomes were compared to a historical cohort before HH-RCM was introduced in our center (n = 94) (2003–2014).
Results
HH-RCM detected subclinical LM in 60% (n = 60) of cases. The median mapping duration was 14 min (range 4–50). In 27% (n = 33), the mapping procedure resulted in modified management, the majority consisting of limited surgery with adjuvant imiquimod (n = 15) or imiquimod monotherapy (n = 14). The remaining cases (n = 84) underwent HH-RCM-assisted surgery. Histological margins were cleared in 96.5% of the patients with a median histological margin of 3.0 mm, significantly higher than 81% in the historical cohort (median 2.0 mm) (p = 0.001). The sensitivity and specificity for detecting the extent of subclinical LM were 94% (95% CI 80.4–99.3) and 84% (95% CI 70.3–92.7), respectively. The negative predictive value for the detection of LMM was 94% (95% CI 84.4–97.7), and 75% of the initially missed LMM (n = 12) were identified during the HH-RCM mapping procedure. The study cohort had a 1.6% local recurrence rate compared with 25% in the historical cohort.
Conclusions
Integrating HH-RCM as the standard of care could lead to more personalized treatment strategies for LM/LMM and allows for the selection of patients suitable for nonsurgical treatment.
INTRODUCTION
Surgical excision is the recommended treatment for head and neck lentigo maligna (melanoma) (LM/LMM).1, 2 However, lesion delineation remains challenging because of the underestimation of lesion extent by clinical examination using dermoscopy and Wood's lamp examination.3-5 This can result in incomplete excisions and contributes to an increased risk of local recurrence.6-8 To address this issue, various staged excisional techniques have successfully reduced the local recurrence risk.9 Even so, these techniques do not predict the definitive size of the surgical defect, which is particularly crucial in the head and neck owing to cosmetic and functional considerations. Nonsurgical options such as topical imiquimod (IMQ) and radiotherapy can be considered in cases where surgical excision is not feasible.10-13
Reflectance confocal microscopy (RCM) is a noninvasive diagnostic device that has been shown to improve the detection of subclinical LM, leading to reduced rates of incomplete excisions and local recurrence following wide local excision (WLE) and staged surgical techniques.9, 14-17 A pilot study in our centre has demonstrated the reliability of handheld reflectance confocal microscopy (HH-RCM) for presurgical mapping.18 In this study, we aim to evaluate the impact of HH-RCM following its introduction as the standard of care in our centre and assess its effect on management decisions and long-term follow-up outcomes.
MATERIALS AND METHODS
This research paper expands on a pilot study (2015–2017) conducted in our centre18 and includes consecutive head and neck LM/LMM patients referred to the Department of Dermatology of the Netherlands Cancer Institute (NKI) with no contra-indication for surgical excision (2018–2023). A historical cohort of consecutive head and neck LM/LMM treated by WLE (n = 92) before HH-RCM was introduced was used as the control group (2003–2014).19 The researchers followed ethical guidelines and received approval from the institutional review board. A single investigator (Y.S. Elshot) with 33 months of experience with HH-RCM at the start of the inclusion period performed all imaging/analyses. Study data were recorded using a standardized form for the extension cohort. Staging was performed according to the eighth edition of the American Joint Committee on Cancer (AJCC).
The HH-RCM mapping procedure was followed by a multidisciplinary consultation with a dermatologist and a head and neck surgeon with the patient present and used in the management decision-making process. The standard of care in NKI for LM/LMM consists of HH-RCM-guided wide local excision (WLE) with a 5–10 mm surgical margin. Excised tissue was cut perpendicular to the long axis (i.e. bread loafing), and immunohistochemical staining was used when necessary for Melan-A, sox10, S100 or PRAME. Diagnostic sampling errors were defined as LM being reclassified as LMM after surgery.
The lesions were analysed for morphological characteristics and invasive LMM missed at initial diagnosis. The clinical border was determined using polarized dermoscopy (DermLite DL4, 3Gen, Inc., San Juan Capistrano, California, USA). The planned surgical margin was delineated using a surgical pen. Adhesive rings were placed around the entire circumference of the margin and examined for subclinical LM. The margin was extended in the case of atypical dendritic cells, and a new ring was applied. This process was repeated until the entire lesion was mapped, and no subclinical LM was found beyond the proposed surgical margins (Figure 1). The mapping procedure is described in detail elsewhere.18 Deviation from the standard of care was discussed with the patient based on the extent of the subclinical component with potential functional or cosmetic consequences and/or suspicion of invasive LMM. The diagnostic accuracy of the HH-RCM in detecting subclinical LM and invasive components was compared to the histological outcome (reference standard). The HH-RCM predicted defect size and the duration of the mapping procedure were recorded.
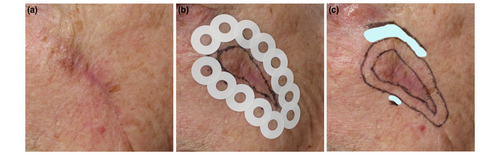
The study's primary endpoint was the surgical outcome or changes in management based on HH-RCM results. Modified management options consisted of imiquimod (IMQ) monotherapy or limited surgery, followed by adjuvant IMQ. Topical IMQ was applied daily for 12 weeks, with intensity reduction (up to a minimum of 5 days per week) in case of side effects. Six months after completing topical IMQ treatment, the entire lesion area was assessed with HH-RCM, and a biopsy was performed when residual LM was suspected. The secondary outcome was the recurrence rate following surgical excision for patients with at least 6 months of follow-up. The pilot study's follow-up outcomes were retrospectively updated. The surgical and follow-up outcomes of the HH-RCM-mapped patients were compared to the historical cohort.
Statistical analysis
Absolute and relative frequencies are described for all study variables. Differences in baseline clinicopathological characteristics between study groups were identified using chi-square or Fisher's exact tests for categorical variables and the unpaired t-test or Mann–Whitney U test for continuous variables, where appropriate. The time to local recurrence was calculated from the surgical treatment date. In the case of IMQ treatment, the recurrence time was calculated from the time of topical treatment completion. Statistical significance was set at p < 0.05. Data were analysed using SPSS 29.0 for Windows (IBM Corp, Armonk, NY).
RESULTS
Clinicopathological characteristics
One hundred-seventeen patients, 26 (22%) from the pilot and 91 (78%) from the extension cohort, were evaluated (Table 1). The predominant diagnostic method involved a single 3 mm punch biopsy (n = 88; 75%). At baseline, 70% (n = 82) were classified as LM, and 30% as LMM (n = 35), with a median Breslow thickness at diagnosis of 0.7 mm (range 0.1–2.5). Following surgical excision, the proportion of LMM increased to 44% (n = 51), as 24% (16/67) of surgically treated LM were histologically reclassified as LMM (median Breslow thickness 0.5 mm; range 0.2–1.2). No LMM showed signs of histological ulceration. Thirteen out of 15 indicated cases underwent SLNB, and isolated tumour cells were found in two of the 13 patients (i.e. N1a, according to the AJCC eighth edition). In the historical cohort, 12 out of 53 (23%) LM were reclassified as LMM.
| No (%) | Study cohort (n = 117) | Historical cohort (n = 92) | p-Value | |||
|---|---|---|---|---|---|---|
| Pilot cohort (n = 26) | Extension cohort (n = 91) | Total (n = 117) | p-Value | |||
| Patient characteristics | ||||||
| Age (years) (mean; range) | 69.5 (48–90) | 72.0 (34–91) | 71.0 (34–91) | NS | 70.1 (45–93) | NS |
| Sex | NS | NS | ||||
| Female | 17 (65.4) | 55 (60.4) | 72 (61.5) | 49 (53.3) | ||
| Male | 9 (34.6) | 36 (39.6) | 45 (38.5) | 43 (46.7) | ||
| Lesion characteristics | ||||||
| LM | 18 (69.2) | 48 (52.7) | 66 (56.4) | NS | 48 (52.2) | NS |
| Primary | Recurrent | 12 (46.2) | 5 (19.2) | 30 (33.0) | 18 (19.8) | 42 (35.9) |23 (19.7) | 34 (37.0) | 14 (15.2) | ||
| LMM | 8 (30.8) | 43 (47.3) | 51 (43.6)a | NS | 44 (47.8) | NS |
| Primary | Recurrent | 5 (19.2) | 4 (15.4) | 33 (36.3) | 10 (11.0) | 38 (32.5) | 14 (12.0) | 41 (44.6) | 3 (3.3) | ||
| Breslow thickness (mm) (median [range]) | 0.5 (0.3–2.2) | 0.6 (0.3–3.6) | 0.5 (0.2–3.6) | NS | 1.5 (0.1–7.0) | <0.001 |
| Localization | NS | NS | ||||
| Facial | 21 (80.8) | 76 (83.5) | 97 (82.9)b | 72 (78.3) | ||
| Ear | 1 (3.8) | 7 (7.7) | 8 (6.8) | 4 (4.3) | ||
| Scalp | 3 (11.5) | 7 (7.7) | 10 (8.5) | 14 (15.2) | ||
| Neck | 1 (3.8) | 1 (1.1) | 2 (1.7) | 2 (2.2) | ||
| Pigmentation | NS | NA | NA | |||
| Pigmented | 9 (34.6) | 39 (42.9) | 48 (41.0) | |||
| Lightly pigmented | 10 (38.5) | 16 (17.6) | 26 (22.2) | |||
| Partly pigmented | 3 (11.5) | 15 (16.5) | 18 (15.4) | |||
| Amelanotic | 2 (7.7) | 8 (8.8) | 10 (8.5) | |||
| Scar | 2 (7.7) | 13 (14.3) | 15 (12.8) | |||
| Demarcation | NS | NA | NA | |||
| Sharp | 3 (11.5) | 15 (16.5) | 18 (15.4) | |||
| Moderate | 8 (30.8) | 32 (35.2) | 40 (34.2) | |||
| Unsharp | 15 (57.7) | 44 (48.4) | 59 (50.4) | |||
| Diameter (mean; range) | ||||||
| Long axis (mm) | 18 (6–55) | 16 (4–48) | 17 (4–55) | NS | 17 (5–57) | NS |
| Short axis (mm) | – | 8.5 (2–40) | 10.5 (2–40) | NA | NA | NA |
- Abbreviations: AJCC, The American Joint Committee on Cancer; HH-RCM, handheld reflectance confocal microscopy; LM, lentigo maligna; LMM, lentigo maligna melanoma; NA, not available; NS, nonsignificant.
- a T-stage according to AJCC 8: T1a (n = 34; 66.7%), T1b (n = 4; 7.8%), T2a (n = 7; 13.7%), T3a (n = 4; 7.8%); Tx (n = 2; 3.9%).
- b Facial (n = 97) included cheek (n = 49), (peri)nasal (n = 18), periorbital (n = 14), forehead (n = 10), nasolabial (n = 4) and chin (n = 2).
Effect of HH-RCM mapping procedure on LM/LMM management
The diagnostic accuracy outcomes are summarized in Table 2. The median mapping duration was 14 min (range 4–50). Subclinical atypical cells beyond the initial surgical margin were detected in 60% of cases (n = 70). For cases with subclinical LM, the median diameter increased from 20 mm (range 4-55 mm) to 33 mm (range 16-60 mm) for the longitudinal axis and from 14 mm (range 2–40 mm) to 25 mm (range 10–50 mm) for the transverse axis. Subclinical LM was significantly associated with increased lesion size (long-axis, p = 0.04; short-axis, p < 0.001). No significant differences were found in lesion type, subtype, localization, demarcation, pigmentation or Breslow thickness.
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | |
|---|---|---|---|---|---|
| Subclinical, presence | 97.0 (84.2–99.9) | 84.6 (71.9–93.1) | 80.0 (67.8–88.4) | 97.8 (86.4–99.7) | 89.4 (80.9–95.0) |
| Subclinical, extenta | 94.3 (80.8–99.3) | 82.0 (68.6–91.4) | 78.6 (66.9–86.9) | 95.3 (84.1–98.7) | 87.1 (78.0–93.4) |
| Detection invasive component | 80.0 (52.0–95.7) | 88.2 (76.1–95.6) | 66.7 (47.5–81.5) | 93.7 (84.4–97.7) | 86.4 (75.7–93.6) |
- Abbreviations: CI, confidence interval; HH-RCM, handheld reflectance confocal microscopy; NPV, negative predictive value; PPV, positive predictive value.
- a In cases with detected subclinical LM, histological margins >5 mm for LM and >10 mm for LMM were considered false-positive (overtreatment); positive histological margins were considered false-negative.
Sixteen of 51 LMM (31%) were initially missed at diagnosis, of which 12/16 (75%) were identified during the HH-RCM procedure. The suspicion of the invasive component was based on the presence of cerebriform nests (n = 2), atypical (i.e. sparse) melanocytic nests at the dermo-epidermal junction/papillary dermis (n = 3), epidermal/junctional disarray (n = 4), large melanocyte size (n = 6), pleomorphic pagetoid/atypical cells (n = 1) and widespread atypical cobblestone pattern (n = 1). The five false-positive cases were based on large melanocyte size (n = 2), disarranged pattern (n = 2) and the single case with widespread atypical cobblestone pattern (n = 1).
Modifications in lesion management occurred in 27% (n = 32) of cases. The remaining patients (n = 85) underwent HH-RCM-guided WLE (Figure 2). The primary reasons for modified management were the extent of the subclinical component (n = 23; 72%), suspicion of an invasive component (n = 1, 3%), suspicion of an invasive component combined with the extent of the subclinical component (n = 5, 16%), or refusal of (further) surgery (n = 3, 9%). In the modified management group, the longitudinal (p = 0.049) and transverse (p = 0.008) diameters were notably larger at baseline. There was no significant difference in the median age (p = 0.748) or Breslow thickness (p = 0.95). The median (IQR) age of patients treated by topical IMQ mono- or adjuvant therapy (n = 26) was 70.5 (65.0–76.0). The majority of lesions (n = 10; 39%) were localized on the cheek, whereas perinasal and orbital lesions represented 35% (n = 9) and 15% (n = 4), respectively.

Surgical outcome
The resection margins were cleared in 96.5% of the HH-RCM-guided group with a median histological margin of 3.0 mm (IQR 2.0–5.0), compared to 81% (p = 0.001) in the historical cohort with a median histological margin of 2.0 mm (IQR 1.0–6.0). There was no difference in the median histological margin between patients with and without subclinical LM detected using HH-RCM (3.0 vs. 2.9; p = 0.643). The invasive component's median (IQR) histological margin was 7.0 (4.9–10.0). For the LMM cases (n = 12) that were treated by diagnostic excisional/incisional biopsy followed by adjuvant treatment, the invasive component's median (IQR) histological margin was 5.0 (4.1–7.3). No residual invasive LMM was found in the excisional specimen in 33% (n = 17) of LMM. The median (range) Breslow thickness for these cases at diagnosis was 0.9 mm (0.2–3.6).
In the HH-RCM-guided WLE group, closure consisted of primary intention (n = 40, 47%), full-thickness grafts (n = 31, 37%), split skin grafts (n = 11, 13%) and local flaps (n = 3, 3%). There was a trend in the rate of primary closure based on the presence or absence of subclinical LM (45% vs. 55%) (p = 0.5).
Follow-up—HH-RCM-assisted wide local excision (n = 84)
Four patients died due to unrelated causes, and one patient was lost to follow-up. Table 3 shows the follow-up outcomes for patients with at least 6 months of follow-up (n = 70; 82%). In two cases, HH-RCM-assisted WLE resulted in positive margins. In the first case, a scalp LM with three previous local recurrences before HH-RCM mapping resulted in the only local recurrence at 11 months of follow-up. The patient was treated with IMQ monotherapy and remained recurrence-free at 52 months. The second case involved a patient with recurrent amelanotic LMM of the cheek who refused further surgical treatment and showed no signs of local recurrence at 35 months of follow-up.
| HH-RCM-assisted WLE | Historical cohort (n = 92) | |||
|---|---|---|---|---|
| Pilot (n = 24) | Extension (n = 46) | Total (n = 70) | ||
| Inclusion period | 2015–2017 | 2018–2023 | 2015–2023 | 2003–2014 |
| Follow-up (months); median (IQR) | 62.0 (41.0–76.7) | 21.0 (12.7–30.5) | 24.5 (16.7–57.3) | 61.0 (35.1–76.0) |
| Recurrence rate No (%) | 1 (4.2) | 0 (0.0) | 1 (1.4) | 23 (25.0) |
- Abbreviations: HH-RCM, handheld reflectance confocal microscopy; IQR, interquartile range; LM, lentigo maligna; LMM, lentigo maligna melanoma; WLE, wide local excision.
- a Only cases with at least 6 months of follow-up were included (70 out of 84).
Among the 51 LMM patients, 1 (1.6%) progressed to stage IIIC and remained recurrence-free at 32 months after adjuvant nivolumab. Two LM patients developed non-LMM-related stage IV melanoma. The first had a history of five prior invasive melanomas and died at 19 months due to metastatic disease. The second, with an unknown primary, achieved complete remission at 78 months after pembrolizumab treatment.
Follow-up—Modified management due to HH-RCM mapping procedure (n = 33)
A single (3%) patient died due to an unrelated cause. Six-month follow-up was available for 75% (n = 24) of patients with modified management with no reported local recurrences at a median follow-up of 18.5 months (IQR 9.0–32.5). For patients treated with IMQ monotherapy (n = 10) or adjuvant IMQ (n = 9), the median follow-up after completion of the IMQ treatment was 18 months (IQR 9.0–23.0). Two patients showed dendritic cells during HH-RCM evaluation after IMQ treatment completion. Residual LM was confirmed in one (4%) of these cases by HH-RCM-guided biopsy, while the other did not have histological signs of LM. A focal dermal sparse nest was observed in a single patient, which was histologically confirmed as a small dermal nevus. A single patient declined adjuvant IMQ and progressed to LMM at 16 months of follow-up (Breslow thickness 0.4 mm without ulceration). One patient with IMQ-treated LM developed regional lymph node metastases, which were attributed to an unknown primary melanoma due to the absence of clonality. The patient was alive and recurrence-free at 34 months of follow-up.
DISCUSSION
Our study demonstrates that implementation of HH-RCM-guided surgery improved the histological clearance rate from 81% to 96.5% (p = 0.001) while maintaining long-term local recurrence-free survival in the pilot cohort. The mapping procedure also changed management strategies in over 25% of patients and identified 75% of LMM missed at initial diagnosis.
Our study's 96.5% histological clearance rate of HH-RCM-guided WLE is notably higher than the reported 78%–83% range for LM/LMM in a recent systematic review when using guideline-recommended WLE surgical margins.9 Negative histological margins do not always correlate with recurrence-free survival, however. Only a limited portion of the surgical margin is evaluated due to transversal tissue processing (‘bread-loafing’).20 In this regard, the extent of the histological margin is the strongest predictor of local recurrence.21 A 3 mm cut-off point has been proposed as a histological margin <3 mm is associated with a 27% risk of local recurrence, compared to 2.6% for ≥3 mm.21, 22 Although the extension cohort's follow-up period was limited, our study's median histological margin of 3 mm aligns with prior data with a limited risk of local recurrence.21, 22 In support of our data, Yélamos et al.23 have shown that the HH-RCM-estimated surgical defect using video mapping correlates well with the eventual surgical outcome.
Approximately one-fourth of surgically treated LM were upstaged to LMM. As HH-RCM has a penetrative depth limited to the level of the papillary dermis in the horizontal plane, it can be challenging to determine the invasion depth of atypical cells.24 Nonetheless, HH-RCM successfully identified 75% of invasive cases that were initially missed. To our knowledge, the study by Melhoranse Gouveia et al.25 is the only study evaluating the diagnostic accuracy of RCM in detecting subclinical invasion components in LMM. A total of 229 LM/LMM were evaluated, and the invasive component was detected in 89% of the cases with a sensitivity of 63% (95% CI 52%–78%) and a specificity of 79% (95% CI 74%–88%). The three features most predictive of an invasive LMM component were epidermal/junctional disarray, melanocytic nests and large melanocyte size. Only including these three predictive criteria would remove two cases from our analysis and result in a modest change in sensitivity from 80% to 79% and specificity from 88% to 90%. These proposed features align with an observational RCM study showing that melanocytic nests presenting as lentiginous perifollicular (i.e. medusa-head-like structures) and dermal nests were significant RCM features distinguishing LM from LMM.26, 27 A possible explanation for false-positive outcomes could be pagetoid cells. While they have been shown to predict invasive LMM in histology,28 they are not a predictive RCM feature for LMM, possibly because intraepidermal Langerhans cells and melanocytic hyperplasia also have dendritic morphology.25, 29 False-negative outcomes are likely the result of the horizontal orientation of the imaging and limited penetration depth. Finally, similar to our data, the NPV (99%) was higher than the PPV (46%). Negative predictive value is a critical diagnostic outcome when selecting LM cases to be treated using nonsurgical modalities.
Over a quarter of the cases had their management strategy changed due to the outcome of the HH-RCM mapping procedure. Most patients underwent limited surgical excision, followed by adjuvant IMQ or IMQ monotherapy. A recent Delphi Consensus paper endorsed topical IMQ as the primary alternative to surgical intervention in monotherapeutic and adjuvant contexts.1 The clinical clearance rates based on data from systematic reviews of IMQ monotherapy range from 63% to 79%. Still, the heterogeneity of the data should be considered when interpreting the reported efficacy of topical IMQ.30, 31 The treatment effectiveness of IMQ highly depends on the intensity, duration and presence of local inflammation. In line with the standard of care in our centre, the greatest odds for clearance are found with 6–7 applications per week with at least 60 applications, with a significant decrease in efficacy when used less than 5 days per week.10, 30
Clinical clearance rates following IMQ treatment may overestimate histological clearance rates due to limited partial sampling by single-punch biopsies. To facilitate early detection of treatment failure, all patients undergoing topical IMQ treatment were evaluated using HH-RCM 6 months after completing treatment. Of the 28 patients, only one case of residual LM was histologically confirmed by HH-RCM-guided biopsy. Previous studies have indicated that RCM is more accurate in detecting treatment failure than clinical-dermoscopic evaluation, with 100% sensitivity and specificity exceeding 92%.32, 33 More than half of the IMQ-treated patients received adjuvant treatment, which appears to increase clearance rates compared to monotherapy and has recurrence rates comparable to micrographically controlled surgical techniques, supporting our low residual LM rate.34, 35
Limitations
This retrospective study has several limitations. First, the limited follow-up may have led to underestimating the recurrence rate. Previous research suggests that a considerable proportion of post-treatment recurrences occur after a median duration of 3 years or more.22, 36-38 This underestimation is likely minimal since our pilot's long-term follow-up data showed a persistent low recurrence rate. The current 1.6% recurrence rate is slightly lower than the 2.6% expected from the median 3 mm histological margin.21, 22 In the case of topical IMQ treatment, we systematically mapped the affected area 6 months post-treatment to detect residual LM. There is a potential for selection bias, as lesions with larger subclinical components were more frequently treated with IMQ monotherapy or adjuvant therapy. Furthermore, even though we compared the clinicopathological features between the historic and study cohorts, other histological or clinical features or management decisions in the historic cohort could have led to bias in our outcomes.
In line with the recent Delphi consensus statement, our centre's primary alternative to surgical treatment is topical IMQ. However, the lack of randomized controlled data on the comparative effectiveness of IMQ and radiotherapy and the optimal usage of topical IMQ (i.e. mono-, adjuvant- or neoadjuvant therapy) still exists.39, 40 Given the complexity of treatment options and the paucity of predictive prognostic data, shared decision-making is essential for patients with LM/LMM.41 Although patients were involved in the multidisciplinary consultation, no pre- or post-consultation questionnaires were administered, preventing us from assessing the impact of the HH-RCM mapping process on decision-making. Lastly, the mapping was conducted by a single confocal user in a tertiary hospital setting; therefore, the generalizability of our results to other patient populations is unclear.
CONCLUSIONS
Our findings support the efficacy of HH-RCM for precisely identifying surgical margins, reducing recurrence rates, and detecting subclinical LMM. This allows for an informed selection of patients suitable for nonsurgical treatment. Although staged micrographically controlled surgical techniques have demonstrated a clear advantage in minimizing local recurrence compared to WLE, they fall short in predicting the final defect size.9 Topical IMQ or radiotherapy may be considered when surgical excision is not feasible.10, 12 However, nonsurgical treatment requires caution, given that clinical features are unreliable in predicting invasive LMM. Implementing HH-RCM as the standard of care could help resolve diagnostic challenges, enabling more tailored treatment approaches for LM/LMM patients.40 To confirm our findings, a prospective, preferably multi-centre, matched-controlled study comparing HH-RCM-assisted excision to micrographically controlled surgery for LM/LMM with long-term follow-up and survival outcomes is needed.
ACKNOWLEDGEMENTS
The authors have nothing to report.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
Approval from the ethics committee was waived following a review by the NKI Institutional Review Board. The patients in this manuscript have given written informed consent to the publication of their case details.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




