Cordyceps cicadae polysaccharides attenuate diabetic nephropathy via the miR-30a-3p/TRIM16 axis
Abstract
Objective
The molecular mechanism of the protective effect of Cordyceps cicadae polysaccharides (CCPs) on renal tubulointerstitial fibrosis in diabetic nephropathy (DN) is still unclear. This study aims to further understand the molecular mechanisms behind the therapeutic benefits of CCP on diabetic nephropathy.
Methods
Mice were randomly assigned into six groups (n = 8). Cordyceps cicadae polysaccharide dissolved in 5% dimethyl sulfoxide was administered by gavage for 12 consecutive weeks. The CCP doses were divided into low, medium, and high, 75, 150, and 300 mg/kg/day, respectively. The efficacy of CCP was determined by assessing the renal function and histological alterations in diabetic db/db mice. The degree of glomerular mesangial dilatation and sclerosis was evaluated using semiquantitative markers. Cell viability, apoptosis, epithelial–mesenchymal transition (EMT), inflammation, oxidative stress, and mitochondrial reactive oxygen species (ROS) in high glucose (HG)-cultured MPC5 podocytes were determined. The interaction of miR-30a-3p and tripartite motif-containing protein 16 (TRIM16) was examined by luciferase reporter assay. Western blotting, reverse transcription-polymerase chain reaction, and immunofluorescence were used to analyze gene and protein expressions.
Results
The in vivo findings illustrated that CCP may protect mice with type 2 diabetes from inflammation and oxidative damage (P < 0.05). Furthermore, CCP has a therapeutic value in protecting renal function and morphology in diabetic nephropathy by reversing podocyte EMT. The in vitro results indicated that CCP dose-dependently inhibited HG-induced apoptosis, EMT, inflammation, oxidative stress, and mitochondrial ROS levels in MPC5 podocytes (P < 0.05). Luciferase reporter assay confirmed the interaction between miR-30a-3p and TRIM16 in MPC5 podocytes cultured in high glucose (P < 0.05).
Conclusion
The protective effect of CCP on HG-induced MPC5 can be achieved by miR-30a-3p/TRIM16 axis.
INTRODUCTION
Diabetic nephropathy (DN) is a major and prevalent complication of diabetes. Its common pathological features include damage to tubular epithelial cells, sclerosis of the glomeruli, apoptosis, inflammatory infiltration, oxidative stress, and fibrosis of renal interstitial tissue1-4. The loss of podocytes in the kidney is an early indication of diabetic nephropathy and is associated with the induction of epithelial–mesenchymal transition (EMT) following podocyte injury, which can induce the generation of fibroblasts, leading to the deposition of extracellular matrix proteins, glomerulosclerosis, and interstitial fibrosis5-10. In addition to oxidative stress, which is driven by an overabundance of reactive oxygen species (ROS), the role of inflammation in diabetic nephropathy has also attracted considerable attention4, 11. MicroRNAs (miRNAs) are endogenous non-coding RNAs that guide miRNP complexes to cleave or translationally inhibit target gene mRNAs by complementary pairing with the 3′-UTR region of target genes, thereby further regulating the expression of proteins related to the translational regulation of target genes. miRNAs are now known to be involved in the development of diabetic nephropathy, and complex regulatory networks were presented in miRNA and target genes12. The miR-30 family members are involved in diabetes or diabetic nephropathy13. Significant downregulation of miR-30a-3p has been reported in patients with diabetic nephropathy compared with normal controls14. Tripartite motif-containing protein 16 (TRIM16) was found to aggravate murine lung fibrosis and was highly expressed in mice with diabetes15, 16. Moreover, TRIM16 is the target gene of miR-30a-3p. Using TargetScan and other database predictions, miR-30a-3p and TRIM16 may have some type of regulatory relationship in diabetic nephropathy.
Cordyceps cicadae (C. cicadae) is a type of fungus, and C. cicadae polysaccharides (CCPs) are believed to have anti-inflammatory, antioxidant, antiangiogenic, and anticonvulsive properties; CCPs are also known to alleviate tubulointerstitial fibrosis in rats with diabetic nephropathy through the suppression of inflammatory and renal fibroblast activation via the TGF-β1/suppressor of mothers against decapentaplegic (SMAD) and nuclear factor (NF)-κB signaling17-20. The objective of this study was to gain a further understanding of the molecular mechanisms behind the therapeutic benefits of CCP in diabetic nephropathy. Type 2 diabetic db/db mice model and HG-induced MPC5 podocyte cell model were used to explore the effect of CCPs on renal morphological changes in mice with diabetic nephropathy and the association of CCPs and the miR-30a-3p/TRIM16 axis.
MATERIALS AND METHODS
Preparation of CCP
Crude polysaccharides were obtained from dried C. cicadae powder (Anhui Cordyceps Source Biotechnology Co. Ltd, Huainan, China)19, 21. The powder (100 g) was dissolved in distilled water (1:10 w/v) at 90°C and then added to 95% ethanol (1:4) and incubated at 4°C overnight. After removing proteins and completing dialysis, CCPs were obtained by freeze-drying, and the polysaccharide content was quantified using a phenol-sulfuric acid method22, 23. The absorbance was measured at 490 nm, and the polysaccharide concentration was calculated by the standard curve.
Animal models
All animal procedures were conducted in accordance with the protocol approved by the Institutional Animal Care and Use Committee of Longhua Hospital, Shanghai University of Traditional Chinese Medicine, China (no. 20180702A). Eight-week-old mice were obtained from the Aier Matt Experimental Animal Company (Suzhou, China). In this study, db/db mice were used to study the effect of a hyperglycemic environment on renal function and renal histopathological changes, and the db/m mice served as controls. Mice were randomly assigned into six groups (n = 8): control group (distilled water, db/m), db/m + CCP high-dose group (CCP-H) (300 mg/kg/day by gavage, db/m + CCP-H), model group (distilled water, DN), DN + CCP low-dose (CCP-L) group (75 mg/kg/day by gavage), DN + CCP medium-dose (CCP-M) group (150 mg/kg/day by gavage), DN + CCP-H group (300 mg/kg/day by gavage). CCP dissolved in 5% dimethyl sulfoxide (DMSO) was administered to animals by gavage for 12 consecutive weeks. During this period, fasting blood glucose was measured weekly, and 24 h urine samples were collected from the mice in metabolic cages to quantify urinary protein. All the mice were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) (i.p.). The corresponding tissue samples were collected.
Measurement of blood glucose, urinary albumin, creatinine, and 8-OHdG assay
The blood glucose content was measured using a glucose meter. Mice were fasted for 12 h before collecting blood samples from the tail vein. After centrifugation (500 g, 15 min, 25°C), the serum was stored at −80°C. Serum creatine (Scr), urinary albumin, and 8-OHdG levels were analyzed using ELISA kits (Cayman Chemical, Ann Arbor, MI, USA; Abcam, Cambridge, UK; CEA660Ge; USCN Life Science, Inc., Wuhan, China, respectively). 50 μL of all blood samples or standard and 50 μL of the antibody cocktail were added to the 96 wells. The plate was then sealed and incubated for 1 h at 25°C at 400 rpm. Each well was washed with 3 × 350 μL 1× wash buffer PT after shaking. The wash buffer PT should remain in the wells for at least 10 s. 100 μL of TMB development solution was added to each well and incubated for 10 min in the dark on a plate shaker set to 400 rpm. Next, 100 μL of a stop solution was added to each well. The plate was shaken on a plate shaker for 1 min to mix the content of the wells. The OD was recorded at 450 nm and used as an endpoint reading.
Renal histopathological analysis
After being perfused in situ with cold saline, the kidneys were fixed in 10% paraformaldehyde and embedded in paraffin. After dewaxing and rehydration, the renal sections (4 μm) were subjected to periodic acid-Schiff (PAS) and Masson trichrome staining to assess the histopathological changes21. The degree of glomerular mesangial dilatation and sclerosis was evaluated using semiquantitative markers24. Each glomerulus in a single segment was assigned a grade between 0 and 4, with 0 indicating the absence of lesions and 1, 2, 3, and 4 indicating the presence of lesions involving 25, 25–50, 50–75, or >75% of the glomerular tuft area, respectively. The images were photographed by microscope (Olympus, Tokyo, Japan) and analyzed using NIH ImageJ (v.1.50; National Institutes of Health, Bethesda, MD, USA) software.
Immunohistochemistry and immunofluorescence of mouse renal tissues
The renal sections, after being deparaffinized and rehydrated, were incubated overnight at 4°C with TRIM16 primary antibodies (A09514-4, 1:500; Boster Biological Engineering Co., International Enterprise Center, Wuhan, China), Bcal-2 (ab182858, 1:500; Abcam), and bcl-2-associated X protein (BAX) (ab53154, 1:500; Abcam); and P-cadherin antibodies (ab242060, 1:500; Abcam) and N-cadherin (ab76011, 1:500; Abcam); then incubated with Alexa Fluor 594-conjugated (red) goat anti-mouse IgG and Alexa Fluor 488-conjugated (green) goat anti-rabbit IgG (1:1000; Invitrogen, Carlsbad, CA, USA) secondary antibodies at 25°C for 1 h. Nuclei were counterstained with a Hoechst solution (Thermo Fisher Scientific, Waltham, MA, USA). The cells were visualized by confocal microscopy (FluoView 300; Olympus) and ImageJ. To identify the specificity used by the antibody, phosphate-buffered saline (PBS) solution was used as a negative control. There was no positive reaction and no fluorescence after the experiment with PBS. All images were quantified using ImageJ software.
Terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling staining
To identify apoptosis, terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling (TUNEL) staining was performed for renal tissue and cultured MPC5 cells were measured using an apoptosis detection kit (Vazyme, Red Maple Hi-tech Industry Park, Nanjing, China) and visualized by confocal microscopy. The average number of positive cell number was calculated based on six independent fields.
Measurement of pro-inflammatory factors and oxidative stress in renal tissue
After homogenization and centrifugation of the serum at 15,000 g for 10 min at 4°C, the supernatant was collected. The levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in the kidney tissue were determined by ELISA kits (Nanjing Jiancheng Biotech Co., Ltd, Nanjing, China), while in-culture medium samples were determined using ELISA kits (Shanghai Xitang Biotechnology Co., Ltd, Shanghai, China).
Oxidative stress biomarkers, including superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) were detected using assay kits (Shanghai Enzyme-linked Biotechnology Institute, Shanghai, China).
Cell culture and transfection
The MPC5 mouse podocyte cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's Modified Eagle Medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco). The cells were cultured with glucose at 5.5 mM (normal glucose, NG) and 30 mM (high glucose, HG). The NG group was additionally treated with 24.5 mM mannitol to establish control osmolarity. The cells were maintained in an incubator with 5% CO2 and 50–60% humidity at 37°C.
A miR-30a-3p inhibitor, miRNA inhibitor negative controls (NC), miR-30a-3p mimics, and miRNA mimic NCs were purchased from Thermo Scientific Co., Ltd. (Pilot Free Trade Zone, Shanghai, China) Small interference RNA-specific targeting TRIM16 (si-TRIM16) and the NCs (si-NCs) were chemically synthesized by GenePharma Co. Ltd (Shanghai, China). The three si-TRIM16 sequences were as follows: (1) si-TRIM16-1: 5′-AUUUUCAUUCAACUUAAGCUU-3′, (2) si-TRIM16-2: 5′-AAAACAGACUUAUGGUUAGCU-3′; (3) si-TRIM16-3: 5′-UUUUGAUGCUGUUGACUUGAU-3′.
Immunofluorescence analysis
Following fixing with 4% paraformaldehyde (30 min, 4°C), washing with PBS, treatment with 0.15% Triton X-100 at 25°C for 15 min, blocking with 5% bovine serum albumin (Hyclone, SH30574.03) for 1 h and goat serum for 30 min at 37°C, MPC5 podocytes were treated overnight at 4°C with antibodies directed against E-cadherin (1:250, ab201499; Abcam) and N-cadherin (1:200, ab119752; Abcam). The cells were treated with fluorescein isothiocyanate (FITC)-labeled secondary antibodies (1:150) for 1 h, and 4′,6-diamidino-2-phenylindole to stain the cell nuclei for 5 min. Images were observed using a confocal microscope (Leica, Shanghai,China) and analyzed using ImageJ software.
Luciferase reporter assay
Podocytes were co-transfected with wildtype (wt) or mutant (mut) 3′-UTR vectors and miR-30a-3p or miR-NC using Lipofectamine 2000 (Invitrogen). After 48 h, podocytes were assessed for luciferase activity using a dual-luciferase reporter assay system (Promega, Fitchburg, WI, USA) according to the manufacturer's protocol. The TRIM16 mutant sequence was CACUUA, and these vectors and their mutants were generated using DH5α competent amplification.
Annexin V-fluorescein isothiocyanate/propidium iodide apoptosis assay
The rate of apoptosis was determined by staining podocytes with fluorescein isothiocyanate (FITC)-labeled Annexin V and propidium iodide (PI) (BD Biosciences, San Jose, CA, USA). The suspended cells were added to tubes containing FITC-labeled Annexin V and PI, incubated in the dark for 15 min and analyzed by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 530 nm using a FACS Calibur system (BD Biosciences).
Cell viability assay
A 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (20 μL 5 mg/mL; Sigma-Aldrich, St Louis, MO, USA) was added to cells (1 × 104) and incubated for 4 h at 37°C. After adding DMSO (150 μL) and shaking for 15 min, the absorbance of solution (at 570 nm) was determined using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of mitochondrial ROS production
Mitochondrial ROS were evaluated using MitoSox Red (Invitrogen). The cells were treated with MitoSox Red (at a final concentration of 5 μM) in the dark for 30 min at 37°C and rinsed with Hank's balanced salt solution. The images were obtained by confocal microscopy (Leica) and analyzed by Image-Pro Plus 6.0 software.
Reverse-transcription polymerase chain reaction analysis
Total RNA was extracted from podocytes and tissue using an RNAsimple total RNA extraction kit (Tiangen, Beijing, China). The RT-PCR was performed using a Mir-X miRNA first-strand synthesis kit (TaKaRa, Dalian, China) and an ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) with SYBR Premix Ex Taq (TaKaRa) and the relevant primers. The primers used in this study are listed in Table 1. The expression of miRNA relative to U6 was determined using the method.
| Gene | Forward | Reverse |
|---|---|---|
| miR-30a-3p | GCGCTTTCAGTCGGATGTT | AGTGCAGGGTCCGAGGTATT |
| TRIM16 | ACACATTAGGGGATGGTGGC | TCAAGACTGTGGGCTATGCG |
| Bax | TCAAGGCCCTGTGCACTAAA | GGGGTCCCGAAGTAGGAGAG |
| Bcl-2 | TCTGGTTGGGATTCCTACGG | TGAATCGGGAGTTGGGGTCT |
| β-Actin | CGTCATTGCACGAAGACACAA | CCTGGTCCACCATTTTAAGGC |
| U6 | CAGGTCTCCAAGACGACATAGA | CGCCTTTTCGATTCATGTACTGC |
Western blot analysis
The total protein was quantified using a BCA kit (Beyotime, Shanghai, China) and the protein concentration was adjusted based on the BCA assay results. Then, the proteins were transferred to PVDF membranes. The membranes were then blocked for 2 h at 25°C with 5% non-fat dry milk and in TBS with 0.1% Tween-20, and incubated overnight at 4°C with TRIM16 primary antibodies (ab72129, 1:1,500; Abcam), cleaved caspase-3 (#9664, 1:1,000; Cell Signaling), caspase-3 (#9662, 1:1,500; Cell Signaling, Shanghai, China), vimentin (#5741, 1:1,000; Cell Signaling), nephrin (ab216341, 1:1,000; Abcam), P-cadherin (#2189, 1:1,000; Cell Signaling), BAX (#2772, 1:1,1,500; Cell Signaling), Bcl-2 (#3498, 1:1,000; Cell Signaling), E-cadherin (#96743, 1:1,500; Cell Signaling), N-cadherin (#84117, 1:1,500; Cell Signaling), and β-actin (#4970, 1:2,000; Cell Signaling). Then, the membranes were incubated with the secondary antibody for 1 h at 25°C. Immunoreactive bands were visualized with ECL western blotting (WB) substrate (Thermo Scientific), and the protein band intensity was quantified with ImageJ software and normalized to the signal intensity of β-actin.
Statistical analysis
Data are expressed as the mean ± standard deviation. The sample data in this study conformed to a normal distribution. A two-tailed Student's t-test was used to compare the differences between the two groups and a one-way anova with Dunnett's post hoc test was used to determine the differences between multiple groups. Statistical analyses were performed using SPSS 26.0 and GraphPad Prism (v.8.0). P < 0.05 was considered statistically significant.
RESULTS
Protective effect of CCP treatment on renal function and morphological changes in mice with DN
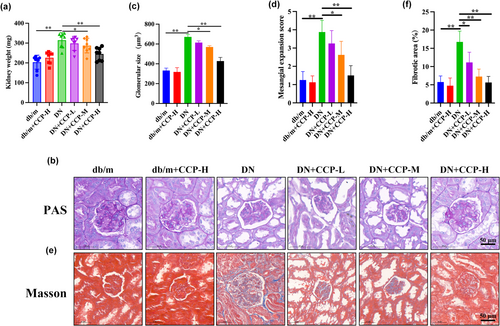
After treating the DN model mice with different concentrations of CCP, the kidney weight (KW) of the different groups of mice were compared. The kidney weight was significantly lower in the CCP-H treatment group than in the DN group but still higher than in the control group (Figure 1a). Levels of fasting blood glucose, blood urea nitrogen, Scr, and urinary albumin in the serum were prominently increased in mice with DN compared with control mice, whereas treatment with CCP-H remarkably reduced the above biochemical parameter levels (Table 2). The blood glucose levels in the DN group increased compared with the db/m group, while CCP-H decreased. PAS staining revealed that mice with DN had a higher rate of glomerular hypertrophy and expansion of the mesangial matrix compared with db/m mice (Figure 1b). The results showed that CCP dose-dependently reduced these renal pathological changes (Figure 1c,d). Masson staining further revealed that mice with DN had significantly worse renal fibrosis performance than db/m mice (Figure 1e,f). These results indicate that CCP had a protective effect on renal function and morphological changes in the DN model.
| Group | FBG (mmol/L) | BUN (mmol/L) | SCR (mmol/L) | Urinary albumin (mg/24 h) |
|---|---|---|---|---|
| db/m | 5.36 ± 1.23 | 3.78 ± 1.23 | 35.23 ± 6.23 | 9.45 ± 2.57 |
| db/m + CCP-H | 6.12 ± 1.41 | 4.12 ± 1.37 | 32.29 ± 4.58 | 8.74 ± 2.09 |
| DN | 25.49 ± 5.32** | 18.45 ± 4.59** | 97.69 ± 15.55** | 35.12 ± 5.23** |
| DN + CCP-L | 24.39 ± 4.97 | 17.32 ± 4.24 | 91.23 ± 13.39 | 31.29 ± 4.40 |
| DN + CCP-M | 21.25 ± 3.87# | 13.24 ± 3.02# | 72.12 ± 11.03# | 22.28 ± 3.39# |
| DN + CCP-H | 16.78 ± 3.02## | 7.69 ± 2.13## | 55.19 ± 8.87## | 18.31 ± 2.76## |
- Data are presented as mean ± SD. n = 8. **P < 0.01 vs db/m group; #P < 0.05, ##P < 0.01 vs DN group.
- BUN, blood urea nitrogen; FBG, fasting blood glucose; SCR, serum creatinine.
Effects of CCP on the parameters of oxidative stress, inflammatory cytokines, apoptosis, and EMT in the kidneys
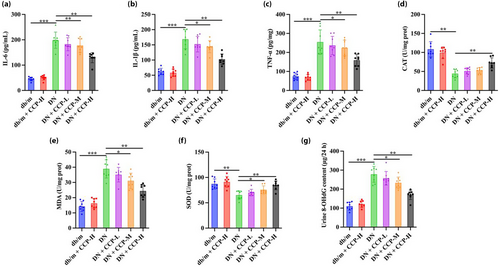
Compared with the db/m group, IL-6 (Figure 2a), IL-1β (Figure 2b), and TNF-α (Figure 2c) in the DN group significantly increased, whereas they were decreased in the CCP-H group. The levels of CAT and SOD in the DN group were significantly lower than in the db/m group, while CCP-H reversed these outcomes (Figure 2d,f). The findings demonstrated a trend in the opposite direction for MDA (Figure 2e). Compared with the CCP treatment groups, the DN group had a significantly higher urine 8-OHdG level, a strong indicator of oxidative tissue damage (Figure 2g)23.
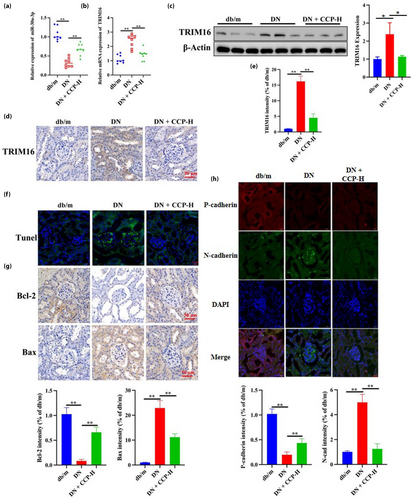
Next, due to the key role played by miR-30a-3p/TRIM16 in the progression of DN, the expression of miR-30a-3p/TRIM16 was further examined in different groups of mice. The results showed that the expression of miR-30a-3p decreased and TRIM16 increased in the DN group, while CCP-H reversed these outcomes (Figure 3a,b). Subsequently, the expression of TRIM16 protein in different mice was detected by WB, and TRIM16 expression was increased in the DN mice model and decreased after CCP-H treatment (Figure 3c), a result consistent with the immunohistochemistry results (Figure 3d,e). TRIM16 is known to be associated with cell apoptosis; the markers of apoptosis included B-cell lymphoma-2 (Bcl-2), BAX and the proteolytic activation of caspase 325-28. TUNEL labeling showed that the DN group had considerably enhanced apoptosis compared with the db/m group, while CCP treatment inhibited this trend (Figure 3f). The immunohistochemistry results showed that CCP-H could consistently reduce the expression of pro-apoptotic BAX proteins and increase the expression of anti-apoptotic Bcl-2 protein in the DN group (Figure 3g). In addition, CCP-H increased the level of P-cadherin, and DN decreased the level of P-cadherin while decreasing the level of N-cadherin (Figure 3h). These outcomes indicate that CCP has protective effects against oxidative and inflammatory damage, apoptosis and EMT in the DN model.
CCP protects MPC5 podocytes from apoptosis, EMT, inflammation, and oxidative stress induced by HG treatment
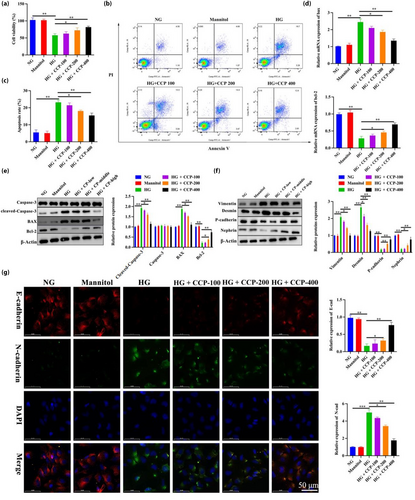
To further validate the in vivo experimental results, the effect of CCP was validated using MPC5 cells in vitro. CCP prevented the loss of viability and apoptosis in MPC5 podocytes grown in high glucose in a dose-dependent manner (Figure 4a–c). RT-PCR results showed that levels of Bcl-2 mRNA were increased with CCP treatment under glycemic conditions, whereas BAX was decreased (Figure 4d). The WB results showed that compared with the NG group, podocyte apoptosis in the HG group was significantly increased, while CCPs reversed this trend (Figure 4e). In addition, CCPs could reduce P-cadherin and nephrin in MPC5 podocytes cultured under HG, which was the opposite of mesenchymal cytoskeletal proteins desmin and vimentin (Figure 4f). The results of immunofluorescence assays for E-cadherin and N-cadherin demonstrated that CCPs protect podocytes from EMT induced by HG (Figure 4g). Similarly, the levels of TNF-α, IL-1β, IL-6, and ROS increased after HG exposure, but CCPs dramatically reversed these effects (Figure 5a–e). High glucose increased the content of MDA but decreased the activities of SOD and CAT, which were restored by CCP treatment (Figure 5f–h). In general, CCPs could reduce apoptosis, EMT, inflammation, and oxidative stress induced by high glucose in MPC5 cells; MiR-30a-3p regulated TRIM16 in MPC5 podocytes.
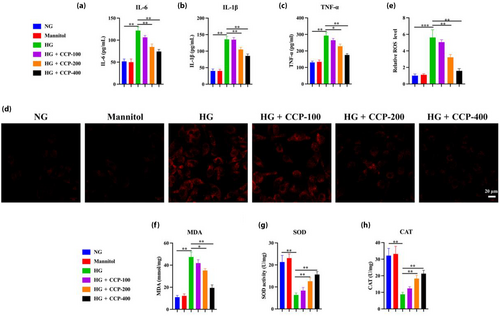
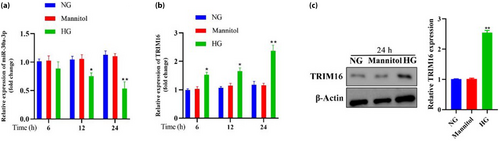
The expression of miR-30a-3p and TRIM16 were detected with an increasing time gradient (6, 12, and 24 h) under different conditions. The results showed that the expression of miR-30a-3p was lower under HG conditions and significantly lower after 12 h, with the lowest expression at 24 h (Figure 6a). In contrast, the levels of TRIM16 expression increased significantly under HG conditions from 6 to 24 h, consistent with the WB results (Figure 6b,c). However, miR-30a-3p and TRIM16 were expressed at a consistent level when grown under controlled conditions with normal glucose and with mannitol. TargetScan predicted TRIM16 would be a target of miR-30a-3p; accordingly, mutations of miR-30a-3p to which TRIM16 could not bind were designed (Figure 7a). The phenomenon of reduced TRIM16 expression was rescued after mutation at the position where TRIM16 mRNA bound to miR-30a-3p, indicating TRIM16 as one of the target genes of miR-30a-3p (Figure 7b). This results denoted that the overexpression of miR-30a-3p led to lower TRIM16 expression, whereas the inhibition of miR-30a-3p led to higher TRIM16 expression (Figure 7c), consistent with the WB results (Figure 7d,e). This indicates that miR-30a-3p was able to control the expression of TRIM16 in MPC5 podocytes cultured under HG conditions.

CCP protects MPC5 podocytes from HG-induced apoptosis, EMT, inflammation, and oxidative stress by controlling miR-30a-3p/TRIM16 expression
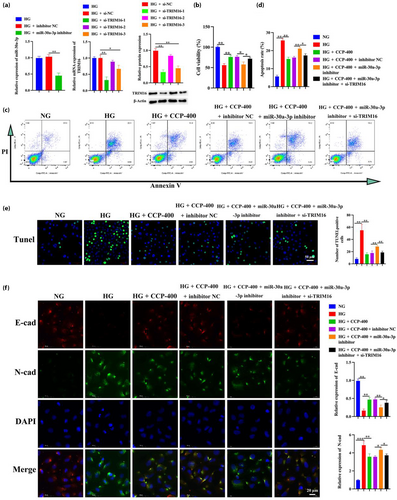
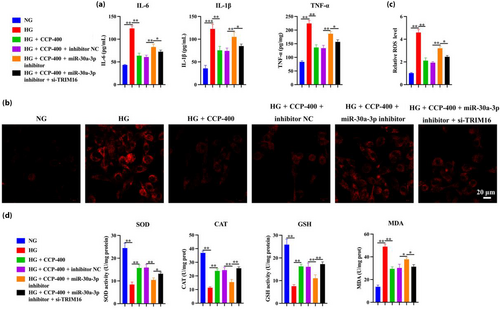
The cells were co-transfected with miR-30a-3p inhibitor, inhibitor NC, and/or si-TRIM16 using Lipofectamine 3000. The interference efficiency was then tested 48 h post-transfection, indicating that si-TRIM16-1 presented the best interference efficiency (Figure 8a). The inhibitor of miR-30a-3p could counteract the effects of CCPs in terms of improving the viability and apoptosis in MPC5 podocytes induced by HG treatment, while TRIM16, a downstream target gene of miR-30a-3p, could reverse the inhibitory effects (Figure 8b–e). Similarly, the miR-30a-3p inhibitor partially counteracted the effect of CCPs on MPC5 EMT podocytes, and further interference with TRIM16 can reverse the effect of the miR-30a-3p inhibitor by measuring the levels of E-cadherin and N-cadherin (Figure 8f). These results indicate that CCPs defend MPC5 podocytes against HG-induced apoptosis and EMT through the regulation of miR-30a-3p/TRIM16 expression. By evaluating the levels of IL-6, IL-1β, TNF-α, ROS, SOD, CAT, GSH, and MDA, suggesting that the miR-30a-3p inhibitor reverses the inflammatory protective effect of CCPs on MPC5 podocytes, while si-TRIM16, in turn, counteracts the effect of the miR-30a-3p inhibitor (Figure 9a–d).
DISCUSSION
At present, the therapeutics for diabetes-related kidney diseases are limited to renin–angiotensin system inhibitors, and patients have a single choice of drugs that must be taken for a long time. Furthermore, these inhibitors can cause side effects such as hyperkalemia, renal impairment, irritable dry cough, and angioneurotic edema in patients29-31. CCP extract from C. cicadae was found to decrease LPS-induced inflammatory cytokine levels and TGF-β1 fibroblast activation to alleviate tubulointerstitial fibrosis in rats with diabetic nephropathy13. This study found that, following CCP treatment, renal function was improved, and concurrent morphological changes were observed in DN mice. In vivo, CCP had protective effects against oxidative, inflammatory damage, apoptosis, and EMT in DN models. In vitro, consistent with the in vivo results, CCP could reduce apoptosis, EMT, inflammation, and oxidative stress induced by HG in MPC5 cells. These results demonstrated that CCPs have therapeutic value in protecting renal function and morphology in mice with diabetic nephropathy and were able to protect MPC5 podocytes from the adverse effects of hyperglycemia.
The miR-30 family members have diverse roles in the pathogenicity of several diseases, particularly hyperglycemia and diabetic nephropathy32-35. The downregulation of miR-30a was found to exacerbate injury to podocytes in focal segmental glomerulosclerosis through increased Notch1/p53 signaling36. A previous study found that miR-30a-3p had a renal effect in DN rat models and patients with diabetic nephropathy37.
In this study, the expression of miR-30a-3p was expressed at low levels in the kidney tissue of mice with diabetic nephropathy and MPC5 podocytes under high glucose conditions, while the addition of CCPs could reverse the decreased expression of miR-30a-3p and led to lower levels of podocyte apoptosis and EMT. Moreover, the levels of E-cadherin, an epithelial marker, were increased by CCPs, whereas levels of N-cadherin were decreased. These results correspond with previous research, and CCPs were also found to improve insulin resistance and glucose tolerance and to subdue tubulointerstitial fibrosis in a DN rat model13.
TRIM16, a target of miR-30a-3p, is believed to regulate EMT-independent metastasis in cancer cells via the polyubiquitination and degradation of vimentin38, 39. A previous study found that the miR-30a-3p inhibitor protected MPC5 podocytes from HG-induced apoptosis and EMT, which contradicts previous results. TRIM16 overexpression was associated with apoptosis through the activation of caspase-240. In this study, the results showed that CCPs could increase the expression of miR-30a-3p, thereby leading to a decrease in the expression of TRIM16. Therefore, the higher expression of TRIM16 may have resulted in increased levels of apoptosis in MPC5 podocytes, independent of EMT.
Oxidative stress and mitochondrial dysfunction are two symptoms that frequently accompany diabetes41. CCPs also alleviated inflammation in a model of high sugar/high fat diet-induced metabolic syndrome in rats by blocking the TLR4/NF-κB and TGF-β1/SMAD signaling pathways42. Furthermore, miR-30a-5p can target GLUD1 under H/R conditions to decrease oxidative stress in vitro43. Similarly, this study demonstrated that CCP could protect MPC5 podocytes from HG-induced inflammation and oxidative stress by controlling miR-30a-3p/TRIM16 expression.
This study has some limitations. First, how CCPs lead to miR-30a-3p changes requires further investigation. Secondly, this study's conclusions concerning both in vivo mice models, as well as in vitro cell lines, were validated; however, further experiments in clinical settings are still needed.
CONCLUSIONS
To conclude, CCPs could obstruct the progress of diabetic nephropathy from apoptosis, EMT, inflammation, and oxidative stress by controlling miR-30a-3p/TRIM16 expression. The results of this study promote the further clinical application of CCPs in the future and can provide new ideas for establishing a cure for diabetic nephropathy.
ACKNOWLEDGMENTS
This study was supported by the National Science Foundation of China (No. 81873245), Senior Talents Program of integrated traditional Chinese and Western Medicine in Shanghai (No. ZY (2018-2020)-RCPY-2002).
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: Research protocol approval has been completed.
Informed consent: N/A.
Registry and the registration no. of the study/trial: Approval date of Registry: 2018-07-02 and 20180702A.
Animal studies: All procedures using animals in this study were performed according to a protocol by the Institutional Animal Care and Use Committee of Longhua Hospital, Shanghai University of Traditional Chinese Medicine, and were approved by the medical ethics committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine (No. 20180702A).




