Current concepts in the management of diabetic polyneuropathy
Abstract
Diabetic sensorimotor polyneuropathy (DSPN) is encountered in approximately one-third of people with diabetes. This, in turn, might markedly impoverish their quality of life, mainly owing to neuropathic pain and foot ulcerations. Painful DSPN might be as frequent as 25% in diabetes patients. Symptoms as a result of DSPN typically comprise pain, paresthesia and numbness in the distal lower limbs. Asymptomatic DSPN might reach 50% among patients with this condition. Unfortunately, DSPN is still not adequately diagnosed and treated. Its management has three priorities: (i) lifestyle improvement, near-normoglycemia and multifactorial cardiovascular risk intervention; (ii) pathogenesis-oriented pharmacotherapy; and (iii) symptomatic alleviation of pain. Intensive diabetes therapy showed evidence for favorable effects on the incidence and deterioration of DSPN in type 1 diabetes, but not type 2 diabetes. Among pathogenesis-oriented treatments, α-lipoic acid, actovegin, benfotiamine and epalrestat are currently authorized to treat DSPN in several countries. Symptomatic therapy uses analgesics, notably antidepressants, opioids and anticonvulsants, reducing pain by ≥50% in approximately 50% of individuals, but might be limited, particularly by central nervous system-related adverse events. Local treatment with the capsaicin 8% patch might offer an alternative. In addition to pain relief, therapy should improve sleep, mobility and quality of life. In conclusion, multimodal treatment of DSPN should consider the individual risk profile, pathogenetic treatment and pain management using pharmacotherapy (combinations, if required), as well as non-pharmacological options.
Epidemiology and Clinical Impact of Diabetic Neuropathy
Diabetic neuropathy represents a condition developing in diabetes patients that cannot be attributed to other causes of peripheral neuropathy1, 2. It manifests in the somatic and/or autonomic parts of the peripheral nervous system1, 2. Diabetic sensorimotor polyneuropathy (DSPN) is the commonest form; it affects approximately 30% of individuals with diabetes and its yearly incidence amounts to approximately 2%3. A recent review reported prevalence rates of DSPN in different South-East Asian countries ranging between 33 and 58% among patients with diabetes4. A retrospective survey of 1,078 patient records and interviews in primary care clinics in Thailand reported a prevalence of DSPN of 34% among diabetes patients5. Data from 1,631 Vietnamese type 2 diabetes patients recorded within the observational DiabCare study show a similar prevalence of DSPN of 38%6. DSPN manifests as a symmetrical, sensorimotor polyneuropathy. It is length-dependent, and results from impaired metabolism and microcirculation after exposure to chronic hyperglycemia and other cardiovascular risk factors7. DSPN is commonly associated with autonomic involvement2, might commence insidiously and if intervention is not successful, it becomes progressive and chronic2. Lower limb long axons appear more amenable to injury2. Impressively, up to 50% of affected patients do not complain of symptoms2. Conversely, 26% of patients with diabetes develop painful DSPN3. The most important underlying factors include inadequate glycemic control, central obesity, diabetes duration, age, hypertension, height, smoking, hypoinsulinemia and an adverse lipid profile7. Accumulating evidence shows that the risk of polyneuropathy is higher in prediabetes8. In the general Augsburg population, the frequency of polyneuropathy was 28.0% among patients with diabetes, 13.0% among those with impaired glucose tolerance and 11.3% among those with impaired fasting glucose, whereas it was 7.4% among those with normal glucose tolerance8.
Measures of DSPN, namely nerve conduction velocity (NCV) and vibration perception threshold (VPT) have been identified as predictors of mortality9, 10. Furthermore, increased VPT heralds future neuropathic foot ulcers11. In regard to cardiovascular morbidity and mortality, DSPN is again decisive. In the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study12, sensory loss and neuropathic pain independently predicted cardiac death and non-fatal myocardial infarction. In a community-based UK study, sensory loss to touch/pressure was a harbinger of cardiovascular morbidity13. In the Action to Control Cardiovascular Risk in Type 2 Diabetes (ACCORD) trial14, DSPN history represented the most important predictor of mortality among individuals with type 2 diabetes who were on a very stringent therapy aiming at glycated hemoglobin <6.0%.
Nevertheless, the importance of DSPN is still not adequately appreciated by physicians and patients alike15. In a German population survey, 77% of participants with DSPN were not aware of this condition, defined as the presence of bilaterally impaired VPT determined by the Rydel Seiffer tuning fork and/or bilaterally impaired pressure sensation assessed by the 10-g monofilament in the feet15. Indeed, they reported that they never had been told of such a diagnosis by their physicians15. In the same way, approximately one out of four patients with diabetes had never had their feet examined15. In a German educational initiative, painful and painless DSPN were previously undiagnosed in 57% and 82% of the participants with type 2 diabetes, respectively16. In that survey, DSPN was defined as abnormal pressure, temperature, and/or vibration sensation in the presence of pain and/or burning (painful DSPN), or paresthesia, numbness or absence of symptoms in the feet (painless DSPN)16. Underdiagnosis and underestimation of DSPN appear to be very common in South-East Asia, as well17. This might be due to a lack of consensus on screening and diagnosis. In most countries, only experts have even simple screening tools, and so many general practitioners do not know how to use them4. According to a survey from Thailand, just 45% of patients with diabetes undergo annual foot examinations, which might explain the high frequency of foot ulcers5, 17. Accordingly, many patients with diabetes are unaware of having DSPN and do not receive adequate professional foot examinations15. The quality of life (QOL) of patients with painful DSPN is clearly affected in many domains. A cross‑sectional survey from Thailand18 showed reduced QOL (especially physical domain) among patients with painful DSPN in comparison with the healthy Thai population. The physical function of patients with painful DSPN was worse than in those with other neurological disorders or in the general population with diabetes18. Impressively, impairment in QOL was similar to the degree found in patients with diabetic foot ulcers18. Hence, effective strategies to improve timely diagnosis of DSPN along with preventive foot care are desirable.
Diagnosis of DSPN
The basic neurological examination assesses sensory (10 g Semmes-Weinstein monofilament for light touch, Tiptherm rod for temperature, calibrated Rydel Seiffer tuning fork for vibration, pin-prick for pain), as well as motor function and tendon reflexes (ankle and knee), also encompassing foot inspection2. It is complemented by general medical and neurological history2. One should be aware of the age-dependent normal range for the tuning fork, starting at ≥5/8 in persons aged 20–29 years, and being reduced for every decade of life to ≥3.5/8 in those aged 70–90 years19. When sensory fibers are affected, “positive” and/or “negative” symptoms develop; the former include burning, stabbing, shooting or lancinating pain, paresthesias, dysesthesias and sensory ataxia (atactic gait); the latter include reduced sensation to temperature, pain, touch and vibration stimuli, and, less frequently, hypersensitivity to tactile (allodynia) and painful stimuli (hyperalgesia)2. Clinical assessment should be standardized using validated examination scores. These quantify DSPN and include the Michigan Neuropathy Screening Instrument20, the Neuropathy Symptom Score21 or Total Symptom Score22 for neuropathic symptoms and the Neuropathy Disability Score for neuropathic signs21.
The Toronto Consensus7 has defined minimal diagnostic criteria as follows: (i) Possible DSPN: symptoms or signs of DSPN; (ii) probable DSPN: a combination of symptoms and signs including two of more of the following: neuropathic symptoms, decreased distal sensation or decreased/absent ankle tendon reflexes; (iii) confirmed DSPN: an abnormality of nerve conduction and a symptom or symptoms or a sign or signs of neuropathy; if nerve conduction is normal, an established attribute measure of small fiber neuropathy might be used; and (4) subclinical DSPN: absence of signs/symptoms with concomitant abnormal NCV or an established attribute of small fiber neuropathy7. The Toronto Consensus recommended using definitions (1), (ii) or (iii) for clinical practice and definitions (iii) or (iv) for research7. Grading of small fiber neuropathy can be as follows: (i) possible: length-dependent symptoms and/or signs of small fiber damage; (ii) probable: length-dependent symptoms, signs of small fiber damage and normal sural nerve conduction; and (iii) definite: length-dependent symptoms, signs of small fiber damage, normal sural nerve conduction and impairments of intra-epidermal nerve fiber density at the ankle and/or abnormal thermal thresholds at the foot7. For patients recently diagnosed with type 2 diabetes from the German Diabetes Study cohort, abnormalities in nerve conduction studies, VPT, thermal thresholds and intra-epidermal nerve fiber density have been described, suggesting a parallel involvement of small and large nerve fibers in the early development of DSPN, highlighting DSPN as an “early” rather than a “late” complication of diabetes23.
Positive neuropathic symptoms appear to differ from deficits in terms of pathophysiology; the former might be linked with nerve fiber regeneration, whereas the latter with their damage24. Consequently, symptom scores are perhaps only suitable to assess pain severity, but not to gauge progression of DSPN24. Pain intensity and its evolution can easily be assessed by an 11-point numerical rating scale (Likert scale) or a visual analog scale24. More elaborated questionnaires, notably PainDetect, Leeds Assessment of Neuropathic Sympoms and Signs (LANSS), Neurophysiology of Pain Questionnaire (NPQ), Douleur Neuropathique 4 Questionnaire (DN4) and Identification Pain Questionnaire (ID-Pain), can quantify neuropathic pain and distinguish it from other pain types24.
Some characteristics point to other causes of peripheral neuropathy and to the need of a detailed investigation. These include: (i) marked asymmetry of neurological deficits; (ii) predominant motor deficits; (iii) mononeuropathy; (iv) cranial nerve involvement; (v) rapid development or progression; (vi) deterioration in spite of optimal glycemic control; (vii) preponderance of symptoms in the upper limbs; (viii) family history of non-diabetic neuropathy; and (ix) failure to document DSPN by clinical examination25.
Differential diagnosis should primarily include neuropathy caused by alcohol abuse, uremia, hypothyroidism, monoclonal gammopathy, vitamin B12deficiency, peripheral arterial disease, cancer, inflammatory and infectious diseases, and neurotoxic drugs25. In practice, full blood count, creatinine, C-reactive protein, thyroid-stimulating hormone, vitamin B12, folic acid, liver enzymes and immunoelectrophoresis are vital for differential diagnosis25. Finally, causes of polyneuropathy might vary between countries, as well as between urban and rural areas4.
Treatment of DSPN and neuropathic Pain
There are three major principles in the management of DSPN: (i) causal treatment including lifestyle modification, intensive glucose control and multifactorial cardiovascular risk intervention; (ii) pathogenesis-oriented therapy; and (iii) symptomatic pain relief.
Causal treatment
Reduction of obesity has been documented to improve nerve function and structure23, 26-29. In type 1 diabetes, the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study has shown that stringent metabolic control to the level of near-normal glucose is successful in delaying and, to some extent, preventing DSPN. Conversely, the efficacy of stringent glucose control in improving the natural history of DSPN is rather limited in type 2 diabetes patients. The Steno-2 trial examined the utility of multiple cardiovascular risk interventions in improving diabetic complications, and found no significant favorable effect on DSPN.
Pathogenesis-derived pharmacotherapy
As confirmed by recent experimental evidence, the pathogenesis of diabetic neuropathy involves several factors25, 26. Figure 1 shows the underlying pathogenetic mechanisms at the cellular level, indicating the room for pathogenesis-oriented treatments. Hyperglycemia and dyslipidemia increase substrates in mitochondria25, 26. This, in turn, leads to their dysfunction with excess production of reactive oxygen species and reactive carbonyls. Both reactive oxygen species and deoxyribonucleic acid damage from carbonyl stress activate poly(adenosine diphosphate-ribose) polymerase-1. As a result, oxidized nicotinamide adenine dinucleotide/adenosine monophosphate is depleted and glyceraldehyde 3-phosphate dehydrogenase is inactivated25, 26. Inactivation of the latter activates the deleterious polyol, hexosamine, protein kinase C and advanced glycation end-products (AGEs) pathways25, 26. Excess production of reactive oxygen species and reactive carbonyls increases endoplasmic reticulum stress. In addition, hyperinsulinemia and inflammation prevent normal insulin signaling. Ultimately, stress and inflammatory pathways become activated, multiple soluble adhesion molecules and genes are abnormally expressed, cytokines/chemokines are upregulated, and apoptosis is promoted26.
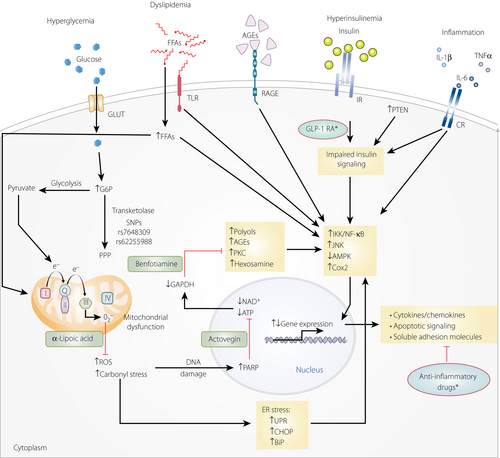
In clinical practice, the aforementioned pathogenic mechanisms suggest corresponding treatment options. Of these, several have been investigated in randomized clinical trials: aldose reductase inhibitors (alrestatin, sorbinil, ponalrestat, tolrestat, epalrestat, zopolrestat, zenarestat, fidarestat, ranirestat); α-lipoic acid and vitamin E (which reduce oxidative stress); ruboxistaurin (which inhibits protein kinase C β); γ-linolenic acid; trandolapril; prostacyclin (PGI2) analogs (iloprost, beraprost); prostaglandin derivatives (PGE1·αCD); nerve growth factor; vascular endothelial growth factor; C-peptide; actovegin (which inhibits poly adenosine diphosphate-ribose polymerase) and benfotiamine (which inhibits AGE accumulation)23, 26, 30. These agents aim at addressing the underlying causal pathways. Importantly, they might be effective even in the setting of hyperglycemia. Another advantage is that they can be combined at low doses with additive effects, at least in experimental models. In humans, only α-lipoic acid31, 32 and benfotiamine33 are licensed for DSPN treatment in many countries, whereas actovegin34 is mainly used in Russia and eastern European countries, and epalrestat is solely marketed in Japan and India.
aldose reductase inhibitors aim to target the activation of the polyol pathway. Several agents of this class have reached phase III clinical trials. However, most were either ineffective or associated with considerable toxicity35. The most recent is ranirestat, which was well tolerated and improved peroneal motor NCV, but was not more efficacious than a placebo at 2 years36. In a 3-year randomized study, epalrestat was again well tolerated, and hindered the deterioration in median motor NCV, minimum F-wave latency and VPT seen in an untreated group with DSPN37. According to subgroup analysis, it was more efficacious in individuals with adequate metabolic control and less severe diabetic complications38. A meta-analysis from China reported favorable effects of epalrestat plus α-lipoic acid combination on DSPN measures, but the sample size of the studies included was small, and their methodological quality was poor39. Another meta-analysis concluded that aldose reductase inhibitors could primarily improve asymptomatic/mild cardiovascular autonomic neuropathy, but this needs to be verified40.
The results of the randomized controlled clinical trials of the anti-oxidant α-lipoic acid for the treatment of DSPN and the corresponding meta-analyses are summarized in Table 1. Collectively, these trials show that intravenous α-lipoic acid (600 mg/day) improved neuropathic symptoms and signs after 3 weeks. Furthermore, oral treatment for 5 weeks (600 mg/day) ameliorated pain, paresthesia and numbness at a clinically important level22, 48. In the Neurological Assessment of Thioctic Acid in Diabetic Neuropathy ( NATHAN ) 1 trial, including 460 participants with diabetes and mild-to-moderate largely asymptomatic DSPN, neuropathic deficits were improved with α-lipoic acid after 4 years32, 41, 47. This trial showed the potential of α-lipoic acid to improve the natural history of DSPN. Throughout the 4-year period studied, the drug was well tolerated32, 41, 47. Postmarketing surveillance reports have confirmed the highly favorable safety of α-lipoic acid31. By contrast, other anti-oxidants, such as vitamin E (mixed tocotrienols), failed to reduce neuropathic symptoms at 1 year49.
| Study (ref.) | n T1D/T2D | Daily dose (mg) | Duration | Effects | Adverse events |
|---|---|---|---|---|---|
| ALADIN22 | 0/328 | 100/600/1,200/placebo | 3 weeks i.v. |
TSS+ NDS+ HPAL+ |
None |
| ALADIN II31 | 65† | 600/1,200/placebo | 2 years p.o. |
Sural SNCV+ Sural SNAP+ Tibial MNCV+ |
None |
| ALADIN III31 | 0/508 | 600 i.v./1,800 p.o./placebo | 3 weeks i.v./6 months p.o. |
TSS(+)/– NIS+/(+) NIS-LL(+)/(+) |
None |
| DEKAN31 | 0/73 | 800/placebo | 4 months p.o. | HRV+ | None |
| ORPIL31 | 0/24 | 1,800/placebo | 3 weeks p.o. |
TSS+ HPAL(+) NDS+ |
None |
| SYDNEY31 | 30/90 | 600/placebo | 3 weeks i.v. |
TSS+ NSC+ NIS+ |
None |
| SYDNEY 231 | 30/151 | 600/1,200/1,800/placebo | 5 weeks p.o. |
TSS+ NSC+ NIS+ |
Dose-dependent |
| NATHAN 132 | 110/344 | 600/placebo | 4 years p.o. |
NIS+, NIS–LL+ NSC+ NCV– |
SAEs increased vs. placebo |
| Mansoura48 | 0/200 | 1,200/placebo | 6 months |
NSS+ NDS+ VPT+ |
Mild nausea |
| Meta-analysis41 | 103/1,155 | 600/placebo | 3 weeks i.v. |
TSS+ NIS+ NIS-LL+ |
None |
| Meta-analysis42 | 60/1,100 |
Trials: ALADIN, ALADIN III, SYDNEY SYDNEY 2, ORPIL |
See individual trials above | See individual trials above | See individual trials above |
| Meta-analysis43 | 60/593 | Trials: ALADIN, SYDNEY, SYDNEY 2, ORPIL, | |||
| Meta-analysis44 | 60/593 | Trials: ALADIN, SYDNEY, SYDNEY 2, ORPIL | |||
| Meta-analysis45 | 170/937 | Trials: ALADIN, SYDNEY, SYDNEY 2, ORPIL, NATHAN 1 | |||
| Meta-analysis46 | 170/585 | Trials: SYDNEY, SYDNEY 2, NATHAN 1 | |||
| Meta-analysis47 | 170/1,445 |
Trials: ALADIN, ALADIN III, SYDNEY SYDNEY 2, ORPIL, NATHAN 1 |
- †Diabetes type not available.
- +, Improvement compared with placebo; (+), trend towards improvement compared with placebo; –, no difference compared with placebo; ALADIN, Alpha-Lipoic Acid in Diabetic Neuropathy; DEKAN, Deutsche Kardiale Autonome Neuropathie; DML, distal motor latency; HPAL, Hamburg Pain-Adjective List; HRV, heart rate variability; i.v., intravenous administration; MNCV, motor nerve conduction velocity; NATHAN, Neurological Assessmentt of Thioctic Acid in Diabetic Neuropathy; NDS, Neuropathy Disability Score; NIS-LL, Neuropathy Impairment Score - lower limbs; NSC, Neuropathy Symptoms and Changes; ORPIL, Oral Pilot; p.o., oral administration; SAEs, severe adverse events; SNAP, sensory nerve action potential; SNCV, sensory nerve conduction velocity; SYDNEY, Symptomatic Diabetic Neuropathy; T1D, type 1 diabetes; T2D, type 2 diabetes; TSS, Total Symptom Score; VPT, vibration perception threshold.
Thiamine (vitamin B1) belongs to the water-soluble vitamins. It is an essential cofactor of several enzymes implicated in carbohydrate metabolism50. Benfotiamine (S-benzoylthiamine O-monophosphate) is a lipid-soluble synthetic S-acyl prodrug of thiamine. It has passive absorption, goes faster through the intestinal barrier faster, and accomplishes higher concentrations in plasma, blood and erythrocytes50. Based on experimental evidence, benfotiamine activates transketolase, shifting hexose and triose phosphates to the pentose phosphate pathway, and inhibiting AGEs inhibitor, thereby improving chronic diabetic complications51. In both diabetes types, thiamine levels are frequently low and thiamine clearance is high52, 53. In patients with diabetes and DSPN, the efficacy and safety of benfotiamine have been investigated in four randomized, double-blind clinical trials54-57. Their duration was 3–12 weeks. Various daily dosages and end-points were studied (Table 2)54-57. The Benfotiamine in Diabetic Polyneuropathy (BENDIP) trial showed a significant improvement of neuropathic symptoms at 6 weeks with a dosing scheme of 300 mg twice daily, but not 300 mg/day54. The Benfotiamine in Diabetic Polyneuropathy (BEDIP) trial reported an improvement in neuropathic symptoms and signs at 3 weeks with the dose of 100 mg four times per day55. Similar efficacy emerged from another trial using a combination of benfotiamine, pyridoxine and cyanocobalamine57. With this therapeutic combination, peroneal motor nerve conduction velocity, but not VPT were improved at 12 weeks56. In all these trials, the safety of benfotiamine was excellent. Taken together, these trials point to the efficacy of benfotiamine in terms of neuropathic symptoms, and, possibly, neuropathic deficits and nerve conduction, as well as to its safety. The optimal dose appears to be 300 mg twice daily.
| References | Compound n/dose (mg) | Duration (weeks) | Primary endpoint | Secondary endpoints | Efficacy: primary end-point | Efficacy: secondary end-points | Adverse events |
|---|---|---|---|---|---|---|---|
| Stracke et al., 2008; BENDIP study54 |
B: 47/300 b.i.d. B: 45/300 q.d. P: 41 |
6 | NSS |
TSS NDS VPT |
B300 b.i.d.: NSS: PP+ ITT(+) |
TSS↔ NDS↔ VPT↔ |
↔ |
| Haupt et al., 2005; BEDIP study55 |
B: 20/100 q.i.d. P: 20 |
3 | Score: Muscle strength, pain, sensory function, coordination, reflexes |
Pain VPT PGIC |
Score+ |
Pain+ PGIC(+) |
↔ |
| Stracke et al., 199656 |
B: 80†-40 q.i.d.+ B6: 180†-90 q.i.d.+ B12: 11/0.5†-0.25 q.i.d. P: 13 |
12 |
MNCV VPT |
Peroneal MNCV+ Median MNCV↔ VPT↔ |
↔ | ||
| Ledermann & Wiedey, 198957 |
B: 80 q.i.d.+ B6: 180 q.i.d.+ B12: 10/0.5 q.i.d. P: 10 |
3 |
Score: Muscle strength, pain, sensory function, coordination, reflexes; VPT, pin-prick, pain |
Score+ Pain+ Pin-prick+ VPT+ |
↔ | ||
- †Dose during the first 2 weeks of study.
- +, Improvement; (+), borderline improvement (P < 0.06); ↔, no difference between active and placebo treatment; B, benfotiamine; B6, vitamin B6; B12, vitamin B12; b.i.d., twice daily; ITT, intention to treat MNCV, motor nerve conduction velocity; NSS, Neuropathy Symptom Score; NDS, Neuropathy Disability Score; P, placebo; PGIC, patient global impression of change; PP, per protocol; q.d., once daily; q.i.d., four times daily; TSS, Total Symptom Score; VPT, vibration perception threshold.
In patients with type 1 diabetes, the therapeutic combination of benfotiamine (300 mg twice daily) plus α-lipoic acid (600 mg twice daily) achieved improvements in biochemical markers of microvascular damage at 4 weeks58. Specifically, it normalized AGEs, it reduced hexosamine-modified proteins in the monocytes and it normalized prostacyclin synthase activity58. These findings suggest that humans share the same underlying pathways of DSPN with rodents58.
A randomized double-blind trial (BOND [EudraCT Number: 2017-003054-16; https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-003054-16/DE]; a randomized double-blind, placebo-controlled parallel group study over a period of 12 months to assess the effects of treatment with benfotiamine on morphometric, neurophysiological and clinical measures in type 2 diabetes patients with mild-to-moderate symptomatic DSPN) will examine the efficacy of benfotiamine on nerve conduction, as well as clinical and morphological parameters in individuals with type 2 diabetes patients and mild-to-moderate symptomatic DSPN. The follow-up period will be 1 year. Finally, in recent-onset diabetes, several transketolase single-nucleotide polymorphisms might be linked with DSPN59. Therefore, targeting thiamine and transketolase, as well as exploiting the role of genetic transketolase variations, holds substantial promise60.
Actovegin is an ultrafiltrate of calf blood, from which proteins have been removed. It contains >200 active substances34. A trial included 567 participants with symptomatic DSPN from several centers. The participants randomly received 20 daily intravenous actovegin administrations (2,000 mg/day) followed by three actovegin tablets daily (1,800 mg/day) or a placebo for 140 days. Efficacy in neuropathic symptoms and VPT with safety similar to the placebo were shown34.
Altogether, given the paucity of long-term clinical trials and thus limited evidence for effective treatments, no major breakthrough in slowing the progression of diabetic neuropathy could be achieved with drugs derived from its pathogenetic concepts. Thus, there is a continuing need for long-term efficacy data with the aforementioned pharmacotherapies and the development of novel drugs tailored to target the pathogenetic mechanisms underlying DSPN.
Symptomatic treatment of painful DSPN
- In every patient, the optimal agent needs to be searched and carefully uptitrated.
- Only after 2–4 treatment weeks with an adequate dose can efficacy or failure be concluded.
- Combinations of analgesic agents might be useful, especially given that a clinically meaningful response to monotherapy amounts to approximately 50%.
- Vigilance for drug interactions is necessary.
A comprehensive evidence-based therapeutic algorithm is presented in Figure 2. Table 3 summarizes the pros and cons of various agents in terms of comorbidities, diabetic complications and drug interactions.

| Duloxetine | Pregabalin/gabapentin | Tricyclic antidepressants | Opioids | Capsaicin 8% patch | α-Lipoic acid/benfotiamine | |
|---|---|---|---|---|---|---|
| Depression | +† | ±† | + | ± | ± | ± |
| Generalized anxiety disorder | + | + | + | + | ± | ± |
| Sleep disturbances | + | + | + | + | + | ± |
| Autonomic neuropathy | (↓) | + | ↓‡ | ↓§ | ± | +¶ |
| Obesity | ± | ↓ | ↓ | ± | ± | ± |
| Coronary heart disease | ± | ± | ↓ | ± | ± | ± |
| Fasting glucose | (↓) | ± | (↓) | ± | ± | (+)¶ |
| Hepatic failure | ↓ | ± | Adapt dose†† | Adapt dose†† | ± | ± |
| Renal failure | ↓ | Adapt dose | Adapt dose†† | Adapt dose†† | ± | ± |
| Drug interactions | ↓ | ± | ↓ | ± | ± | ± |
| Pathogenesis-derived therapy | No | No | No | No | No | Yes |
- †Additional anxiolytic effect in generalized anxiety disorder. ‡Caveat: anticholinergic side effects might aggravate impaired bladder voiding or cardiovascular autonomic neuropathy. §Caveat: slowing of gastrointestinal passage might aggravate gastrointestinal neuropathy. ¶Applies only to α-lipoic acid.††Dependent on individual agent.
- +, Favorable effects; ↓, unfavorable effects; ±, no relevant effects.
The Special Interest Group on Neuropathic Pain (NeuPSIG) has updated its guidelines on pain management61. According to these, tricyclic antidepressants, serotonin-noradrenaline reuptake inhibitors, pregabalin and gabapentin are strongly recommended as first-line agents61. Lidocaine patches, capsaicin high-concentration patches and tramadol are weakly recommended as second-line treatments. Strong opioids and botulinum toxin A are weakly recommended as third-line treatments61. Conversely, a recent systematic review concluded that the strength of evidence is moderate for duloxetine and venlafaxine, and is moderate and low for tricyclic antidepressants and pregabalin/oxcarbazepine. For example, eight out of 15 pregabalin trials in painful DSPN did not find significantly more pain reduction compared with placebo, and gabapentin was rated as ineffective63. Likewise, in the Comparative Effectiveness Review Number 187 prepared for the Agency for Healthcare Research and Quality (USA), only serotonin-noradrenaline reuptake inhibitors had moderately strong evidence, whereas the efficacy of pregabalin and oxcarbazepine, atypical opioids, botulinum toxin, and α-lipoic acid was assigned a low strength of evidence47.
An interesting alternative is topical analgesic therapy. Its advantages include fewer untoward effects and drug interactions. Capsaicin is a highly selective agonist of transient receptor potential vanilloid-1. It has been approved as an 8% dermal patch64. However, it should not be used in active skin lesions or in areas with severe sensory loss64. Lower concentrations (0.025–0.075%) in gel, lotions or creams were not consistently superior to placebo65-68. Lidocaine 5% patch is being used for postherpetic neuralgia69, but has not been authorized for the treatment of painful DSPN60-62.
Overall, just 50% of patients with painful DSPN respond to single-agent analgesic therapy61, 62. Hence, patients with inadequate response and/or those who cannot tolerate higher doses will benefit from multiple-agent pharmacotherapy. This approach might target several pathogenic mechanisms and achieve superior pain relief with fewer adverse effects61, 62. There is evidence that combination therapy is superior to monotherapy, but this finding was not consistent in all trials70, 71.
Summary of therapy
Management of chronic painful DSPN is still challenging. Incomplete evidence on long-term efficacy and combination therapies, poor experience with head-to-head comparisons, and intolerability of some drugs by some patients are important drawbacks70, 72. It looks attractive to combine symptomatic and pathogenesis-oriented agents, but again little is known73.
Granted that pharmacotherapy is still suboptimal, non-pharmacological treatments have also been used, despite their low level of evidence45. These include psychological support, acupuncture, physical therapy and transcutaneous electrical nerve or muscle stimulation45. In more demanding situations, invasive spinal cord stimulation significantly mitigates pain and improves QOL74.
Conclusions
DSPN still remains poorly diagnosed and treated. Hence, effective strategies to improve these deficiencies, along with adequate foot care, need to be pursued. Multimodal treatment of DSPN should consider the individual risk profile, pathogenesis-derived treatment and pain management using pharmacotherapy (combinations, if required), as well as non-pharmacological options. The evidence for interventions in neuropathic pain, as derived from systematic reviews on which recommendations are based, is often inconclusive75. Therefore, therapeutic algorithms need to be harmonized and constantly updated to foster suitable and efficacious treatments in everyday practice.
The increasing burden of diabetes constitutes important public health challenges both at regional and global levels. European countries, for example, made variable progress toward investing in and implementing of comprehensive strategies for the prevention and treatment of diabetes. The key challenge for the future will be to ensure that national diabetes plans can be monitored and evaluated by increasing the capacity of information systems to allow for adequate assessment of the health outcomes of such interventions76. To reduce the burden resulting from DSPN and its sequela, adequate consideration and implementation of strategies aimed at early detection and prevention of the condition in national diabetes plans is imperative.
Disclosure
Dan Ziegler is a member of advisory boards of the companies Wörwag Pharma, Teva and Astellas; owns shares/equity of the companies Bayer and Pfizer; has received remunerations/fees for activities on behalf of the companies Wörwag Pharma, Meda, AstraZeneca, Mitsubishi Tanabe, Takeda, Pfizer, Lilly, Trigocare, Allergan, Novartis, Biogen, Berlin-Chemie, Novaremed, Mundipharma and Astellas; and has received third-party funds/project grants from Wörwag Pharma. Nikolaos Papanas has been an advisory board member of AstraZeneca, Boehringer Ingelheim, MSD, Novo Nordisk, Pfizer, Takeda and TrigoCare International; has participated in sponsored studies by AstraZeneca, Eli Lilly, GSK, MSD, Novo Nordisk, Novartis and Sanofi-Aventis; has received honoraria as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elpen, MSD, Mylan, Novo Nordisk, Pfizer, Sanofi-Aventis and Vianex; and attended conferences sponsored by TrigoCare International, Eli-Lilly, Galenica, Novo Nordisk, Pfizer and Sanofi-Aventis. Oliver Schnell is a member of advisory boards of Wörwag Pharma. Bich Dao Thi Nguyen is a member of advisory boards of Wörwag Pharma. Khue Thy Nguyen is a member of advisory boards of the companies Wörwag Pharma, Boehringer Ingelheim, Novo Nordisk, AstraZeneca and Abbott; and has received remunerations/fees for activities on behalf of the companies Boehringer Ingelheim, Tosoh, MSD, Servier, Urgo and Sanofi-Aventis. Kongkiat Kulkantrakorn is a member of advisory boards of Wörwag Pharma. Chaicharn Deerochanawong is a member of advisory boards of Wörwag Pharma.




