Phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of teneligliptin monotherapy in Chinese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise
Clinical Trial Registry
ClinicalTrials.gov
NCT02916706
Abstract
Aims/Introduction
Although the efficacy of teneligliptin, a highly selective dipeptidyl peptidase-4 inhibitor, has been amply studied for the treatment of type 2 diabetes, no clinical trials of teneligliptin have been carried out in China. We evaluated the efficacy and safety of teneligliptin monotherapy compared with a placebo in Chinese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise.
Materials and Methods
This multicenter, randomized, double-blind, placebo-controlled, parallel-group study, carried out at 42 sites, enrolled type 2 diabetes patients with glycosylated hemoglobin 7.0 to <10.0% and fasting blood glucose <270 mg/dL. Patients were randomly assigned, in a 1:1 ratio, to treatment with 20 mg teneligliptin or a placebo (n = 127, each) administered orally once daily before breakfast for 24 weeks. Change in glycosylated hemoglobin from baseline to week 24 was the primary efficacy end-point. Safety was assessed by the incidence of adverse events and adverse drug reactions.
Results
The least square mean (LSM) change in glycosylated hemoglobin from baseline to week 24 was −0.95% with teneligliptin versus −0.14% with a placebo, yielding an LSM difference (teneligliptin vs placebo) of −0.80% (P < 0.0001). For the secondary end-point, from baseline to week 24, the LSM change in fasting blood glucose was −21.9 mg/dL with teneligliptin versus −1.4 mg/dL with a placebo, yielding an LSM difference (teneligliptin vs placebo) of −20.5 mg/dL (P < 0.0001). The adverse event and adverse drug reaction incidence rates, including hypoglycemia, were similar in both groups.
Conclusions
At 24 weeks, teneligliptin was generally well tolerated and effective in Chinese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise.
Introduction
In China, the prevalence of diabetes has increased markedly over the past three decades1. A recent meta-analysis of Chinese inland residents with type 2 diabetes mellitus reported an overall prevalence of 9.1%2, which translates into >90 million adults with type 2 diabetes3. Alarmingly, the prevalence was as high as 14.1% among those aged 65–74 years, 11.6% among women and 11.4% in urban areas1.
Blood glucose levels gradually increase with the progression of the disease, leading to a plethora of both microvascular and macrovascular complications4, 5 that can significantly affect overall health and quality of life. Therefore, treatment intensification is required to control hyperglycemia. Current treatment recommendations for type 2 diabetes inadequately controlled with lifestyle changes, such as diet and exercise6, suggest initial interventions comprising the addition of therapy with hypoglycemic agents, of which metformin remains the first choice. Intensification strategies with several drugs, such as dual combination therapy (i.e., addition of insulin secretagogues, alpha-glucosidase inhibitors, dipeptidyl peptidase [DPP]-4 inhibitors, thiazolidinedione, sodium–glucose cotransporter 2 inhibitors, insulin or glucagon-like peptide-1 receptor agonists), triple-combination therapy (i.e., combined use of three drugs with distinct mechanisms of action) or the addition of insulin (e.g., basal insulin + mealtime insulin, or multiple daily administrations of premixed insulin) can be used as required7. Nevertheless, only approximately half of patients with diabetes achieve glycemic control with these intensification schemes8. Treatment failure is attributed to poor medication adherence9, adverse drug reactions (ADRs; e.g., weight gain and hypoglycemia) or attenuated efficacy after prolonged use10.
For patients for whom metformin therapy is not suitable, alternatives are alpha-glucosidase inhibitors, insulin secretagogues, DPP-4 inhibitors, thiazolidinedione, sodium–glucose cotransporter 2 inhibitors, insulin or glucagon-like peptide-1 receptor agonists. Among these, DPP-4 inhibitors have been associated with a low risk of hypoglycemia and a neutral effect on bodyweight11; thus, it might have several advantages over other agents as a monotherapy. Furthermore, DPP-4 inhibitors have been widely used in east Asia, as some reports have shown that DPP-4 inhibitors exert greater glycosylated hemoglobin (HbA1c)-lowering effects in this population than in non-Asians12, 13. Teneligliptin is a J-shaped DPP-4 inhibitor with an anchor lock domain14. This structure sets teneligliptin apart from other DPP-4 inhibitors, as it strengthens DPP-4 enzyme binding and results in a long-lasting inhibitory effect that provides stable glycemic levels throughout the day15.
In previous studies carried out in Japan, teneligliptin was well tolerated, and resulted in significant reductions of HbA1c and fasting plasma glucose (FPG) alone16 or in combination with other drugs17, 18. Teneligliptin was reported to be safe among patients with renal impairment19, which is important as diabetes predisposes patients to a higher risk of developing renal complications. It can also be used safely in patients with hepatic impairment20. Additionally, pleiotropic effects of teneligliptin on vascular function, lipids and, potentially, obesity might result in added benefits to obese diabetes patients and patients with a high risk of vascular diabetic complications15.
Teneligliptin, which was developed in Japan, is currently available, and indicated for treating type 2 diabetes in Japan, Korea, India and Argentina21, 22. As no clinical trials of teneligliptin have been carried out in China, we evaluated the efficacy and safety of teneligliptin monotherapy, compared with a placebo, in Chinese patients with type 2 diabetes mellitus that is inadequately controlled with diet and exercise.
Methods
Study design, setting, randomization and blinding
This was a multicenter, randomized, placebo-controlled, double-blind, parallel-group study (registered at Clinicaltrials.gov: NCT02916706) carried out at 42 sites in China. The study lasted 30 weeks, including a screening period (2 weeks), a placebo run-in period (2 weeks), a treatment period (24 weeks) and a follow-up period (2 weeks; Figure S1). Patients with ≥75% treatment compliance with the investigational product (placebo) during the placebo run-in period were randomly assigned to either 20 mg teneligliptin or placebo once daily administration.
Patients were randomly assigned to treatment in a 1:1 ratio according to a computer-generated randomization code, which was managed through static blocked randomization, and an Interactive Web Randomization System was used. Patients, investigators, laboratory personnel and the sponsor were blinded to treatment.
Patients could be discontinued from the study at any time and were also free to discontinue their participation in the study at any time without prejudice to further treatment. Patients lacking glycemic control during double-blind treatment were discontinued from the study and referred to appropriate antihyperglycemic therapy.
Participants
The main inclusion criteria were the following: patients with a documented type 2 diabetes mellitus diagnosis for ≥3 months, age ≥18 years, an HbA1c value ranging from ≥7.0% to <10.0%, an FPG <270 mg/dL (15 mmol/L) at the screening visit (day −28) and on day −14, who were under a diet and exercise therapy regimen, and whose diet and exercise regimen at the time of the screening visit had remained unchanged for ≥8 consecutive weeks.
The following exclusion criteria applied: history of type 1 diabetes; previous insulin treatment within 1 year before the screening visit; treatment with any prohibited concomitant medication within 8 weeks before screening; history of cardiovascular, renal, hepatic or neurological diseases; history of severe diabetic complications; history of malignancy; history of drug abuse and/or alcoholism; women or men of childbearing potential unwilling to use appropriate contraception methods; pregnant or lactating women, or those planning to become pregnant; <75% treatment compliance with the investigational product (placebo) during the run-in period; and history of joint pain with DPP-4 inhibitors. All participants gave informed consent, and patient anonymity was preserved.
Interventions
The treatment intervention in the present study consisted of the oral administration of 20 mg teneligliptin (Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) or a placebo (formulated and packaged identically to the active drug), once daily before breakfast for the treatment period (24 weeks).
Patients were undertaking diet and exercise therapy, which had been unchanged for at least eight consecutive weeks at the day −28 screening visit. Patient HbA1c levels ranged from 7.0% to 10.0% at the screening visit and on day −14, and FPG was <270 mg/dL (15 mmol/L) at the screening visit and on day −14.
Prohibited concomitant medications were insulin, sulfonylureas, biguanides, alpha-glucosidase inhibitors, glinides, thiazolidinediones, glucagon-like peptide-1 receptor agonists, DPP-4 inhibitors, herbal medicines that lower blood glucose levels, new drugs intended for diabetes, fixed-dose combination tablets including the aforementioned active ingredients and adrenocorticosteroids (excluding for external use). Medications not mentioned here could be used concomitantly for the treatment of complications and adverse events (AEs). In principle, medications already in use at the screening visit were used until 2 weeks after the final study drug dose without any change to the prescription. No dose adjustments were planned.
Outcomes
Changes from baseline to week 24 in HbA1c and FPG levels were the primary and secondary efficacy end-points, respectively. Other end-points were the proportion of patients who achieved HbA1c <7.0% at week 24; changes from baseline to week 12 in HbA1c and FPG; and changes from baseline to week 24 in fasting insulin, glucagon, C-peptide, bodyweight and homeostatic model assessment-insulin resistance (HOMA-IR) and β-cell function (HOMA-β).
Safety end-points were AEs, as classified using the Medical Dictionary for Regulatory Activities version 21.0; ADRs; treatment-emergent AEs; cardiovascular events; hypoglycemic episodes; vital sign measurements (pulse rate, blood pressure and body temperature); laboratory measurements; and 12-lead electrocardiogram. Definitions of hypoglycemia and details of the methods used for the measurement of glucagon are provided in Appendix S1.
Statistical analysis
Sample size
A sample size of 240 patients was planned, with 120 patients to be randomly assigned to each treatment group. Further details are provided in Appendix S1.
Analysis
The full analysis set (FAS) and the safety analysis set are defined in Appendix S1. Efficacy and safety analyses used the FAS and safety analysis set, respectively.
All statistical tests were two-sided, and the significance level set to 5%. The 95% confidence intervals (CI) were calculated for each treatment effect and difference between groups. Descriptive statistics (number of non-missing values [n], mean, standard deviation, median, minimum and maximum) were used for continuous variables, with frequency counts and percentages used for discrete variables.
Analysis of covariance (ancova) was used to analyze the primary, secondary and other efficacy end-points, with treatment as the fixed effect and baseline as a covariate. Using the last observation carried forward (LOCF) method, missing values at week 12 or 24 were imputed, before analysis. Additionally, the robustness of the primary analysis results was assessed using the mixed effects model for repeated measures, with treatment, visit, and interaction of treatment and visit as fixed effects, and baseline as a covariate. For the patients who achieved HbA1c <7.0% by week 24, a logistic regression analysis was carried out with treatment as a fixed effect, and baseline HbA1c value as a covariate. Subgroup analyses by baseline characteristics were carried out for change from baseline to week 24 in HbA1c levels. Multiplicity due to multiple testing was not adjusted within and between the primary, secondary, and other efficacy end-points. SAS Version 9.2 or higher (SAS Institute, Cary, NC, USA) was used for the analyses.
Ethical considerations
The research protocol and all other appropriate documents were reviewed and approved by an independent ethics committee and regulatory authorities. The study was carried out in accordance with the 1964 Declaration of Helsinki (2013 Fortaleza revision), Good Clinical Practice (as required by the International Council on Harmonisation guidelines), and regional and local legislation.
Results
Participants
Of the total of 397 patients who provided informed consent, 254 patients were randomly assigned (teneligliptin and placebo groups, n = 127 each), whereas 143 patients were not eligible for randomization (Figure 1). Of the 254 patients randomized, 208 (81.9%) completed the study. In the teneligliptin group, the main reasons for premature study discontinuation were patient withdrawal (4.7%), other (3.9%) and protocol violation (2.4%); in the placebo group, the main reasons were other (15.0%) and patient withdrawal (7.1%). Of 19 (15.0%) patients who discontinued the placebo arm for “other reasons,” 16 patients discontinued because of hyperglycemia (increase of FPG), and the other three patients discontinued for the following reasons: one patient used another antihyperglycemic treatment; one patient used insulin, a prohibited concomitant medication, during the occurrence of a serious AE (SAE); and one patient felt the effect of the study drug was not ideal.
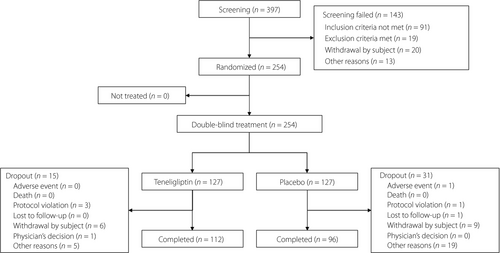
Baseline data
In the FAS, there were 125 (98.4%) patients in the teneligliptin group and 126 (99.2%) in the placebo group. The mean ± standard deviation HbA1c level was 7.89 ± 0.80% and 8.04 ± 0.71% in the teneligliptin and placebo groups, respectively. The mean ± standard deviation of the duration of diabetes was 2.66 ± 2.58 and 2.86 ± 3.36 years in the teneligliptin and placebo groups, respectively (Table 1). Both treatment groups were generally comparable.
| Teneligliptin (n = 125) | Placebo (n = 126) | |
|---|---|---|
| Mean age, years (SD) | 52.1 (10.2) | 56.0 (10.2) |
| Median (range) | 52.0 (28, 77) | 57.0 (29, 77) |
| Sex, n (%) | ||
| Male | 85 (68.0) | 79 (62.7) |
| Alcohol consumption, n (%) | ||
| Abstainer | 89 (71.2) | 95 (75.4) |
| ≤28 units alcohol/week | 36 (28.8) | 31 (24.6) |
| >28 units alcohol/week | 0 | 0 |
| Mean height, cm (SD) | 166.7 (7.7) | 164.4 (7.9) |
| Median (range) | 167.0 (146, 189) | 165.0 (147, 181) |
| Mean weight, kg (SD) | 71.51 (12.10) | 68.70 (10.94) |
| Median (range) | 72.00 (43.0, 115.0) | 69.35 (41.3, 109.7) |
| Mean body mass index, kg/m2 (SD) | 25.64 (3.43) | 25.33 (3.00) |
| Mean HbA1c, % (SD) | 7.89 (0.80) | 8.04 (0.71) |
| Mean duration of diabetes, years† (SD) | 2.66 (2.58) | 2.86 (3.36) |
- Percentages in the table are calculated based on the number of patients in each treatment group.
- SD, standard deviation.
- † Duration of diabetes (years) = (year of screening visit − year of diagnosis) + (month of screening visit − month of diagnosis) / 12.
Treatment compliance (measured by tablet count) was good. The median compliance rate was 100% in both groups, and ≥96.8% of patients in the FAS in both groups were >75% compliant.
Outcomes
Primary efficacy end-point
The mean changes from baseline to week 24 in HbA1c in the teneligliptin and placebo groups are shown in Figure 2. The least square mean (LSM) ± standard error (SE) change from baseline to week 24 in HbA1c was −0.95 ± 0.06% (95% CI −1.07, −0.82) in the teneligliptin group and −0.14 ± 0.06% (95% CI −0.27, −0.02) in the placebo group. The difference (LSM ± SE) between the placebo group and the teneligliptin group was −0.80 ± 0.09% (95% CI −0.98, −0.63). Statistically significant differences, in favor of teneligliptin, were observed at week 24 (ancova, P < 0.0001; Table S1) and in the sensitivity analysis (mixed effects model for repeated measures, P < 0.0001).
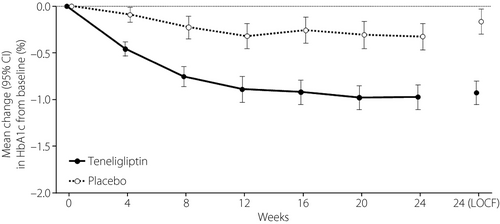
Secondary efficacy end-point
The changes from baseline to week 24 in FPG in the teneligliptin and placebo groups are shown in Figure 3. The LSM ± SE change in FPG was −21.9 ± 2.5 mg/dL (95% CI −26.8, −17.0) in the teneligliptin group and −1.4 ± 2.5 mg/dL (95% CI: −6.3, 3.5) in the placebo group. The difference (LSM ± SE) between the placebo and teneligliptin groups was −20.5 ± 3.5 mg/dL (95% CI −27.4, −13.5). At week 24, a significant difference in FPG, in favor of teneligliptin, was observed (teneligliptin vs placebo, ancova, P < 0.0001; Table S1).
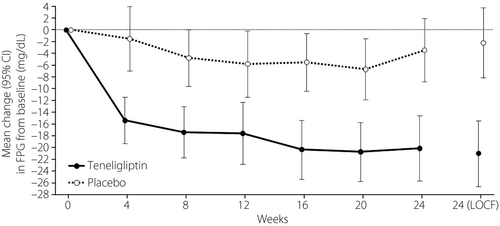
Other efficacy end-points
The number of patients with HbA1c ≥7.0% at baseline was 112 of 125 (89.6%) in the teneligliptin group, and 120 of 126 (95.2%) in the placebo group. The proportions of patients who achieved HbA1c levels <7.0% at week 24 were 50.0% (56/112) and 13.3% (16/120) in the teneligliptin and placebo groups, respectively. The odds ratio of HbA1c levels <7.0% at week 24 for teneligliptin was 7.96 (95% CI 3.79, 16.73; P < 0.0001).
Table S1 summarizes the results of efficacy end-points in the placebo versus teneligliptin groups. Mean changes from baseline to week 12 in HbA1c and FPG were −0.26% and −0.83%, and −7.2 mg/dL and −17.9 mg/dL in the placebo and teneligliptin groups, respectively. The difference (LSM ± SE) of the change from baseline to week 24 in HOMA-β between the groups was 6.78 ± 1.86, which was statistically significant in favor of teneligliptin (ancova, P = 0.0003). There were no significant differences in changes from baseline to week 24 in fasting insulin (P = 0.6619), fasting C-peptide level (P = 0.6737), glucagon level (P = 0.0929), HOMA-IR (P = 0.0917) or bodyweight (P = 0.4192) between the teneligliptin and placebo groups.
The results of the subgroup analyses for changes from baseline to week 24 in HbA1c, stratified by baseline characteristics, showed statistically significant differences (P < 0.0001 to <0.01) favoring teneligliptin in both sexes, all age ranges evaluated (<40 to ≥70 years) and body mass index ≥20 kg/m2; for abstainers and those consuming ≤28 units of alcohol per week, HbA1c ≥7 to <9%, all FPG levels, and for those with disease durations of <1 year up to <10 years (Table S2).
Safety
The safety analysis set comprised 127 patients in the teneligliptin group and 127 patients in the placebo group. During the treatment period, 97 patients (76.4%) and 91 patients (71.7%) in the teneligliptin and placebo groups, respectively, had AEs (Table 2). The most frequently reported AEs in the teneligliptin and placebo groups, respectively, were infections and infestations (29.9% and 22.8%), comprising upper respiratory tract infection (23.6% and 15.7%), and metabolism and nutrition disorders (27.6% and 21.3%).
|
Teneligliptin (n = 127) n (%) |
Placebo (n = 127) n (%) |
|
|---|---|---|
| TEAE | 97 (76.4) | 91 (71.7) |
| Severe TEAE† | 5 (3.9) | 1 (0.8) |
| Serious TEAE | 6 (4.7) | 4 (3.1) |
| ADR‡ | 32 (25.2) | 23 (18.1) |
| Serious ADR | 1 (0.8) | 2 (1.6) |
| Treatment-emergent cardiovascular events | 1 (0.8) | 1 (0.8) |
| Study drug-related treatment-emergent cardiovascular events | 0 | 1 (0.8) |
| AE leading to discontinuation | 0 | 2 (1.6) |
| ADR leading to discontinuation | 0 | 1 (0.8) |
| Hypoglycemia§ | 8 (6.3) | 7 (5.5) |
| Study drug-related hypoglycemia§ | 6 (4.7) | 4 (3.1) |
|
System organ class Preferred term ¶ |
||
| Infections and infestations | 38 (29.9) | 29 (22.8) |
| Upper respiratory tract infection | 30 (23.6) | 20 (15.7) |
| Metabolism and nutrition disorders | 35 (27.6) | 27 (21.3) |
| Hyperuricemia | 11 (8.7) | 6 (4.7) |
| Hypoglycemia§ | 8 (6.3) | 7 (5.5) |
| Hyperlipidemia | 5 (3.9) | 9 (7.1) |
| Investigations | 23 (18.1) | 19 (15.0) |
| Urine ketone body present | 9 (7.1) | 1 (0.8) |
| Protein urine present | 7 (5.5) | 5 (3.9) |
| Gastrointestinal disorders | 19 (15.0) | 17 (13.4) |
| Diarrhea | 7 (5.5) | 6 (4.7) |
| Renal and urinary disorders | 19 (15.0) | 11 (8.7) |
| Proteinuria | 9 (7.1) | 5 (3.9) |
| Musculoskeletal and connective tissue disorders | 12 (9.4) | 13 (10.2) |
| Hepatobiliary disorders | 12 (9.4) | 3 (2.4) |
| Hepatic function abnormal | 7 (5.5) | 2 (1.6) |
| Blood and lymphatic system disorders | 10 (7.9) | 8 (6.3) |
| Thrombocytopenia | 8 (6.3) | 4 (3.1) |
| Injury, poisoning and procedural complications | 10 (7.9) | 2 (1.6) |
| Nervous system disorders | 7 (5.5) | 10 (7.9) |
| Cardiac disorders | 3 (2.4) | 8 (6.3) |
| Vascular disorders | 2 (1.6) | 8 (6.3) |
- Percentages are based on the number of patients in each treatment group.
- TEAE, treatment-emergent adverse event.
- † The severity of an adverse event (AE) was graded by an investigator as 1 = mild, 2 = moderate and 3 = severe. If any AE occurred more than once, the highest severity was summarized. For AEs with missing severity, the most severe assessment was imputed.
- ‡ Adverse drug reactions (ADRs) were defined as AEs where the causal relationship to the study drug was classified as a reasonable possibility. Any missing relationship of an AE to the study drug was considered a reasonable possibility.
- § Investigator-assessed hypoglycemia was a sum of probable symptomatic hypoglycemia, relative hypoglycemia and documented symptomatic hypoglycemia, as an AE in this study referred to symptomatic hypoglycemia. Documented symptomatic hypoglycemia was defined as an event during which typical symptoms of hypoglycemia were accompanied by a measured plasma glucose concentration ≤70 mg/dL (3.9 mmol/L). Probable symptomatic hypoglycemia was defined as an event during which symptoms of hypoglycemia were not accompanied by a plasma glucose determination, but that was presumably caused by a plasma glucose concentration ≤70 mg/dL (3.9 mmol/L). Relative hypoglycemia was defined as an event during which the person with diabetes reported any of the typical symptoms of hypoglycemia, and interpreted the symptoms as indicative of hypoglycemia, but with a measured plasma glucose concentration >70 mg/dL (3.9 mmol/L).
- ¶ All AEs as described by the investigators (verbatim) were coded using the Medical Dictionary for Regulatory Activities version 21.0.
Hypoglycemia occurred in eight patients (6.3%) and seven patients (5.5%) in the teneligliptin and placebo groups, respectively. Of these, six patients (4.7%) in the teneligliptin group and four (3.1%) in the placebo group reported study drug-related hypoglycemia. All of these events were considered mild or moderate and resolved/recovered without dose changes.
Only one patient (0.8%) in each of the placebo and teneligliptin groups reported treatment-emergent cardiovascular events. Of these, the only study drug-related treatment-emergent cardiovascular event was a case of moderate angina pectoris in the placebo group, which required hospitalization, but was resolved without any dose changes.
An ADR and an SAE leading to discontinuation (blood glucose increase and cerebral ischemia, respectively) occurred only in the placebo group. Six patients (4.7%) reported six SAEs in the teneligliptin group, and four patients (3.1%) in the placebo group reported five SAEs. Of these events, four cases were possibly related to the study drug: moderately severe angina pectoris and atherosclerotic heart disease were exacerbated in the same patient; severe gastritis; and severe blood glucose increased. All cases were reported as recovered/resolved. No deaths occurred during the study, and thus, no AEs or ADRs resulted in death. No cases of pancreatitis, heart failure, bullous pemphigoid or prolonged QT occurred during the study. We observed no notable changes or differences between groups from baseline to week 24 in laboratory values, vital signs, physical examination or 12-lead electrocardiogram parameters.
Discussion
An estimated 90 million Chinese adults have type 2 diabetes3, and poor levels of glycemic control have been reported8. Furthermore, currently approved pharmacological treatments are limited, as many are associated with ADRs and attenuated efficacy10. Teneligliptin, a highly selective DPP-4 inhibitor, was found to improve outcomes in Japanese patients with diabetes16, 17. However, clinical trial data on the use of teneligliptin for Chinese treatment-naïve patients with poor glycemic control with diet and exercise have been lacking. To the best of our knowledge, this is the first clinical trial in China to assess the efficacy and safety of teneligliptin in type 2 diabetes inadequately controlled with diet and exercise.
In the present study, Chinese patients received 20 mg teneligliptin once daily for 24 weeks. The most relevant findings in this study were the statistically significant change from baseline to week 24 in the LSM change in HbA1c between teneligliptin (−0.95%) and a placebo (−0.14%), yielding an LSM difference of −0.80% (P < 0.0001). Statistically significant differences in the secondary end-point, LSM change in FPG from baseline to week 24, were −21.9 mg/dL with teneligliptin versus −1.4 mg/dL with placebo, yielding an LSM difference of −20.5 mg/dL (P < 0.0001). Other significant differences between the teneligliptin and placebo groups were the proportion of patients who achieved HbA1c <7.0% at week 24 (P < 0.0001), and changes in HOMA-β (P = 0.0003) at week 24, HbA1c at week 12 (P < 0.0001) and FPG at week 12 (P = 0.0001). However, over the 24 weeks of treatment, there were no significant effects of teneligliptin on bodyweight, fasting insulin, C-peptide, glucagon or HOMA-IR, compared with placebo.
The LSM changes in HbA1c (−0.95%) with teneligliptin observed in the present study resembled those reported in a recent meta-analysis of 30 DPP-4 inhibitor studies in Chinese patients with type 2 diabetes23. In that analysis, weighted mean differences in the changes in HbA1c levels from baseline were −1.28% with saxagliptin, −1.17% with sitagliptin, −0.84% with linagliptin, −0.77% with vildagliptin and −0.91% with alogliptin. Furthermore, as in the present study, the aforementioned DPP-4 inhibitors were weight neutral. Although the HbA1c-lowering effect among DPP-4 inhibitors might be similar, switching from other DPP-4 inhibitors to teneligliptin for 24 weeks reportedly leads to a decrease in DPP-4 activity, resulting in a decrease in albuminuria among type 2 diabetes patients suffering from diabetic kidney disease24.
When comparing the present LSM differences in HbA1c (−0.80; P < 0.0001) and FPG (−20.5 mg/dL; P < 0.0001) between the teneligliptin and placebo groups after monotherapy for 24 weeks in Chinese patients with those in Korean patients after 24 weeks (HbA1c −0.94%, FPG −21.8 mg/dL; both P < 0.0001)24 and in Japanese patients after 12 weeks (HbA1c −0.9% to −1.0% according to teneligliptin dose, FPG −16.9% to −20.0% mg/dL; all P < 0.001)16, it can be seen that teneligliptin monotherapy performed similarly across Asian populations. Overall, the present findings relating to teneligliptin treatment resemble those of previous studies in Chinese23 and other Asian populations16, 17, 25-27. In a study of European patients with type 2 diabetes who received teneligliptin concomitantly with metformin, the changes in HbA1c at week 24 (−0.76%; P < 0.001)28 also resembled the changes in HbA1c observed in the present study and other Asian teneligliptin studies16, 17, 25-27. Although direct comparisons are not possible given the differences between the studies and populations, it seems that the efficacy of teneligliptin for HbA1c reductions was similar between Asian and non-Asian patients. A recent pooled analysis to determine the effect of race and ethnicity of another DPP-4 inhibitor, vildagliptin, arrived at similar conclusions29.
Notably, recent studies of Japanese patients with type 2 diabetes showed that greater weight increase after 24 weeks, dietary saturated fat intake and treatment with higher doses of glibenclamide were associated with deterioration of the HbA1c-lowering effects produced by DPP-4 inhibitors30, 31.
The most common AEs and ADRs reported in the present study were upper respiratory tract infections, and metabolism and nutrition disorders, with similar incidences between the groups. The incidences of hypoglycemia (6.3% vs 5.5%) and cardiovascular events (0.8% each) were also similar in both the teneligliptin and placebo groups. Of these cases, hypoglycemia was only considered to be study drug-related in six patients (4.7%) in the teneligliptin group, and four (3.1%) in the placebo group, but all were mild or moderate and resolved without the need to modify the dose of teneligliptin. Notably, just two discontinuations due to AEs occurred in the present study, both of which occurred in the placebo group. In the past, some concerns have been raised regarding cardiovascular AEs with teneligliptin, such as QT prolongation at high concentrations32. However, in the present study, as in previous trials of teneligliptin16, 25, 26, 33-35, none of the patients presented any study drug-related cardiovascular events, suggesting its safety in this regard. Overall, 20 mg teneligliptin taken once daily was well tolerated during the 24 weeks of treatment in the population studied.
The main study limitation of the present study was the lack of an active comparator. As diabetes is a chronic condition, patients require ongoing treatment; however, the observation period of 24 weeks in this study precludes analysis of the possible long-term outcomes with this treatment regimen. Another limitation is that only Chinese patients were enrolled, and this might limit the generalizability of the present findings to other ethnicities. Statistically significant results of non-primary efficacy end-points should be considered only as signals of possible treatment effects, because the alpha levels were not adjusted for multiple testing. Finally, when teneligliptin is used as combination therapy for diabetes or in conjunction with therapies for comorbidities, such as hypertension, its safety and efficacy profile remain unclear.
In conclusion, compared with a placebo, teneligliptin once daily at a dosage of 20 mg for 24 weeks significantly decreased HbA1c and FPG levels in Chinese patients with type 2 diabetes inadequately controlled with diet and exercise. No new safety concerns were raised. The findings show that monotherapy with teneligliptin could improve glycemic control without significant safety issues.
Acknowledgments
The authors thank Keyra Martinez Dunn, MD, of Edanz Evidence Generation for providing medical writing assistance, which was funded by Mitsubishi Tanabe Pharma, Tokyo, Japan. This study was sponsored by Mitsubishi Tanabe Pharma, Osaka, Japan.
Disclosure
Linong Ji has received personal fees from Mitsubishi Tanabe Pharma Development (Beijing) Co., Ltd. Yi Wang is an employee of Mitsubishi Tanabe Pharma Development (Beijing) Co., Ltd. Gen Takayanagi is an employee of Mitsubishi Tanabe Pharma Development America, Inc. The other authors declare no conflict of interest.




