Diagnostic utility of corneal confocal microscopy in type 2 diabetic peripheral neuropathy
Abstract
Aims/Introduction
The early pathological changes of diabetic peripheral neuropathy (DPN) are mainly small nerve fiber injuries. Corneal confocal microscopy (CCM) is an easy, rapid, non-invasive and repeatable technique to detect the damage of small nerve fibers. The purpose of this study was to explore the application of CCM in DPN and other chronic complications of type 2 diabetes mellitus.
Materials and Methods
A total of 220 individuals (48 normal healthy control participants and 172 patients with type 2 diabetes mellitus) were included in the study. All participants were assessed and scored for neurological symptoms and neurological deficits, quantitative sensory test, neuroelectrophysiological test, and CCM.
Results
Corneal nerve fiber density, corneal nerve fiber length and corneal nerve branch density were significantly reduced in patients with type 2 diabetes mellitus compared with normal healthy control subjects (P < 0.001, P < 0.001 and P < 0.001, respectively). In the DPN group, corneal nerve fiber density, corneal nerve branch density and corneal nerve fiber length were significantly lower than for patients without DPN (P < 0.001, P < 0.001 and P < 0.001, respectively). Receiver operating characteristic analysis showed that the optimal cut-off values were 24.68, 39 and 15.315, respectively, in which corneal nerve fiber density and corneal nerve fiber length had moderate sensitivity and specificity.
Conclusion
This study provides more support for the clinical use of CCM to diagnose type 2 diabetes mellitus-related complications, especially DPN.
Introduction
The incidence rate of diabetes is increasing worldwide, of which approximately 90% is type 2 diabetes mellitus1, 2. Meanwhile, the average life expectancy of patients with type 2 diabetes mellitus is increasing as a result of the improvement of economic level and the improvement of healthcare3, 4; chronic complications of diabetes are more common, such as diabetic peripheral neuropathy (DPN), diabetic nephropathy (DN), diabetic retinopathy (DR) and so on. DPN is one of the most common chronic and long-term complications of type 2 diabetes mellitus5. It is reported that nearly 50% of patients with type 2 diabetes mellitus suffer from DPN6, and nearly 20% of patients with type 2 diabetes mellitus have chronic painful neuropathy. Therefore, early accurate diagnosis of DPN is crucial.
At present, the commonly used methods to diagnose DPN include peripheral neuropathy assessment scale, quantitative sensory testing, neuroelectrophysiological evaluation and intra-epidermal nerve fiber density (IENFD) in skin biopsy. The peripheral neuropathy assessment scale has strong subjectivity and poor repeatability6. Quantitative sensory testing has strong repeatability, but is still subjective7. Neuroelectrophysiological evaluation is a widely used diagnostic method8. However, these assessment methods are mainly used to evaluate the large nerve fibers injury, but have low sensitivity to diagnose DPN in the early stage because early stage of DPN is more likely injured in small fibers9, 10. IENFD is considered to be the best standard for the diagnosis of small fiber injury, and an increasing number of studies have further emphasized the important value of the technique11-14. However, IENFD is an invasive, complex and highly technical inspection; meanwhile, the acceptance by patients is low, which makes it unsuitable for repeated investigations and limits its large-scale clinical application. Because of this, the reliability and ability of IENFD to diagnose DPN have not been confirmed in a large cohort study of diabetes patients15. Thus, an easy, non-invasive and repeatable method for detecting small nerve fiber damage of DPN is urgently required.
Many studies have shown that corneal confocal microscopy (CCM) is an easy, rapid, non-invasive and repeatable technique for making a quantitative assessment of small fiber injury, and has been proven to be useful for the diagnosis and follow up of DPN development16-18. More studies have used CCM to diagnose diabetic neuropathy, trying to find a suitable cut point for the early diagnosis of DPN16, 17, 19. Unfortunately, there is no unified standard, and more research is required to find possible cut points. The present research might provide more clinical evidence for this, and we also discuss the ability of CCM to diagnose DN and DR.
Methods
Study population
In the present study, we evaluated 220 individuals from December 2016 to June 2020 in Qilu Hospital of Shandong University, Qingdao district and Ji’nan District, in which, 48 normal healthy control individuals (Controls), 172 type 2 diabetes mellitus patients (including 100 patients with DPN, 37 patients with DN and 89 patients with DR). All individuals had no history of wearing contact lenses, or refractive surgery, malignancy, deficiency of vitamin B12, familial hereditary peripheral neuropathy, systemic disease known to affect the cornea, active diabetic foot ulceration, chronic corneal pathologies, ocular trauma or previous ocular surgery. The ethics committee of Qilu Hospital of Shandong University approved this study, and the research adhered to the tenets of the Declaration of Helsinki. Each participant signed the informed consent before the study. The basic demographic characteristics of each participant, such as sex, age, bodyweight, height, duration of type 2 diabetes mellitus and blood pressure, were obtained by trained research staff. Meanwhile, all participants underwent assessment of fasting blood glucose, glycated hemoglobin (HbA1c), total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, triglycerides, blood urea nitrogen, blood creatinine and urinary albumin creatinine ratio. All participants were photographed with a binocular fundus camera (Canon CR-2; Canon, Tokyo, Japan), and the diabetic fundus lesions were evaluated by professional ophthalmologists.
Assessment of DPN
All patients and control participants were assessed and scored for Diabetic Neuropathy Symptom20 (DNS) and Neurological Deficit Score21 (NDS). Quantitative sensory testing included warm sensation threshold and cold sensation threshold (CST) using the method of limits with the TSA-II NeuroSensory Analyzer (Medoc Ltd., Ramat-Vishay, Israel) on the dorsolateral aspect of the left foot. The vibration perception threshold (VPT) was tested by using a Horwell Neurothesiometer (Scientific Laboratory, Wilford, UK). Neuroelectrophysiological diagnosis was tested by using a D-K system (Dante Dynamics Ltd., Bristol, UK) equipped with a DISA temperature regulator to keep the limb temperature constantly between 32 and 35°C. Sural sensory nerve conduction velocity (SSNCV), peroneal motor nerve conduction velocity (PMNCV), sural nerve sensory nerve amplitude potential (SSNAMP) and peroneal compound muscle action potential (PCMAP) were tested in the right lower limb by an experienced neurophysiologist.
DPN diagnosis
DPN was defined according to the Toronto Diabetic Neuropathy Expert Group recommendation22. If a participant met the following criteria: clinical symptom or symptoms, or a sign or signs of neuropathy; or abnormal nerve electrophysiology, we defined them as having neuropathy. The normal reference ranges of nerve conduction parameters are as follows: PMNCV ≥42 m/s; SSNCV ≥42 m/s; SSNAMP ≥6 µV; PCMAP ≥2 mV.
DN diagnosis
DN was defined as a urinary albuminuria creatinine ratio ≥30 mg/g according to the recommendations of the 2012 Kidney Disease Improving Global Outcomes guidelines for CKD23.
CCM
All participants were examined by two qualified optometrists (INP and MT) using the Heidelberg Retina Tomograph Rostock Cornea Module (HRT-III, Heidelberg, Germany) to capture CCM images, as described24, 25. Based on depth, focus position and contrast, the sub-basal nerve plexus of the cornea from each participant were captured. Each eye captured at least 10 images, and five images from each eye were selected for analysis. We used an automatic software (CCMetrics; Imaging Science, University of Manchester, Manchester, UK) to analyze the selected images, as described before26. Three specific parameters were measured per frame: corneal nerve fiber density (CNFD; n/mm2), corneal nerve fiber length (CNFL; mm/mm2) and corneal nerve branch density (CNBD; n/mm2).
Statistical analysis
Normally distributed data are expressed as the mean ± standard deviation. Receiver operating characteristic (ROC) curves were generated, and the area under the ROC curve (AUC) values, 95% confidence intervals, optimal cut-off, and sensitivity and specificity were calculated. The means were compared using one-way anova, The Bonferroni post-hoc test was used for normally distributed variables, and the non-parametric Kruskal–Wallis test was used for non-normally distributed variables. Statistical analysis was carried out using SPSS 16.0 (Armonk, NY, USA). A P-value <0.05 was considered significant.
Results
Basic clinical data
The basic clinical data of normal healthy control participants and type 2 diabetes patients with or without chronic complications (DPN, DN and DR) are shown in Tables 1–3. Patients with type 2 diabetes mellitus were significantly older than the control participants (P < 0.001). Body mass index, systolic blood pressure (SBP), diastolic blood pressure, total cholesterol, high-density lipoprotein, triglycerides, blood urea nitrogen, creatinine and fasting blood glucose were higher in patients with type 2 diabetes mellitus than in normal healthy control participants (P < 0.001, P < 0.001, P = 0.010, P = 0.008, P = 0.002, P = 0.008,P < 0.001, P = 0.004 and P < 0.001, respectively). HbA1c was also significantly higher in patients with type 2 diabetes mellitus than in normal healthy control participants (P < 0.001).
| Variable | Control | Type 2 diabetes mellitus | P-value for non-DPN vs DPN | |
|---|---|---|---|---|
| Non-DPN | DPN | |||
| n | 48 | 72 | 100 | – |
| Female (%) | 47.92 | 36.11 | 46.00 | – |
| Age (years) | 51.90 ± 14.86 | 54.85 ± 11.09 | 56.23 ± 12.40 | 0.452 |
| Duration of diabetes (years) | – | 7.71 ± 5.92 | 10.33 ± 7.32 | 0.013 |
| BMI (kg/m2) | 24.37 ± 2.26 | 25.78 ± 2.62** | 26.74 ± 4.10 | 0.061 |
| Systolic BP (mmHg) | 122.98 ± 13.31 | 135.57 ± 15.48*** | 140.92 ± 17.50 | 0.039 |
| Diastolic BP (mmHg) | 73.19 ± 8.66 | 79.14 ± 11.30** | 80.94 ± 12.70 | 0.338 |
| HbA1c (%) | 5.07 ± 0.44 | 7.90 ± 1.96*** | 8.70 ± 2.71 | 0.035 |
| Total cholesterol (mmol/L) | 4.27 ± 0.68 | 4.44 ± 1.23 | 4.79 ± 1.27 | 0.075 |
| LDL-C (mmol/L) | 2.96 ± 0.33 | 2.76 ± 0.91 | 2.98 ± 0.91 | 0.128 |
| HDL-C (mmol/L) | 1.18 ± 0.20 | 1.22 ± 0.26 | 1.35 ± 0.31 | 0.003 |
| Triglycerides (mmol/L) | 1.56 ± 0.58 | 1.91 ± 1.70 | 2.06 ± 1.94 | 0.603 |
| BUN (mmol/L) | 4.64 ± 0.68 | 5.13 ± 1.38 | 5.55 ± 2.03 | 0.124 |
| Cr (µmol/L) | 55.27 ± 13.62 | 64.08 ± 15.82 | 66.98 ± 28.15 | 0.432 |
| FBG (mmol/L) | 5.07 ± 0.46 | 7.22 ± 2.09*** | 7.99 ± 2.28 | 0.026 |
| NDS (–/10) | 0.39 ± 0.84 | 1.36 ± 1.83*** | 4.82 ± 3.66 | <0.001 |
| DNS (–/4) | 0.13 ± 0.61 | 0.37 ± 0.69* | 2.08 ± 1.40 | <0.001 |
| CNFD (n/mm2) | 27.93 ± 9.47 | 23.27 ± 10.01* | 17.67 ± 7.95 | <0.001 |
| CNBD (n/mm2) | 49.15 ± 25.55 | 37.38 ± 17.76** | 27.05 ± 12.09 | <0.001 |
| CNFL (mm/mm2) | 19.52 ± 3.11 | 15.69 ± 5.26*** | 12.56 ± 3.80 | <0.001 |
| SSNCV (m/s) | 50.04 ± 5.85 | 48.82 ± 3.82 | 40.61 ± 4.98 | <0.001 |
| PMNCV (m/s) | 48.27 ± 4.36 | 48.44 ± 3.49 | 33.55 ± 5.02 | <0.001 |
| PCMAP (mV) | 5.25 ± 1.27 | 4.17 ± 1.46*** | 1.38 ± 1.07 | <0.001 |
| SSNAMP (µV) | 19.93 ± 4.82 | 8.88 ± 2.35*** | 4.74 ± 3.48 | <0.001 |
| VPT (V) | 5.89 ± 3.05 | 6.74 ± 3.71 | 24.74 ± 4.30 | <0.001 |
| WST (°C) | 36.23 ± 1.14 | 38.10 ± 2.20*** | 42.44 ± 2.59 | <0.001 |
| CST (°C) | 28.75 ± 0.98 | 27.63 ± 1.19*** | 18.43 ± 5.31 | <0.001 |
- Data are presented as mean ± standard deviation or n (%).
- *P < 0.05, **P < 0.01, ***P < 0.001, control versus type 2 diabetes mellitus without diabetic peripheral neuropathy (non-DPN).
- BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; Cr, creatinine; CST, cold sensation threshold; DNS, Diabetic Neuropathy Symptom score; DPN, diabetic peripheral neuropathy; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NDS, Neurological Deficit Score; PCMAP, peroneal compound muscle action potential; PMNCV, peroneal motor nerve conduction velocity; SSNAMP, sural nerve sensory nerve amplitude potential; SSNCV, sural sensory nerve conduction velocity; VPT, vibration perception threshold; WST, warm sensation threshold.
| Variable | Control | Type 2 diabetes mellitus | P-value for non-DN vs DN | |
|---|---|---|---|---|
| Non-DN | DN | |||
| n | 48 | 135 | 37 | – |
| Female (%) | 47.92 | 40.74 | 45.95 | – |
| Age (years) | 51.90 ± 14.86 | 55.62 ± 11.90 | 55.76 ± 11.87 | 0.951 |
| Duration of diabetes (years) | – | 8.76 ± 6.41 | 10.97 ± 8.21 | 0.082 |
| BMI (kg/m2) | 24.37 ± 2.26 | 26.20 ± 3.61*** | 26.85 ± 3.48 | 0.333 |
| Systolic BP (mmHg) | 122.98 ± 13.31 | 136.73 ± 16.54*** | 145.81 ± 16.22 | 0.003 |
| Diastolic BP (mmHg) | 73.19 ± 8.66 | 78.60 ± 11.37** | 85.97 ± 13.17 | 0.001 |
| HbA1c (%) | 5.07 ± 0.44 | 8.15 ± 1.98*** | 9.16 ± 3.62 | 0.026 |
| Total cholesterol (mmol/L) | 4.27 ± 0.68 | 4.54 ± 1.20 | 5.02 ± 1.45 | 0.044 |
| LDL-C (mmol/L) | 2.96 ± 0.33 | 2.83 ± 0.85 | 3.09 ± 1.10 | 0.132 |
| HDL-C (mmol/L) | 1.18 ± 0.20 | 1.28 ± 0.27* | 1.38 ± 0.38 | 0.142 |
| Triglycerides (mmol/L) | 1.56 ± 0.58 | 2.01 ± 1.90* | 1.95 ± 1.65 | 0.853 |
| BUN (mmol/L) | 4.64 ± 0.68 | 5.37 ± 1.86*** | 5.40 ± 1.53 | 0.922 |
| Cr (µmol/L) | 55.27 ± 13.62 | 65.56 ± 25.23** | 66.54 ± 17.66 | 0.824 |
| FBG (mmol/L) | 5.07 ± 0.46 | 7.58 ± 2.33*** | 7.99 ± 1.80 | 0.328 |
| NDS (–/10) | 0.39 ± 0.84 | 2.15 ± 2.81*** | 2.02 ± 2.92 | 0.631 |
| DNS (–/4) | 0.13 ± 0.61 | 0.79 ± 1.84*** | 0.91 ± 2.01 | 0.126 |
| CNFD (n/mm2) | 27.93 ± 9.47 | 19.85 ± 9.38*** | 20.61 ± 8.95 | 0.662 |
| CNBD (n/mm2) | 49.15 ± 25.55 | 31.40 ± 15.92*** | 31.27 ± 14.29 | 0.963 |
| CNFL (mm/mm2) | 19.52 ± 3.11 | 13.77 ± 4.80*** | 14.26 ± 4.43 | 0.576 |
| SSNCV (m/s) | 50.04 ± 5.85 | 44.39 ± 5.93*** | 42.78 ± 6.50 | 0.154 |
| PMNCV (m/s) | 48.27 ± 4.36 | 40.47 ± 8.83*** | 37.27 ± 7.27 | 0.027 |
| PCMAP (mV) | 5.25 ± 1.27 | 2.60 ± 1.93*** | 2.36 ± 1.57 | 0.479 |
| SSNAMP (µV) | 19.93 ± 4.82 | 6.72 ± 3.81*** | 5.56 ± 3.02 | 0.088 |
| VPT (V) | 5.89 ± 3.05 | 16.30 ± 9.99*** | 20.50 ± 8.33 | 0.011 |
| WST (°C) | 36.23 ± 1.14 | 40.37 ± 3.31*** | 41.55 ± 2.82 | 0.050 |
| CST (°C) | 28.75 ± 0.98 | 22.81 ± 6.17*** | 20.36 ± 5.66 | 0.031 |
- Data are presented as the mean ± standard deviation or n (%).
- *P < 0.05, **P < 0.01, ***P < 0.001, control versus type 2 diabetes mellitus without diabetic nephropathy (non-DN).
- BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; Cr, creatinine; CST, cold sensation threshold; DNS, Diabetic Neuropathy Symptom score; DN, diabetic nephropathy; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NDS, Neurological Deficit Score; PCMAP, peroneal compound muscle action potential; PMNCV, peroneal motor nerve conduction velocity; SSNAMP, sural nerve sensory nerve amplitude potential; SSNCV, sural sensory nerve conduction velocity; VPT, vibration perception threshold; WST, warm sensation threshold.
| Variable | Control | Type 2 diabetes mellitus | P-value for non-DR vs DR | |
|---|---|---|---|---|
| non-DR | DR | |||
| n | 48 | 83 | 89 | – |
| Female (%) | 47.92 | 44.58 | 39.33 | – |
| Age (years) | 51.90 ± 14.86 | 54.86 ± 13.61 | 56.39 ± 9.97 | 0.402 |
| Duration of diabetes (years) | – | 6.75 ± 5.54 | 11.55 ± 7.21 | <0.001 |
| BMI (kg/m2) | 24.37 ± 2.26 | 26.48 ± 3.26*** | 26.20 ± 3.86 | 0.609 |
| Systolic BP (mmHg) | 122.98 ± 13.31 | 135.92 ± 15.97*** | 141.26 ± 17.31 | 0.037 |
| Diastolic BP (mmHg) | 73.19 ± 8.66 | 79.71 ± 10.16*** | 80.63 ± 13.76 | 0.618 |
| HbA1c (%) | 5.07 ± 0.44 | 8.12 ± 2.03*** | 8.60 ± 2.78 | 0.199 |
| Total cholesterol (mmol/L) | 4.27 ± 0.68 | 4.52 ± 1.10 | 4.76 ± 1.40 | 0.215 |
| LDL-C (mmol/L) | 2.96 ± 0.33 | 2.87 ± 0.91 | 2.91 ± 0.92 | 0.785 |
| HDL-C (mmol/L) | 1.18 ± 0.20 | 1.27 ± 0.26* | 1.33 ± 0.33 | 0.185 |
| Triglycerides (mmol/L) | 1.56 ± 0.58 | 1.91 ± 1.68 | 2.08 ± 1.99 | 0.552 |
| BUN (mmol/L) | 4.64 ± 0.68 | 5.06 ± 1.26* | 5.67 ± 2.14 | 0.023 |
| Cr (µmol/L) | 55.27 ± 13.62 | 63.55 ± 11.94*** | 67.83 ± 30.91 | 0.239 |
| FBG (mmol/L) | 5.07 ± 0.46 | 7.57 ± 2.05*** | 7.76 ± 2.40 | 0.577 |
| NDS (–10) | 0.39 ± 0.84 | 2.08 ± 2.39*** | 2.78 ± 2.15 | 0.037 |
| DNS (–/4) | 0.13 ± 0.61 | 0.72 ± 2.02*** | 0.93 ± 1.86 | 0.043 |
| CNFD (n/mm2) | 27.93 ± 9.47 | 21.79 ± 10.10** | 18.36 ± 8.13 | 0.015 |
| CNBD (n/mm2) | 49.15 ± 25.55 | 33.09 ± 16.95*** | 29.78 ± 14.02 | 0.164 |
| CNFL (mm/mm2) | 19.52 ± 3.11 | 14.77 ± 5.00*** | 13.03 ± 4.30 | 0.015 |
| SSNCV (m/s) | 50.04 ± 5.85 | 46.29 ± 6.14** | 41.96 ± 5.24 | <0.001 |
| PMNCV (m/s) | 48.27 ± 4.36 | 42.60 ± 7.72*** | 37.16 ± 8.59 | <0.001 |
| PCMAP (mV) | 5.25 ± 1.27 | 2.87 ± 1.89*** | 2.25 ± 1.78 | 0.026 |
| SSNAMP (µV) | 19.93 ± 4.82 | 6.86 ± 3.56*** | 6.11 ± 3.77 | 0.182 |
| VPT (V) | 5.89 ± 3.05 | 14.40 ± 9.28*** | 19.81 ± 9.57 | <0.001 |
| WST (°C) | 36.23 ± 1.14 | 40.24 ± 3.13*** | 40.98 ± 3.32 | 0.137 |
| CST (°C) | 28.75 ± 0.98 | 23.95 ± 5.67*** | 20.72 ± 6.17 | <0.001 |
- Data are presented as the mean ± standard deviation or n (%).
- *P < 0.05, **P < 0.01, ***P < 0.001, control versus type 2 diabetes mellitus without diabetic retinopathy (non-DR).
- BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; Cr, creatinine; CST, cold sensation threshold; DNS, Diabetic Neuropathy Symptom score; DR, diabetic retinopathy; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NDS, Neurological Deficit Score; PCMAP, peroneal compound muscle action potential; PMNCV, peroneal motor nerve conduction velocity; SSNAMP, sural nerve sensory nerve amplitude potential; SSNCV, sural sensory nerve conduction velocity; VPT, vibration perception threshold; WST, warm sensation threshold.
Neuropathy assessment
The NDS and DNS were significantly higher in type 2 diabetes mellitus patients than normal healthy control participants (P < 0.001 and P < 0.001, respectively). VPT and warm sensation threshold were significantly greater in type 2 diabetes mellitus patients compared with normal healthy control participants (P < 0.001 and P < 0.001, respectively), whereas CST was significantly lower in type 2 diabetes mellitus patients compared with normal healthy control participants (P < 0.001). PMNCV, SSNCV, PCMAP and SSNAMP were significantly lower in type 2 diabetes mellitus patients compared with normal healthy control participants (P < 0.001, P < 0.001, P < 0.001 and P < 0.001, respectively).
Subgroup analysis
DPN analysis
We divided patients with type 2 diabetes mellitus into the DPN group (n = 100) and the non-DPN group (n = 72). We found that duration of diabetes was longer in the DPN group than non-DPN group (P = 0.013). SBP, HbA1c, high-density lipoprotein and fasting blood glucose were higher in the DPN group than the non-DPN group (P = 0.039, P = 0.035, P = 0.003 and P = 0.026, respectively). The DNS and NDS were higher in the DPN group than the non-DPN group (P < 0.001 and P < 0.001, respectively). SSNCV, PMNCV, SSNAMP and PCMAP were significantly slower in the DPN group than the non-DPN group (P < 0.001, P < 0.001, P < 0.001 and P < 0.001, respectively). VPT and warm sensation threshold were significantly greater in the DPN group compared with the non-DPN group (P < 0.001 and P < 0.001, respectively), whereas CST was significantly lower in the DPN group than the non-DPN group (P < 0.001; Table 1).
DN and DR analysis
We divided patients with type 2 diabetes mellitus into the DN group (n = 37) and patients without DN group (non-DN; n = 135; Table 2), the DR group (n = 89) and non-DR group (n = 83; Table 3). We found that SBP, diastolic blood pressure, HbA1c and total cholesterol were greater in the DN group compared with the non-DN group (P = 0.003, P = 0.001, P = 0.026 and P = 0.044, respectively). PMNCV was slower in the DN group than the non-DN group (P = 0.027). VPT was greater in the DN group compared with the non-DN group (P = 0.011), whereas CST was lower in the DN group than the non-DN group (P = 0.031), and similar in the DR group compared with the non-DR group (P < 0.001 and P < 0.001, respectively). In the DR group, the duration of diabetes was longer compared with the non-DR group (P < 0.001). SBP, blood urea nitrogen, NDS and DNS were higher in the DR group than the non-DR group (P = 0.037, P = 0.027, P = 0.037 and P = 0.043, respectively). SSNCV, PMNCV and PCMAP were slower in the DR group than the non-DR group (P < 0.001, P < 0.001 and P = 0.026, respectively).
CCM
Corneal nerve fiber density, CNFL and CNBD in type 2 diabetes mellitus patients were significantly lower than normal healthy control participants (P < 0.001, P < 0.001 and P < 0.001, respectively), and similar in the DPN group compared with the non-DPN group (P < 0.001, P < 0.001 and P < 0.001, respectively). CNFD and CNFL were lower in the DR group than non-DR group (P = 0.015 and 0.015), whereas CNBD showed no difference. However, CNFD, CNBD and CNFL showed no differences between the DN group and non-DN group.
ROC analysis
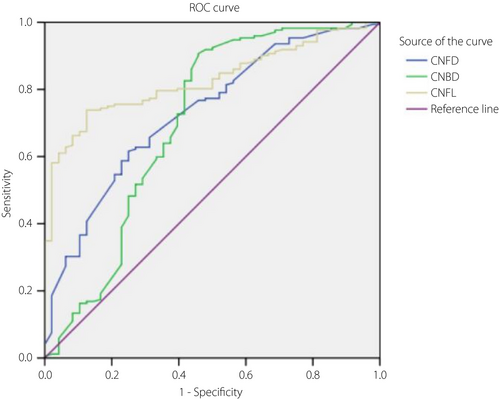
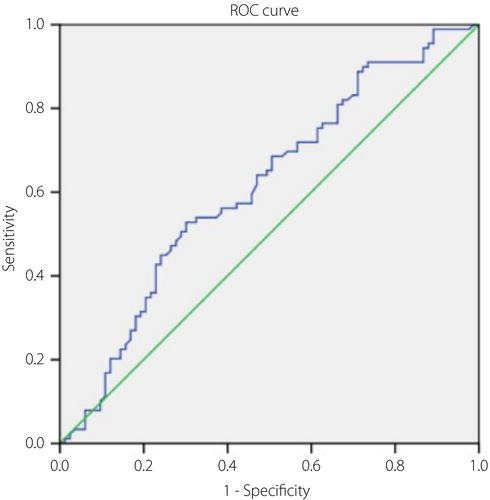
To evaluate the ability of CCM to diagnose type 2 diabetes mellitus, DPN and DR, ROC analysis was undertaken, including AUC values, 95% confidence intervals, optimal cut-off, and its sensitivity and specificity (Table 4). In using CCM to identify type 2 diabetes mellitus, the optimal cut-off of CNFD, CNBD and CNFL were 22.125, 51.875 and 16.29, respectively, with the AUC values of 0.731, 0.7 and 0.827, in which, CNFD and CNFL showed better sensitivity and specificity (Figure 1). In using CCM to identify DPN, the optimal cut-off of CNFD, CNBD and CNFL were 24.68, 39 and 15.315, respectively, with the AUC values of 0.668, 0.675 and 0.701, in which CNFD and CNFL showed moderate sensitivity and specificity (Figure 2). In using CCM to identify DR, the optimal cut-off of CNFL was 12.4, with AUC values of 0.613, and showed a low sensitivity and specificity (Figure 3).
| Subgroups | CCM parameters | AUC | P | 95% CIs | Cut-off value | Sensitivity | Specificity | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes mellitus | CNFD | 0.731 | <0.001 | 0.653, 0.810 | 22.125 | 0.616 | 0.750 | 2.464 | 0.512 |
| CNBD | 0.700 | <0.001 | 0.599, 0.801 | 51.875 | 0.907 | 0.542 | 1.980 | 0.172 | |
| CNFL | 0.827 | <0.001 | 0.773, 0.882 | 16.29 | 0.738 | 0.875 | 5.904 | 0.299 | |
| DPN | CNFD | 0.668 | <0.001 | 0.585, 0.751 | 24.68 | 0.780 | 0.528 | 1.653 | 0.417 |
| CNBD | 0.675 | <0.001 | 0.592, 0.759 | 39 | 0.850 | 0.472 | 1.610 | 0.318 | |
| CNFL | 0.701 | <0.001 | 0.618, 0.785 | 15.315 | 0.800 | 0.597 | 1.985 | 0.335 | |
| DR | CNFL | 0.613 | 0.011 | 0.528, 0.697 | 12.4 | 0.528 | 0.699 | 1.754 | 0.675 |
- AUC, area under the curve; CCM, corneal confocal microscopy; CI, confidence interval; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; DPN, diabetic peripheral neuropathy; DR, diabetic retinopathy.

Discussion
The exact pathogenesis of DPN is not clear. DPN can reduce the quality of life of patients with diabetes, and can lead to diabetic foot, amputation or even death27. Therefore, early detection and diagnosis of DPN is required, so as to start treatment as early as possible, to reduce the occurrence of serious complications, which helps to improve the quality of life of patients with diabetes and reduce medical expenses. Although IENFD is one of the examinations to prove small nerve fiber neuropathy to diagnose DPN in the guidelines28, it is difficult for large-scale clinical application, because it is an invasive and complex technique with low patient acceptance.
At present, neuroelectrophysiological detection – that is, nerve conduction measurement – is often used to evaluate and diagnose DPN29; however, in the early stage of DPN, small nerve fibers injury and sensory nerve damage are the main pathogenesis, and the sensitivity of nerve conduction measurement to evaluate these nerves is weak30, 31. Early identification of unmyelinated small nerve fibers injury will likely provide the best opportunity for effective therapy. CCM is an easy, novel, rapid, easy, non-invasive and repeatable technique that quantifies small nerve fibers, and is expected to be a powerful tool for early diagnosis of DPN. In particular, the recognition of CCM image by automatic analysis software is becoming more and more accurate, which not only saves manpower, material resources and time, but also reduces human errors.
Many studies show that the nerve fiber features captured from CCM are associated with DPN and even other diabetic chronic complications, such as DR and DN32-35. CNFD, CNFL and CNBD are the most commonly used indicators for CCM to evaluate diabetes mellitus and its complications25. In the present study, we found that CNFD, CNBD and CNFL were significantly lower in patients with type 2 diabetes mellitus than control participants, and ROC analysis showed that the optimal cut-off values were 22.125, 51.875 and 16.29, respectively, in which CNFD and CNFL showed moderate sensitivity and specificity. In subgroup analysis, we found that CNFD, CNBD and CNFL in the DPN group were significantly lower than those in the non-DPN group. ROC analysis showed that the optimal cut-off values were 24.68, 39 and 15.315, respectively, in which CNFD and CNFL had moderate sensitivity and specificity. In the DR group, CNFL was significantly lower than those in the non-DR group. ROC analysis showed that the optimal cut-off value was 12.4, but the sensitivity and specificity were low. It has been reported that CCM-related indicators of DN patients significantly differed from those of healthy individuals33. However, in the present study, there was no significant difference in CCM measurements between the DN group and non-DN group; this might be related to the mild condition of the diabetes patients with kidney disease in our study.
Therefore, based on the present results, CNFD and CNFL are valuable for DPN diagnosis, with the optimal cut-off values of 28.44 and 16.325, which are similar to the previous study36-38. The sensitivity and specificity of CNFD are 78 and 52.8%, the positive likelihood ratio is 1.653, and the negative likelihood ratio is 0.417; the sensitivity and specificity of CNFL are 80 and 59.7%, and the positive likelihood ratio is 1.985 and the negative likelihood ratio is 0.335. According to our research, CCM-related indicators are insufficient in the diagnosis of DR.
In previous studies, researchers reported a high sensitivity and specificity in using CCM measurements to assess type 1 diabetic peripheral neuropathy39, 40, as well as in patients with prediabetes41 and even children with type 1 diabetes42. In the present study, it is feasible to evaluate type 2 diabetes mellitus-related chronic complications by using CCM measurements, especially CNFL, but the sensitivity and specificity still need to be improved, which might be related to CCM image acquisition, automatic analysis software and slight diabetic complications in this study. At the same time, with the development of artificial intelligence technology, the software for automatic analysis of CCM images will become more and more accurate and intelligent. Some researchers have developed more accurate analysis software by using artificial intelligence technology43. However, more clinical research evidence is still required to evaluate the use of CCM in the early diagnosis of type 2 diabetes mellitus chronic complications, but as a new, convenient, repeatable and non-invasive examination, it has gained increasing attention by clinicians. Meanwhile, the cost of CCM detection equipment is expensive, which hinders its large-scale clinical application. However, with the development of economy and the reduction of the cost of CCM detection equipment, we believe that in the near future, the application of CCM in the diagnosis of chronic complications of type 2 diabetes mellitus, especially for the early diagnosis of DPN, will gradually become a reality.
Acknowledgment
This research was funded by the Hospital Youth Foundation of Qilu Hospital of Shandong University, Qingdao (QDKY2017QN12), and the Clinical New Technology Development Found of Qilu Hospital (2017).
Disclosure
The authors declare no conflict of interests.




