Association between gut microbiota composition and glycoalbumin level during pregnancy in Japanese women: Pilot study from Chiba Study of Mother and Child Health
Abstract
Aims/Introduction
Gut microbiota have various effects on human health. Some previous reports have shown that gut microbiota change during pregnancy and affect metabolism, but others have shown that microbiota do not change. Here, we examined the gut microbiota and glycoalbumin levels of 45 healthy Japanese women during pregnancy.
Materials and Methods
We carried out 16S rRNA gene sequencing analyses of maternal stool samples and compared the gut microbiota composition of samples from women in early and late pregnancy. We also examined the association between gut microbiota and maternal characteristics, including glycoalbumin.
Results
Microbiota composition in early and late pregnancy did not differ, according to principal coordinate analysis of weighted and unweighted UniFrac distances. Shannon indices were not different between early and late pregnancy. The proportion of one phylum, TM7, significantly decreased in late pregnancy compared with early pregnancy, but the proportions of other major phyla did not change. The Shannon index of late pregnancy was negatively associated with pregestational body mass index and positively correlated with glycoalbumin level, with adjustment of covariates.
Conclusions
We concluded that Japanese women did not show obvious differences in gut microbiota during pregnancy, except for TM7, and that the diversity of gut microbiota might affect maternal metabolism. As this study had limited statistical power, further large-scale studies are required.
Introduction
Gut microbiota are reported to have various effects on human health and diseases1. They play an important role in human health by producing vitamins and short-chain fatty acids2. Some gut bacteria produce B vitamins, such as folate. Others produce short-chain fatty acids through fermentation, such as butylate, which is reported to regulate the immune system3 and metabolism4. An imbalance in gut microbiota is called dysbiosis, and is characterized by decreased diversity of gut microbiota, a reduced number of beneficial bacteria and an increased number of pathobionts or bacterial species associated with disease5. Dysbiosis is reported to be associated with obesity and metabolic diseases6-8.
Several studies have investigated gut microbiota during pregnancy. Most of those studies reported that gut microbiota is altered during pregnancy9-13. Koren et al.10 reported that gut microbiota alterations are associated with dramatic changes in metabolic status during pregnancy. They reported an increase in Proteobacteria and Actinobacteria from the first to the third trimester in Finnish women10. In their study, microbiota from the third trimester affected the adiposity and glucose tolerance of germ-free mice to which the stool was transferred. Another report showed that Bacteroidetes is dominant in pregnant women in South China, and that Vercomicrobia declines and Tenericutes increases during late pregnancy11. The same study noted a significant structural shift in gut microbiota in pre-eclampsia patients11. In contrast, DiGiulio et al.14 reported no dramatic changes in gut microbiota during pregnancy. Other studies have shown differences in the microbial diversity or abundance of some phyla15, 16. It is unclear how gut microbiota are affected by pregnancy and whether the changes are associated with metabolic status in pregnant women.
Gut microbiota composition differs among different areas or populations, and is influenced by ethnicity and diet17, 18. For example, gut microbiota during pregnancy is related to dietary fat and fiber intake in Finnish pregnant women19. To understand the role of gut microbiota in pregnancy, more information is required regarding various populations of different ethnicities and those that consume different diets.
Thus, the aim of the present study was to examine the changes in gut microbiota during pregnancy in healthy Japanese women, and the association between gut microbiota and maternal metabolic status according to glycoalbumin, total cholesterol and triglyceride levels in early and late pregnancy.
Methods
Study design and participants
The participants were members of the Chiba Study of Mother and Child Health (C-MACH), a hospital-based birth cohort study20. Participants were recruited at three hospitals in Chiba and Saitama prefectures in Japan. Approximately 400 pregnant women participated between February 2014 and June 2015, and all provided blood samples. Of those women, 66 participants also consented to collection of their stool samples; however, 15 participants were excluded because they did not provide stool samples during either early or late pregnancy, and 13 were excluded because their anthropometric or blood chemical data were lacking. We analyzed pairs of samples from early and late pregnancy from 45 women.
Ethics
The study was carried out according to the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Japanese Ministry of Health, Labor and Welfare. The study protocol was approved by the Biomedical Research Ethics Committee of the Graduate School of Medicine, Chiba University. In addition, written informed consent was obtained from the participants.
Questionnaires and biochemical data
Self-administered questionnaires were carried out during early and late pregnancy, as described previously20.
The blood of participants was collected around 12 and 32 weeks of gestation. The blood was separated into serum and stored at −80°C in Chiba University Center for Preventive Medical Sciences BioBank before the measurement. The maternal serum levels of glycoalbumin, total cholesterol, and triglycerides during early and late pregnancy were measured using Lucica GA-L (Asahi Kasei Pharma Corporation, Tokyo, Japan), Cholestest CHO (Sekisui Medical Co., Ltd., Tokyo, Japan) and Pureauto S TG–N (Sekisui Medical Co., Ltd.), respectively, at SRL (SRL, Inc., Shinjuku, Tokyo, Japan).
Gut microbiota composition
Stool samples were collected to analyze the composition of the gut microbiota in early (~12 weeks) and late (~32 weeks) pregnancy. Stool samples were collected at home and frozen at −18°C. The samples were then transferred to Chiba University Center for Preventive Medical Sciences Biobank and kept frozen at −80°C until DNA extraction.
DNA extraction from stool samples and 16S rRNA gene sequencing were carried out, as described previously21.
Stool samples were blended with methanol and filtrated. The filtrate was centrifuged at 15,000 g and the supernatant was stored for later analysis. Fecal DNA extraction was carried out according to the literature, with minor modifications22. The pellet was suspended and incubated with lysozyme (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) and achromopeptidase (Fujifilm Wako Pure Chemical Corporation), sequentially. The suspension was added to sodium dodecyl sulfate and proteinase K (Merck, Darmstadt, Germany), then incubated. The bacterial DNA was purified using phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation. RNase treatment and polyethylene glycol precipitation were carried out.
The V1-2 variable region (27Fmod-338R) was sequenced on an Illumina MiSeq (Illumina, San Diego, CA, USA)22. The 16S rRNA V1–V2 amplicon was amplified using universal bacterial 16S rRNA gene primers: forward primer TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAGRGTTTGATYMTGGCTCAG and reverse primer GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGATTACCGCGGCTGCTGG. We attached dual indexes and Illumina sequencing adapters to polymerase chain reaction products using the Nextera XT Index Kit (Illumina). After purification of the amplicon, mixed samples were prepared by pooling approximately equal amounts of polymerase chain reaction amplicons from each sample. A sample library with 20% denatured PhiX spike-in (SeqMatic LLC, Fremont, CA, USA) was sequenced by MiSeq using MiSeq Reagent Kit v.2 (2 × 250 bp).
Taxonomic assignments and estimation of the relative abundance of sequencing data were carried out using the analysis pipeline of the QIIME software package23. Chimera checking was carried out using UCHIME24. An operational taxonomic unit was defined at 97% similarity. The operational taxonomic unit was assigned a taxonomy based on a comparison with the Greengenes database using RDP Classifier25, 26. We summarized the proportions of identified taxa in each sample, and calculated the amount of bacterial diversity. The Shannon index, a measurement of within-sample community diversity, was used to evaluate alpha diversity. The similarity of samples was calculated using weighted or unweighted UniFrac distances as implemented in R software27 (R Foundation for Statistical Computing, Vienna, Austria) and visualized for the beta-diversity analysis using principal coordinate analysis plots. Differences in microbial communities between early and late pregnancy were assessed by permutational analysis of variance (permanova) of weighted or unweighted UniFrac distances using the adonis function from the R package vegan28. Effect sizes (R2) and statistical significance (P-value) were determined by 1,000 permutations.
Statistical analysis
Data are presented as medians (1st and 3rd quartiles). The differences in the measurement values between different groups were evaluated by Wilcoxon rank-sum test. Spearman’s rank correlation coefficient was calculated using the maternal microbiota composition and other characteristics. We carried out statistical calculations using R version 3.4.027. Partial correlation coefficients were calculated using the package “ppcor”29. As the sample size of the present study was relatively small, correlation coefficients between maternal characteristics or blood data and each phylum were calculated without adjustment for multiple tests.
Results
Participant characteristics
The median characteristics of the mothers were as follows: age 34.0 years (1st and 3rd quartile values of 31.0 years and 36.0 years); pregestational weight 52.0 kg (49.0 kg and 56.0 kg); height 159.0 cm (155.0 cm and 162.0 cm); pregestational body mass index (BMI) 20.4 kg/m2 (19.1 kg/m2 and 22.2 kg/m2) and gestational bodyweight gain 8.5 kg (5.9 kg and 10.6 kg). In early pregnancy, maternal serum glycoalbumin, total cholesterol and triglyceride values were 13.8% (13.2 and 15.5%), 174.0 mg/dL (152.0 and 193.0 mg/dL) and 81.0 mg/dL (60.0 and 110.0 mg/dL), respectively (Table 1). In late pregnancy, maternal serum glycoalbumin, total cholesterol and triglyceride values were 12.9% (12.5 and 13.6%), 265.0 mg/dL (244.0 and 295.0 mg/dL) and 208 mg/dL (166.0 and 244.0 mg/dL), respectively (Table 1). Pregestational BMI was almost the same as that reported in national survey data, but gestational weight gain was slightly lower than in previous reports30, 31. Biochemical data during pregnancy were comparable with previous reports32, 33.
| Characteristic | |
|---|---|
| n | 45 |
| Age (years) | 34.0 [31.0, 36.0] |
| Height (cm) | 159.0 [155.0, 162.0] |
| Pregestational weight (kg) | 52.0 [49.0, 56.0] |
| Pregestational BMI | 20.39 [19.07, 22.22] |
| Gestational weight gain (kg) | 9.05 [7.85, 12.05] |
| Glycoalbumin, early (%) | 13.8 [13.2, 14.5] |
| Glycoalbumin, late (%) | 12.9 [12.5, 13.6] |
| Total cholesterol, early (mg/dL) | 174.0 [152.0, 193.0] |
| Total cholesterol, late (mg/dL) | 265.0 [244.0, 295.0] |
| Triglyceride, early (mg/dL) | 81.0 [60.0, 110.0] |
| Triglyceride, late (mg/dL) | 208.0 [166.0, 244.0] |
- Values are shown as median [1st, 3rd quartile]. BMI, body mass index.
Alteration of maternal gut microbiota during pregnancy
A total of 45 pairs of stool samples taken in early and late pregnancy were analyzed. Principal coordinate analysis using weighted and unweighted UniFrac distances did not clearly distinguish early and late pregnancy microbiota (Figure 1). To determine differences in the composition of gut microbiota between early and late pregnancy, we carried out permanova. Results showed that the composition of gut microbiota was not affected by the stage of pregnancy (unweighted UniFrac: R2 = 0.00974, P = 086; weighted UniFrac: R2 = 0.00581, P = 0.987). The Shannon index, which is an alpha diversity index, did not differ between early and late pregnancy.
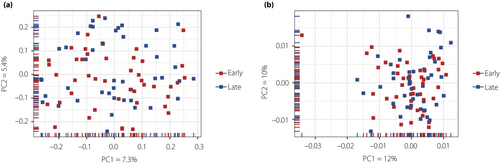
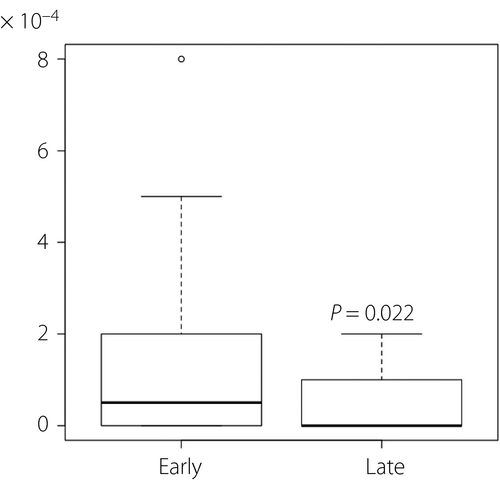
At the phylum level, the relative abundance of the four dominant phyla in women during early pregnancy were Firmicutes 69.2% (62.6 and 76.7%), Bacteroidetes 15.0% (6.8 and 23.2%), Actinobacteria 11.3% (5.8 and 20.2%) and Proteobacteria 0.8% (0.4 and 1.5%). In late pregnancy, the relative abundance of Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria was 64.7% (57.4 and 70.8%), 18.5% (11.6 and 26.3%), 13.6% (8.5 and 21.5%) and 0.8% (0.5 and 1.5%), respectively (Table 2). The proportion of phylum TM7 was significantly lower in late pregnancy than in early pregnancy (Figure 2). No other phyla showed significant alterations.
| Phylum | Early | Late | P-value |
|---|---|---|---|
| Actinobacteria | 11.3 [5.8, 20.2] | 13.6 [8.5, 21.5] | 0.663 |
| Bacteroidetes | 15.0 [6.8, 23.2] | 18.5 [11.6, 26.3] | 0.147 |
| Firmicutes | 69.2 [62.6, 76.7] | 64.7 [57.4, 70.8] | 0.075 |
| Proteobacteria | 0.8 [0.4, 1.5] | 0.8 [0.5, 1.5] | 0.831 |
- Values are shown as median [1st, 3rd quartile]. Phyla detected in more than half of the participants at any time are shown in this table.
Association of gut microbiota with maternal characteristics and blood chemistry
The Shannon index for samples taken in early pregnancy did not show any correlation with maternal age (r = −0.092, P = 0.549), pregestational BMI (r = −0.210, P = 0.167) or gestational weight gain (r = 0.180, P = 0.238). Although the Shannon index for late pregnancy also had no correlation with maternal age (r = −0.006, P = 0.968) or gestational weight gain (r = 0.064, P = 0.677), it showed a significant negative correlation with pregestational BMI (r = −0.458, P = 0.002). The Shannon index showed no correlation with blood chemistry data in early pregnancy. It also showed no significant correlation with total cholesterol or triglyceride levels, but there was a positive correlation with serum glycoalbumin levels in late pregnancy (Table 3). The correlation between the Shannon index and glycoalbumin in late pregnancy was significant after adjustment for maternal age, pregestational BMI and gestational weight gain (Table 3).
| Crude | Adjusted† | |||
|---|---|---|---|---|
| Rho | P | Rho | P | |
| Early | ||||
| Glycoalbumin | 0.056 | 0.717 | 0.122 | 0.443 |
| Total cholesterol | −0.151 | 0.321 | −0.238 | 0.129 |
| Triglyceride | −0.238 | 0.116 | −0.008 | 0.962 |
| Late | ||||
| Glycoalbumin | 0.423 | 0.004 | 0.345 | 0.025 |
| Total cholesterol | −0.276 | 0.067 | −0.224 | 0.155 |
| Triglyceride | −0.327 | 0.029 | −0.215 | 0.171 |
- † Adjusted by maternal age, pregestational body mass index and gestational weight gain.
- Statistically significant values are in bold.
Next, we examined the association between the proportions of five specific phyla and maternal characteristics during each period. After adjustment for multiple tests, there were no significant correlations, so we expressed the data without adjustment for multiple testing. None of the phyla showed an association with maternal pregestational BMI, gestational weight gain or serum levels of total cholesterol and triglyceride during early pregnancy. The serum level of glycoalbumin showed a negative correlation with the proportion of TM7 phylum in early pregnancy (Figure 3).
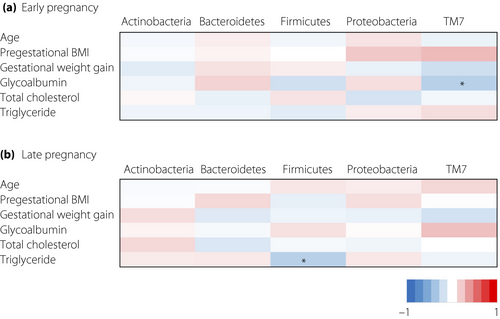
None of the phyla showed an association with maternal pregestational BMI, gestational weight gain or serum levels of total cholesterol and glycoalbumin during late pregnancy. The serum levels of triglyceride showed a negative correlation with the proportion of Firmicutes in late pregnancy (Figure 3).
To analyze the correlation between TM7 and glycoalbumin in detail, we evaluated partial correlation coefficients using maternal age and pregestational BMI. The partial correlation coefficient between TM7 and glycoalbumin was not statistically significant in early pregnancy (r = −0.211, P = 0.17). In addition, changes in the proportion of TM7 phylum from early to late pregnancy did not correlate with changes in maternal serum glycoalbumin levels from early to late pregnancy (r = −0.179, P = 0.24). To analyze the correlation between Firmicutes and triglyceride in detail, we evaluated partial correlation coefficients using maternal age, pregestational BMI and gestational weight gain (until 32 weeks of gestational age). The partial correlation coefficient between Firmicutes and triglyceride was not significant (r = −0.265, P = 0.090).
Discussion
In the present study, we examined microbiota changes during pregnancy, and their association with maternal characteristics and blood chemistry related to glucose/lipid metabolism in pregnant Japanese women. Principal coordinate analysis and permanova did not show significant changes in microbiota composition between early and late pregnancy. There were no obvious differences among four major phyla between early and late pregnancy, although the proportion of the TM7 phylum decreased in late pregnancy compared with that in early pregnancy. The Shannon index showed a significant negative correlation with pregestational BMI in late pregnancy and a positive correlation with glycoalbumin, also in late pregnancy.
Several previous reports showed that the microbiota composition changes during pregnancy9-12, 14, 16. In the present study, unlike that in a previous study10, Proteobacteria and Actinobacteria did not change during pregnancy, but the proportion of the TM7 phylum did change. Previous studies reported that the gut microbiota in different populations are distinct from one another16-18, 34, and that long-term dietary habits affect human gut microbiota35. The differences between previous studies and the present study might be due to different ethnicities and dietary habits of the study populations. Although pregestational weight, BMI and gestational weight gain reportedly correlate with microbiota composition during pregnancy36, 37, we did not detect any correlation of microbiota composition with these variables. The participants showed relatively small pregestational BMI values, which might contribute to the differences between previous reports and the present results. Sample size was relatively small, so statistical power was not enough to detect a significant difference. Larger studies of Japanese pregnant women are required.
The diversity of gut microbiota plays an important role in health. Patients with inflammatory bowel disease have less diverse gut microbiota than do healthy individuals38. Decreased gut microbiota diversity is also associated with metabolic disorders, such as obesity and glucose intolerance39, 40. In the present study, the Shannon index, which reflects the diversity of gut microbiota, had a significant negative correlation with pregestational BMI, and a positive correlation with serum glycoalbumin levels. Gut microbiota might directly affect adiposity or blood glucose levels. Another possibility is that maternal nutrition affects both gut microbiota and the blood glucose level. However, there was only a narrow range of glycoalbumin levels in the participants of the present study. This narrow distribution might have affected the trend in correlations. Although several other parameters (e.g., fasting plasma glucose levels, hemoglobin A1c levels and triglyceride/high-density lipoprotein cholesterol ratios) also reflect glucose metabolism or insulin resistance, we did not obtain those parameters. Thus, this result should be verified to clarify the relationship between gut microbiota diversity and glucose metabolism during pregnancy.
The proportion of the TM7 phylum during early pregnancy was negatively associated with glycoalbumin levels in a simple correlation, but the relationship was not significant after adjustment for confounding factors. Because the proportion of TM7 was small, we compared glycoalbumin levels with or without detection of TM7, in addition to correlation analysis. This analysis showed a significant difference between both groups (Figure S1). Previous studies reported that the composition of gut microbiota can affect glucose metabolism41-43. TM7 has not been reported to be associated with glucose metabolism; instead, the intestinal environment that favors TM7 might also affect maternal glucose metabolism. Further studies are required to clarify the relationship of the TM7 phylum to glucose metabolism.
The proportion of the Firmicutes phylum during late pregnancy was negatively associated with serum triglyceride levels in a simple correlation, but the relationship was not significant after adjustment for confounding factors. Gut microbiota might affect serum lipid profiles during pregnancy44. In one study, pregnant women who received supplementation with probiotics showed lower serum triglyceride levels45. In contrast, Hoppu et al.46 did not detect any probiotic effects in the serum lipid profiles of pregnant women. The association between gut microbiota and lipid profiles during pregnancy remains unclear. In the present study, the association between Firmicutes and serum triglyceride levels was not significant after adjustment for pregestational BMI and gestational weight gain. Thus, the results might have been affected by those confounding factors. In addition, the study participants were not fasting when blood sampling was carried out, which might have affected the results. Further studies are required to clarify the relationship between gut microbiota and lipid profiles during pregnancy.
The present study was subject to several limitations. Although dietary habits can affect gut microbiota, we did not evaluate the diets of the participants. The study was carried out in a small region of Japan and evaluated a relatively small number of participants. A large-scale study spanning multiple regions is required to confirm the present results in the overall Japanese population.
In conclusion, the proportion of the TM7 phylum within the gut microbiota of Japanese women changed during pregnancy. Gut microbiota might affect maternal metabolic status during pregnancy. The present study contributes to understanding the role of gut microbiota during pregnancy, but further studies are required.
Acknowledgments
We express our sincere appreciation for the cooperation and support of all study participants and members of C-MACH. The authors thank Professor Hideoki Fukuoka (Waseda University) for his contribution toward planning and conducting C-MACH. This work was partly supported by JSPS KAKENHI (grant numbers 16H01781, 17K00577), AMED-CREST (grant number 18gm0710009h0004), Chiba Foundation for Health Promotion & Disease Prevention and Yakult Bio-Science Foundation.
Disclosure
The authors declare no conflict of interest.




