The Uncoupling Protein 2 -866G > A Polymorphism is Associated with the Risk of Ischemic Stroke in Chinese Type 2 Diabetic Patients
The first two authors contributed equally to this work.
SUMMARY
Aims: To determine genetic predispsitions for diabetic cerebral ischemia, we investigated the relationship between the -866G>A polymorphism of uncoupling protein (UCP) 2 and the risk of ischemic stroke in two cohorts of type 2 diabetic patients. Methods: A total of 844 type 2 diabetic patients with 4-year prospective study were examined using a case-control methodology. And 404 cases with ischemical stroke, 440 cases without ischemical stroke. The -866G>A polymorphism in UCP2 was genotyped by TaqMan MGB probe method. Results: The -866G>A SNP in UCP2 was significantly associated with diabetic ischemical stroke (odds ratio [OR]= 1.94; 95% confidence interval [CI]= 0.68 to1.31; P < 0.037). Similar results were observed for baseline cases of IS. Stratification by sex confirmed an allelic association with IS in women, whereas no association was observed in men. Conclusions: The A allele of the -866G>A variant of UCP2 was associated with increased risk of IS in Chinese diabetic women with type 2 diabetes in a 4-year prospective study. This association was independent of other common IS risk factors.
Introduction
Stroke is the second leading cause of death worldwide and a major burden on health care [1]. The consequence of stroke is often enhanced by risk factors such as diabetes, which increases the incidence of stroke in the ischemic stroke by 4–12 folds particularly [1]. Acute hyperglycemia and diabetes could aggravate brain damage, because the transient focal or forebrain ischemia by both glutamate excitotoxicity and increased reactive oxygen species (ROS) generation. The ROS generation is suggested to be a connecting link between high glucose, mitochondrial dysfunction, and apoptosis [2]. Additionally, the excessive glucose supply is suggested to upregulate the glucose transporters levels and ΔΨm (hyperpolarization), which might be detrimental to the brain cells [2]. The results of these above risk factors is the extension of core region or conversion of penumbra into the core in a short time.
Several studies have shown that uncoupling protein 2 (UCP2) plays an antiatherogenic role in the vascular wall [5–7] and may improve the tolerance of brain to ischemia [8]. UCP2 acts as a physiological downregulator of ROS generation in both endothelial and smooth muscle cells of the vascular wall as well as in macrophages [3, 4]. A series of clinical trials have shown the -866G>A (rs659366), a functional single nucleotide polymorphism (SNP) in the promoter region of UCP2, is related to obesity [9], glucose homeostasis [10, 11], and dyslipidemia [12]. The aim of the present study was to investigate the association of -866G>A polymorphism with ischemic stroke in type 2 diabetic patients.
Materials and Methods
Subjects
This study was conducted with two groups: type 2 diabetic subjects with ischemic stroke (n = 404, male 246, female 158), type 2 diabetic subjects without ischemic stroke (n = 440, male 240, female 200). The ischemic stroke population consisted of ischemic stroke patients who were selected from a sample of over 2000 stroke patients between May 2005 and March 2006 in the first affiliated hospital of Nanjing Medical University and Nanjing Brain Hospital in Jiangsu, China. The clinical diagnosis of ischemic stroke was defined as an acute focal or global neurological deficit lasting more than 24 h without apparent cause other than that of vascular origin, consecutively confirmed by brain computed tomography (CT) or magnetic resonance imaging (MRI) scan within 72 h from the onset of the symptoms. Subjects with cerebral hemorrhage, brain tumor, and external injury were excluded. T2DM group subjects were composed of patients who were free of ischemic stroke and with the disease duration of at least 5 years, regardless of age and sex in the same center (Table 1). Data collected included age, sex, body mass index (BMI), A1C, known duration of diabetes, age at diagnosis of diabetes, fasting plasma glucose, TC, TG, HDL-C, LDL-C, systolic blood pressure (SBP), diastolic blood pressure (DBP). The study was approved by the ethics committee of the first affiliated hospital of Nanjing Medical University.
| Incident IS | P | ||
|---|---|---|---|
| Yes (n = 404) | No (n = 440) | ||
| Gender (% male:%female) | 60.9 | 54.6 | 0.06 |
| Age (years) | 67.6 ± 11.9 | 62.8 ± 14.2 | <0.001 |
| Age at diagnosis of diabetes (years) | 59.5 ± 10.5 | 54.6 ± 12.4 | <0.001 |
| Known duration of diabetes (years) | 8.08 ± 6.76 | 8.24 ± 7.51 | 0.77 |
| A1C (%) | 7.75 ± 1.92 | 8.00 ± 2.23 | 0.61 |
| BMI (kg/m2) | 25.02 ± 3.02 | 23.50 ± 3.48 | 0.30 |
| Triglycerides (mmoL/L) | 4.66 ± 1.39 | 4.61 ± 1.50 | 0.58 |
| Total cholesterol (mmoL/L) | 1.90 ± 1.94 | 1.68 ± 1.71 | 0.01 |
| LDL cholesterol (mmoL/L) | 2.88 ± 0.95 | 2.85 ± 1.00 | 0.62 |
| HDL cholesterol (mmoL/L) | 1.07 ± 0.31 | 1.18 ± 1.32 | 0.06 |
| SBP (mmHg) | 121 ± 9.71 | 124 ± 14.9 | 0.80 |
| DBP (mmHg) | 86.4 ± 6.58 | 84.8 ± 5.64 | 0.59 |
| Tobacco smoking (%) | 93 (23.0) | 80 (18.2) | 0.09 |
| Drinking (%) | 46 (11.5) | 37 (8.51) | 0.17 |
- Data are means ± SD. Statistics of quantitative parameters are Student's t-test performed with log-transformed data. Data available for 404 subjects with IS and 440 subjects without IS.
Genotyping
Genomic DNA was isolated from peripheral blood according to standard procedures. The SNP at position -866 in the promoter region of the UCP2 gene (rs659366) was genotyped by TaqMan MGB probe method using Gene-Amp 7300 Sequence Detector (Applied Biosystem).
The PCR amplifications were performed using the following primers: F: CCAGCCTTCTTCTACTCCCCA; R: GGGCCTGGTTCGCCTTTA; Probe1: FAM-CACGCGTCAGTTAC-MGB; Probe2: HEX-TTCACGCATCAGTTAC-MGB. The conditions for TaqMan reaction were as follows: 95°C for 10 min, and 45 cycles of 95°C for 15 s and 60°C for 1 min. Genotype was directly obtained with the GeneAmp 5700 SDS software. The genotyping call rate was 98%.
Statistical analyses
Differences between groups were assessed by Student's t-test, contingency table χ2 test, and Fisher's exact test. Before genotype-related statistical analyses were performed, it was verified that genotypes were in Hardy-Weinberg equilibrium in all groups of subjects. Genotype associations with IS were assessed by regression models. Cox proportional hazards survival regression analyses were used to examine the effect of explanatory variables on time-related survival (disease-free) rates in prospective analyses. Kaplan-Meier curves were used to plot survival (disease-free) rates over time according to genotype. Logistic regression analyses were used for cross-sectional analyses. Odds ratios (ORs), respectively, with their 95% CIs were computed in these analyses for the minor A allele. For dominant (XA vs. GG genotypes) and recessive (AA vs. XG genotypes) models, ORs were considered to be 1 for GG or XG genotypes, respectively. For the codominant model, genotype data were coded as the number of A-allele copies (0, 1, or 2), and OR was computed for the number of A alleles as a quantitative variable (1 vs. 0 and 2 vs. 1 copies, with the lower number of copies of each pair considered to present an OR of 1). P < 0.05 was considered significant. Statistics were performed with the JMP software (SAS Institute, Cary, NC).
Results
The incidence of IS during the follow-up of the two cohort was 47.87%. Main baseline characteristics of subjects with or without IS during the follow-up (incident cases) are shown in Table 1. Excepting age, age at diagnosis of diabetes and TG, Is risk factors, such as hyperglycaemia, smoking, obesity, dyslipidemia, hypertension, were no difference in two groups.
Incidence of IS according to genotype was 48.18% for GG, 48.22% for GA, and 45.05% for AA. UCP2 -866A genotype was associated with survival in the overall cohort (mortality: AA 15.9%, GA 16.8%, GG 19.1%, P= 0.541). Subjects of GA or AA genotype had no significantly worse survival than GG diabetic patients (P > 0.05) (Figure 1). Cox proportional hazards survival regression analyses showed an inverse association of the A allele with the incidence of IS in a dominant model (HR 1.94 [95% CI 0.68–1.31]; P= 0.037) (Table 2). A significant interaction between genotype and sex (P= 0.029) was observed in this association with IS. Stratification by sex confirmed the allelic association with IS in women, whereas no association was observed in men (Table 2). The association persisted after adjustment for allocation group in age, BMI, HDL cholesterol, triglycerides, A1C, duration of diabetes, and arterial hypertension (Table 2). Similar association in a dominant model was observed when we considered the prevalent cases of IS at baseline, defined as a previous history of IS or TIA (Table 3). XA(AA+GA) of genotype frequencies polymorphism of UCP2 increase the risk of ischemical stroke in women but not in men (P= 0.003). After adjustment for allocation group in age, BMI, HDL cholesterol, triglycerides, A1C, duration of diabetes, and arterial hypertension, the association persisted (Table 3).
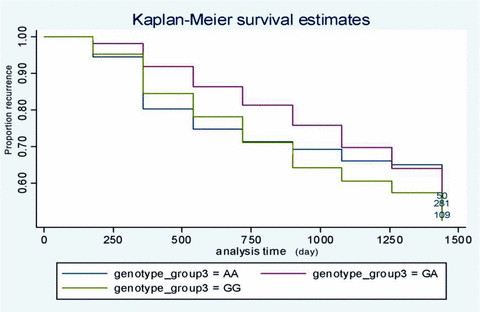
Kaplan-Meier survival (disease-free) curves for the cohort during follow-up according to genotype. Survival (y) axis represents absence of IS. The cumulated incidence of IS can be computed as (100%-survival).
| Subjects | IS | (Genotype frequency, %) | A allele in a dominant model | |||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | Unadjusted model | Adjusted model | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | |||||
| All | No | 109 (24.7) | 281 (63.9) | 50 (11.4) | 1.94 (0.68–1.31) | 0.04* | 1.90 (0.64–1.26) | 0.03* |
| Yes | 111 (27.5) | 252 (62.4) | 41 (10.2) | |||||
| Men | No | 56 (23.3) | 156 (65.0) | 28 (11.7) | 1.15 (0.71–1.86) | 0.57 | 1.12 (0.68–1.86) | 0.65 |
| Yes | 68 (27.2) | 157 (61.8) | 21 (11.0) | |||||
| Women | No | 58 (29.0) | 120 (60.0) | 22 (11.0) | 1.74 (0.47–1.18) | 0.03* | 1.74 (0.46–1.19) | 0.02* |
| Yes | 38 (24.1) | 100 (63.3) | 20 (12.7) | |||||
- HRs for the A-allele in a dominant model (XA vs. GG) determined by Cox proportional hazards survival regression analyses. Time to event was defined either as the number of days of follow-up until the occurrence of an IS event for subjects with IS or as the duration of follow-up for right-censored subjects without IS at the end of follow-up. Computations performed without adjustment for covariables or *adjusted for allocation group in age, BMI, total cholesterol, HDL cholesterol, triglycerides, creatinine clearance, A1C, duration of diabetes, and arterial hypertension.
| Subjects | IS | (Genotype frequency, %) | A allele in a dominant model | |||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | Unadjusted model | Adjusted model | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | |||||
| All | No | 111 (25.2) | 284 (64.6) | 45 (10.2) | 2.13 (1.29–3.53) | 0.003* | 2.32 (1.29–4.20) | 0.005* |
| Yes | 109 (27.0) | 249 (61.6) | 46 (11.4) | |||||
| Men | No | 57 (23.8) | 161 (67.1) | 22 (9.17) | 1.43 (0.74–2.75) | 0.27 | 1.33 (0.61–2.90) | 0.47 |
| Yes | 67 (27.2) | 152 (61.8) | 27 (11.0) | |||||
| Women | No | 54 (27.0) | 123 (61.5) | 23 (11.5) | 3.70 (1.58–8.70) | 0.003* | 5.14 (1.82–14.51) | 0.002* |
| Yes | 42 (26.6) | 97 (61.4) | 19 (12.0) | |||||
- OR for the A allele in a dominant model (XA vs. GG) determined in logistic regression analyses. Computations performed without adjustment for covariables or *adjusted for age, BMI, total cholesterol, HDL cholesterol, triglycerides, creatinine clearance, A1C, duration of diabetes, and arterial hypertension.
To investigate the role of possible intermediate phenotypes in these allelic associations with IS, we compared clinical and biological profiles at baseline according to genotype. Age of diagnosis of diabetes, severity of hyperglycemia, BMI, prevalence of arterial hypertension, plasma levels of triglycerides, total cholesterol, and HDL cholesterol were similar in carriers of different genotypes (data were not shown). Odds ratios for the incidence of ischemic stroke are shown in Table 3. The -866G/A SNP in UCP2 increased the risk of ischemic stroke in women (age- and sex-adjusted odds ratio = 5.14; 95% CI, 1.82 to 14.51; P= 0.005) under a dominant model.
Discussion
The consequence of stroke is often enhanced by risk factors such as diabetes, which increases the incidence of stroke in the ischemic stroke by 4 to 12 folds particularly [1]. Acute hyperglycemia and chronic diabetes can aggravate brain damage [13–17] by glutamate excitotoxicity [18, 19] and increase ROS generation in metabolically challenged tissue [20–22]. The ROS generation is suggested to be a connecting link between high glucose, mitochondrial dysfunction, and apoptosis [23]. In the other hand, the excessive glucose supply is suggested to upregulate the glucose transporters levels and ΔΨm (hyperpolarization), which might be detrimental to the brain cells [24]. The consequence of these effects is the extension of core region or conversion of penumbra into the core in a short time.
The mitochondrial UCP families are expressed in various human tissues. Particularly UCP2 can provide a controlled leak of protons across the inner membrane of the mitochondria and thus uncouple oxidative phosphorylation from respiration, with a concomitant decrease in inner mitochondrial membrane potential [25] and free radical generation [26,27] in response to glucose. The expression of UCP2 is stimulated in vitro and in vivo by hyperglycemia, FFA, and O2−[28–30], which are increased after acute brain injury. In mice overexpressing human UCP2, brain damage was diminished after experimental stroke or traumatic brain injury, and neurological recovery was enhanced. In cultured cortical neurons, UCP2 reduced cell death and inhibited caspase-3 activated by oxygen and glucose deprivation. Mild mitochondrial uncoupling by 2,4-dinitrophenol (DNP) reduced neuronal death, and UCP2 activity was enhanced by palmitic acid in isolated mitochondria [31]. Glucose sensing negatively also controlled by UCP2. It also reduces neurons less sensitive to glucose. The study conducted on wild-type and UCP2−/− mice fed on a high fat diet revealed that the abolished glucose-sensing in the wild type is reversed in UCP2−/− animals [32]. These evidence (ROS, potential and glucose sensing alteration) demonstrated UCP2 as a neuromodulator and neuroprotector.
Previous studies have shown that the -866G>A SNP is a functional polymorphism [33,34]. The A allele has been associatied with decreased insulin secretion and increased the risk of type 2 diabetes [34]. This SNP polymorphism also causes deterioration of peripheral nerve function in Japanese type 2 diabetes patients [35]. Dhamrait et al. reported a doubling in the risk of coronary heart disease for -866A/A homozygotes in a prospective study of 2695 healthy men [36]. The -866G/A site alone or in a haplotype context with the 45nt-del/ins polymorphism is associated with asymptomatic carotid artery atherosclerosis in female subjects of the SAPHIR population [37]. There are, however, no reports on the association between UCP2 gene polymorphism and risk of stroke in type 2 diabetes.
In this study, we investigated the association of this variant with IS in type 2 diabetic patients. We have observed in two independent cohorts of Chinese subjects that the common -866G>A SNP in the UCP2 promoter region strongly modulates the risk of IS in type 2 diabetic women. Inverse associations of the A allele with both baseline and incident cases of IS were observed in a dominant model with a 12%–25% increase in IS risk for A allele carriers. Our study notes that the risk in IS afforded by the A allele was independent from effects on other known IS risk factors, including obesity, arterial hypertension, and dyslipidemia. Interestingly, the association showed interaction with sex and -866G>A polymorphism were observed only in women. But it was impossible to exclude true sex-related biological differences with the large amount of male patients. There was no deferent survival in three genotype because the observed timelimit was too short. In Table 3, the span of CI in women was too wide maybe because enrolled number of women subjects was too small.
However, the mechanism of why -866G>A polymorphism associated with IS is not clear. It's reported that no matter in THP-1 cells or in HUVECs cells the transcription ability of G allele was better than A allele, so we supposed that the G allele could increase the UCP2 gene mRNA transcription in endothelial cell and decreases the ROS production.
In summary, we found that the A allele of the -866G>A SNP in the UCP2 promoter region was associated with a significantly increased risk of IS in Chinese type 2 diabetic women. The result was independent of effects of other risk factors, such as duration of diabetes, BMI, dyslipidemia, arterial hypertension, smoking, and drinking. The mechanisms about this allelic association need to be investigated in further studies. Carrying out UCP2 gene -866G>A polymorphism detection could be related to IS early screening.
Acknowledgment
We thank Honggang Yi for statistical analysis support, and Qian Zhu, Chun Qiao for technical assistance.
Conflict of Interest
The authors declare no conflict of interest.




