Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes
Abstract
Individual studies indicate that kidney transplantation is associated with lower mortality and improved quality of life compared with chronic dialysis treatment. We did a systematic review to summarize the benefits of transplantation, aiming to identify characteristics associated with especially large or small relative benefit. Results were not pooled because of expected diversity inherent to observational studies. Risk of bias was assessed using the Downs and Black checklist and items related to time-to-event analysis techniques. MEDLINE and EMBASE were searched up to February 2010. Cohort studies comparing adult chronic dialysis patients with kidney transplantation recipients for clinical outcomes were selected. We identified 110 eligible studies with a total of 1 922 300 participants. Most studies found significantly lower mortality associated with transplantation, and the relative magnitude of the benefit seemed to increase over time (p < 0.001). Most studies also found that the risk of cardiovascular events was significantly reduced among transplant recipients. Quality of life was significantly and substantially better among transplant recipients. Despite increases in the age and comorbidity of contemporary transplant recipients, the relative benefits of transplantation seem to be increasing over time. These findings validate current attempts to increase the number of people worldwide that benefit from kidney transplantation.
Abbreviations:
-
- ESRD
-
- end-stage renal disease
-
- EQ-5D
-
- European quality of life-5 dimensions
-
- GHQ
-
- general health questionnaire
-
- HR
-
- hazard ratio
-
- HUI
-
- health utility index
-
- KDQoL
-
- kidney disease quality of life
-
- QoL
-
- quality of life
-
- SES
-
- socio-economic status
-
- SF-36
-
- Medical Outcomes Study 36-item short-form health survey
-
- SIP
-
- sickness impact profile
-
- TTO
-
- time trade off
-
- WHO-QoL
-
- World Health Organization quality of life
-
- 15D
-
- Finnish 15 dimensions
Introduction
Available treatments for end-stage renal disease (ESRD) include dialysis and kidney transplantation (1,2). Increasing prevalence of ESRD, together with stable or declining rates of organ donation have led to a critical shortage of kidneys available for transplantation (3,4). The median interval between placement on a transplant waiting list and receipt of a kidney transplant from a deceased donor has dramatically increased in recent years, and currently ranges between 3 and 7 years for North American patients with kidney failure, depending on region of residence (5). At the same time, the age and comorbidity of patients who are treated with dialysis continues to increase (6).
Individual studies indicate that kidney transplantation is associated with lower mortality and improved quality of life compared with chronic dialysis treatment (7,8). However, factors that are associated with greater or lesser benefit from transplantation are poorly described. In addition, there has been little or no systematic exploration of how the relative benefits of transplantation (compared to dialysis) have varied over time, given that contemporary dialysis patients are older and sicker (6), but must wait longer to receive a kidney transplant than those in previous years (3,9).
We did a systematic review to summarize the anticipated clinical benefit associated with kidney transplantation (compared with dialysis) in the current era. We also aimed to identify characteristics associated with especially large or small relative benefit, compared to dialysis.
Materials and Methods
Data sources and searches
This systematic review is reported according to published guidelines (10). An expert librarian conducted a comprehensive search to identify all relevant studies regardless of publication status. Nonenglish articles were included where an appropriate translator was available. Three electronic databases, MEDLINE (1950–February 25, 2010), EMBASE (1980–February 25, 2010), and all evidence-based medicine reviews (September 7, 2007) were searched. The detailed search strategies are included in the Supporting Information. A content expert and a methodologist screened each citation or abstract. Any study considered potentially relevant by at least one reviewer was recovered for further review.
Study selection
The full text of each potentially relevant study was independently assessed by two reviewers for inclusion in the review using predetermined eligibility criteria on a preprinted form. Studies were eligible for inclusion if they reported important clinical outcomes (mortality, cardiovascular events, hospitalization and quality of life [QoL]) in both a chronic dialysis population and a kidney transplantation population. Pediatric studies (age < 16 years) and studies including multi-organ transplantation were excluded. Studies had to include at least 30 participants in each relevant treatment modality group. This minimum sample size was set to improve the efficiency of the work without an appreciable loss of power and to minimize bias (robust calculations of standard deviation and to prevent small study bias). Multigroup cohort studies were included; crossover, case-control and cross-sectional studies were excluded with one exception—we included cross-sectional studies when QoL was reported. Disagreements were resolved by discussion and consultation with a third party. Reviewers agreed on study selection for 89% of the articles (κ= 0.66).
Data extraction and risk of bias assessment
We assessed and reported risk of bias in included studies using items from the Downs and Black checklist (11). These include items of study design (selection of participants, allocation of participants and outcome definitions), statistical analysis (calculation of sample size and adjustment for potential confounding) and results (losses to follow up). Post hoc, we included three items specifically relevant to the time-to-event analyses in these studies: (1) selected time of origin (e.g. dialysis initiation or transplantation) and destination (e.g. modality failure, death), (2) adjustment for prior time spent on renal replacement therapy and (3) modeling time-dependency of modality (i.e. attributing the time on dialysis for eventual transplant recipients and graft failure patients to the dialysis hazard). Two reviewers independently assessed each included study, and resolved disagreements with the aid of a third party.
The following properties were extracted from each study: characteristics (country, data source, era of accrual, duration of follow-up, special subgroups or populations, sample size and setting), participants (age, gender, race, body mass index, socio-economic status and comorbidities), renal replacement modality (living or deceased donor, hemodialysis, peritoneal dialysis, etc.) and results (both unadjusted and adjusted, covariates, interactions and subgroups). The following outcomes were considered: all-cause mortality, cardiac events (limited to myocardial infarction, stroke, heart failure and to the aggregate of any cardiac event), hospitalization (incidence, rate, mean counts; limited to infection and all-cause) and QoL. Specifically, the following QoL instruments were included: European quality of life-5 dimensions (EQ-5D), time trade off (TTO), standard gamble, health utility index (HUI), Finnish 15 dimensions (15D), Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), kidney disease quality of life (KDQoL), Karnofsky, sickness impact profile (SIP), general health questionnaire (GHQ), and World Health Organization quality of life (WHO-QoL). A second reviewer checked the data for accuracy.
Data synthesis and analysis
Because identified studies were expected to be observational and, therefore, both methodologically and clinically diverse, we decided a priori not to statistically combine results. To facilitate comparison, we present individual study summary statistics in unpooled metagraphs using R software. Dichotomous outcomes (e.g. mortality) are summarized using the unadjusted risk ratio and all available adjusted ratios (i.e. hazard ratio [HR], risk ratio, odds ratio, rate ratio; depending on what was reported). Continuous outcomes (e.g. QoL) are summarized using the mean difference. Given the presence of large heterogeneity, we did not formally assess for the presence of publication bias (12).
We planned a priori to examine the following subgroups for evidence of effect modification on the association between transplantation and mortality: diabetic patients, elderly patients, patients with chronic infections (human immunodeficiency virus, hepatitis B or hepatitis C) and patients with cardiovascular disease. We were primarily interested in the results of formal tests for effect modification in the primary studies. Unfortunately, we did not identify eligible studies for all of these subgroups. However, using multivariable meta-regression models (weighted least squares linear regression), we examined whether era (midyear of the interval for inclusion of participants), restricting analyses to dialysis patients who were active on the kidney transplantation waiting list, or elements of study design (prospective, retrospective or registry) modified the relationship between unadjusted mortality and modality. To minimize participant overlap (and ensure independence of data) between studies in our meta-regression analyses, we restricted our pool of studies to unique combinations of region (or registry) and era of accrual. More current studies were given precedence; studies with special populations were excluded (e.g. hepatitis positive).
Results
Search yield
From 32 166 identified citations, 732 articles were recovered for detailed evaluation (Figure 1). Of these, 110 studies were eligible for inclusion in this review (Table 1). Study sample sizes ranged from 39 to 468 681 (median 501); enrolment of study participants ranged from 1960 to 2006 and maximum duration of follow up ranged from 6 months to 19 years.

PRISMA flow diagram. PRISMA = preferred reporting items for systematic reviews and meta-analyses); ESRD = end-stage renal disease. Details of the search strategies including data sources are in the Supporting Information.
| Author and year | Country | Source | Accrual era | Maximum follow-up (years) | Population | Treatment | Sample size | Mean age (years) | Percent male | Percent diabetic |
|---|---|---|---|---|---|---|---|---|---|---|
| Bayat (43) 2010 | France | Regional registry | 1997–2003 | 7 | 60–80 years | Tx/D | 54/940 | 63 | 57 | 41 |
| <60 years | 280/221 | 62 | 21 | |||||||
| Ansell (96) 2009 | UK | UKRR | 1997–2006 | 1 | − | Tx/D | 17,545/22, 115 | − | − | − |
| Buturovic-Ponikvar (65) 2009 | Slovenia | ERA-EDTA | 1998–2006 | 9 | − | Tx/HD; PD | 461/1271; 103 | 52/65; 532 | 57/55; 59 | 14/24; 21 |
| Chauveau (27) 2009 | France | − | 1985–2000 | 111 | Low protein diet | Tx/HD | 101/102 | 42/62 | 52/- | − |
| Griva (88) 2009 | UK | Two sites | − | None6 | − | Tx/HD; PD | 117/77; 68 | 50/50 | 60/65 | 5/17 |
| Jain (37) 2009 | UK | Multiple sites | 1996–2000 | 42 | White; Black; South Asian | Tx/HD; PD | 157/598 | 622 | 63 | 25 |
| Kramer (70) 2009 | Europe | ERA-EDTA | 1997–2006 | 10 | − | CTx; LTx/HD; PD | 20, 321; 2965/398, 871; 46, 524 | 46/63 | 63/61 | 12/22 |
| Martin Navarro (16) 2009 | Spain | − | 2000–2005 | 22 | WL; nWL; ECD | CTx/WL HD | 31/164 | 68/74 | 64 | 35/20 |
| Pauly (59) 2009 | Canada; USA | USRDS; Regional registry | 1994–2006 | 42 | − | CTx; LTx/Night | 531; 531/177 | 47; 44/46 | 58; 57/65 | 14; 14/144 |
| Stel (97) 2009 | Europe | ERA-EDTA | 2000–2004 | 2 | − | CTx; LTx/HD; PD | 13,753; 3505/7,137 | − | − | − |
| Patel (98) 2008 | UK | Single site | 2002–2005 | 32 | − | CTx/WL HD; PD [nWL HD; PD] | 80/142 [78] | 51 [54] | 64 | 19 [39] |
| Visnja (14) 2008 | Serbia | Single site | 1987–2001 | 17 | HBV+; | Tx/WL HD | 67/128 | 35/50 | 75/58 | 0/84 |
| HCV+; | 39/83 | 36/55 | 69/48 | 5/8 | ||||||
| Hep– | 278/192 | 33/50 | 68/53 | 2/5 | ||||||
| Chavers (52) 2007 | USA | USRDS | 1996–2001 | 3 | − | Tx/HD; PD | 31,663/333,453 | <65/65–742 | 60/53 | 29/454 |
| Gill (77) 2007 | USA | USRDS | 1995–2003 | − | − | CTx; LTx[GF]/WL D | 47,433 [5,461]/41, | 49 [48]/50 | 62 [61]/60 | 30 [25]/364 769 |
| Sorenson (30) 2007 | Denmark | ERA-EDTA; Multi-national registry | 1994–2005 | 71 | DM; nDM | Tx/WL HD; PD [WL HD; PD] | 1,239/517 [4,737] | 41/49 [66] | − | 17/22 [24] |
| Cheawchanwattana (81) 2006 | Thailand | Single site | 2005 | None | − | Tx/HD; PD | 133/107; 62 | 45/45; 57 | 65/60; 55 | 23/18; 47 |
| Snyder (31) 2006 | USA | USRDS | 1995–2003 | − | DM; | Tx/WL D | 11,418/19,107 | 50–642 | 60/59 | 100 |
| nDM | 32,009/34,202 | 35–49 | 60/59 | 0 | ||||||
| Yildirim (92) 2006 | Turkey | Multiple sites | 2004–2005 | None | − | Tx/HD; PD | 356/104; 186 | 44/55; 46 | 56/46; 53 | − |
| Zimmerman (55) 2006 | Brazil | Single site | 1996–1997 | None3 | − | Tx/HD | 64/40 | 45 | 55 | − |
| Borentain (25) 2005 | France | Single site | 1995–2001 | 4 | PTCA | Tx/D | 37/75 | 52/61 | 95/69 | 11/32 |
| Buturovic-Ponikvar (99) 2005 | Slovenia | Multiple sites | 2003 | 1 | − | Tx/HD; PD | 374/1171; 116 | − | − | −/19 |
| Ho (68) 2005 | China | Regional registry | 1995–2004 | 7 | − | Tx/HD; PD | 3,174/1,119; 5,149 | 49–552 | ∼59/52; 49 | 12/23; 39 |
| Lee (83) 2005 | Wales | Single site | 2002 | None | − | Tx/HD; PD | 209/99; 74 | 53/63; 59 | 60/61; 51 | − |
| Massad (26) 2005 | USA | Single site | 1992–2004 | − | CABG | Tx/HD; PD | 32/62 | 58/58 | 59/63 | 50/76 |
| Merion (17) 2005 | USA | National registry | 1995–2002 | 10 | ECD; | CTx; LTx/D | 7,790 [41,052]; | 40–59 [40–59]; −/40–592 | 62 [61]; −/58 | 27 [22]; −/354 |
| nECD | 15,203/45,082 | |||||||||
| Niu (100) 2005 | Taiwan | Two sites | 2002 | None | − | Tx/HD; PD | 80/80; 80 | 43/55; 51 | 44/40; 43 | − |
| Oniscu (49) 2005 | UK | Multiple regional registries | 1989–1999 | 3 | − | CTx/WL HD; PD | 1,095/641 | 43/53 | 61/62 | 12/194 |
| Schaubel (101) 2005 | USA | National registry | 1999 | 3 | − | CTx; LTx/D | 7,773/8,011 | − | − | − |
| Abbott (15) 2004 | USA | USRDS | 1995–2000 | 6 | HCV+donor; HCV–donor | CAD/WL D | 389; 16,495/17,044 | 51; 46/50 | 75; 63/60 | 30; 34/404 |
| Moe (102) 2004 | USA | Single site | − | 2 | − | Tx/HD | 38/30 | 45/55 | 76/61 | 37/94 |
| Oniscu (46) 2004 | UK | Multiple regional registries | 1989–1999 | 11 | ≥60 years | Tx/HD; PD | 128/197 | 64/66 | 65/59 | 13/16 |
| Schon (103) 2004 | Sweden | National registry | 1991–2002 | 12 | − | CTx; LTx/HD; PD | 721/3,052; 1,294 | − | − | 17/33; 35 |
| Sezer (13) 2004 | Turkey | Single site | <1996 | 5 | nDM: HCV+ HCV– | CTx; LTx/HD CTx; LTx/HD | 116/136 | 34/43 | 71/60 | 0 |
| Abbott (104) 2003 | USA | USRDS | 1994–1997 | − | − | Tx/HD; PD | 19,033/16,182 | 43/47 | 61/58 | 31/364 |
| Brunkhorst (19) 2003 | Germany | Regional registry | 1978–1996 | 19 | ESRD-DM; DM1 | DM1; DN | 46/46 | 43/45 | 69/69 | 100 |
| Glanton (35) 2003 | USA | USRDS | 1995–1999 | 7 | Obese | CTx; LTx/HD; PD | 1,719; 552/5,172 | 48 | 54 | 40 |
| nObese | CTx; LTx/HD; PD | 4,795; 1,528/16,896 | ||||||||
| McDonald (40) 2003 | Australia; New Zealand | ANZDATA | 1991–2000 | 9 | nIndigenous; ATSI; | Tx/HD; PD | 12,984 | 602 | 59 | 174 |
| Pacific; | 1,061 | 48 | 43 | 47 | ||||||
| Maori | 502 | 51 | 48 | 55 | ||||||
| 935 | 44 | 57 | 63 | |||||||
| Tomasz (105) 2003 | Poland | Two sites | − | None | − | Tx/HD | 83/61 | 43/58 | 52/56 | − |
| Baiardi (80) 2002 | Italy | Single site | 1997–1999 | None3 | − | Tx/HD; PD | 34/171; 30 | 44/62; 64 | 65/63; 63 | 3/6; 7 |
| McDonald (48) 2002 A | Australia; New Zealand | ANZDATA | 1991–2001 | 10 | − | CTx/WL HD; PD [nWL HD; PD] | 2,362/2,782 [5,052] | 44/46 [53] | 63/58 [53] | 89/75 [50] |
| McDonald (106) 2002 B | Australia; New Zealand | ANZDATA | 2000 | 1 | Australia; New Zealand | Tx/HD; PD | 1,764 417 | − | − | − |
| Piccoli (107) 2002 | Italy | Regional registry | 1995–1999 | None | nDM | Tx;GF/HD; PD | 92; 40/56 | 51; 52/60 | 59; 73/54 | 0 |
| Abbott (18) 2001 | USA | USRDS | 1994–1997 | 21 | ESRD-DM | Tx/HD; PD | 5,683/5,686 | 47 | 60 | 100 |
| Bakewell (39) 2001 | UK | Single site | 1998 | None | South Asian; White | Tx/HD; PD | 40/40; 40 | 46/53; 49 | 70/65; 70 | 10/30; 30 |
| Rebollo (45) 2001 | Spain | Multiple sites | − | None | <65 years | Tx/HD | 213/116 | 49/56 | 66/58 | 7/10 |
| Schmidt (24) 2001 | Austria | Single site | 1994–1998 | 5 | PTCA | Tx/HD | 42/42 | 59/57 | 81/76 | 17/26 |
| Staathof-Galema (79) 2001 | Netherlands | Two sites | 1990–1996 | 7 | − | CTx; LTx/HD | 54/102 | 50/48 | 61/63 | 3/3 |
| Choi (32) 2000 | South Korea | Single site | − | 0.5 | DM; nDM | Tx/PD | 71/101 | 59/58 | − | 30/18 |
| Fujisawa (82) 2000 | Japan | Two sites | − | None | − | CTx; LTx/WL HD [nWL HD] | 117/49 [65] | 44/46 [46] | 43/76 [69] | 4/4[5] |
| Johnson (44) 2000 | Australia | Single site | 1993–1997 | 6 | >60 years | CTx/D | 67/107 | 66/66 | 49/41 | 8/20 |
| Rabbat (50) 2000 | Canada | CORR | 1990–1994 | 6 | − | CTx/D | 722/1,156 | 44 | 63/62 | 18/224 |
| Rebollo (86) 2000 | Spain | Multiple sites | 1996 | None | − | CTx/HD | 210/170 | 51/67 | 67/51 | 7/124 |
| Ward (20) 2000 | USA | USRDS | 1987–1994 | 32 | Females: ESRD-Lupus | Tx/D | 946/3,431 | 36/40 | 0 | − |
| ESRD | 5,173/66,202 | 41/61 | 1004 | |||||||
| ESRD | 12,733/100,980 | 43/62 | − | |||||||
| Mazzuchi (53) 1999 | Uruguay | Multiple sites | 1981–1998 | 10 | − | Tx/HD | 460/695 | 37/60 | 67/63 | 7/17 |
| Wolfe (62) 1999 | USA | USRDS | 1991–1996 | 7 | − | CTx/D | 23,275/22,889 | 40–59/40–592 | 63/58 | 31/354 |
| Lim (73) 1998 | Malaysia | National registry | 1992–1995 | 4 | − | Tx/HD[Home]; PD | 909/1,260; 307 | 34/42; 46 | 57/66; 54 | 9/26; 34 |
| Schnuelle (8) 1998 | Germany | Regional registry | 1989–1997 | 8 | − | Tx/HD | 144/167 | 48/44 | 60/62 | 5/14 |
| Segoloni (78) 1998 | Italy | Single site | 1992–1996 | 5 | − | Tx/WL D | 344/916 | 45/46 | 60/64 | 1/24 |
| Spencer (41) 1998 | Australia | − | 1978–1996 | 18 | Indigenous; nIndigenous | Tx/D | 56/158 | 45 | 46 | 41 |
| Wight (87) 1998 | UK | Single site | 1995 | None | − | Tx/HD; PD; Home; Sat | 228/100; 109; 42; 41 | 40–49/50–59; 50–59; 40–49; 60–692 | 62/56; 57; 69; 59 | 10/11; 22; 0; 204 |
| Bonal (56) 1997 | Spain | Regional registry | 1984–1993 | 10 | − | CTx/HD | 157/395 | 62/61 | 58/62 | 04 |
| Lui (108) 1997 | China | Regional registry | 1995–1996 | 1 | − | CTx; LTx/HD; PD | 957/495; 1,885 | 41/48; 57 | 57/52; 50 | 6/11; 24 |
| Ozminkowski (85) 1997 | USA | National registry | 1992–1995 | None | − | Tx/D | 211/304 | 45–592 | 54 | 294 |
| Boni (63) 1996 | Brazil | Single site | − | 3 | − | CTx; LTx/HD; PD | 36/36 | 33/33 | 67/61 | 3/34 |
| Agodoa (109) 1995 | USA | USRDS | 1980–1990 | − | − | CTx; LTx/HD; Home; Self; PD | 9,065/121,987 | 60–642 | 622 | 30 |
| Disney (66) 1995 | Australia | ANZDATA | 1983–1992 | 3 | − | Tx/HD; PD | 4,381/6,635 | −/45–642 | −/54–602 | 8–264 |
| Locatelli (61) 1995 | Italy | Regional registry | 1983–1992 | − | − | Tx/HD; PD | 1,450/6,823 | 57 | 58 | 3–74 |
| Schaubel (54) 1995 | Canada | CORR | 1987–1993 | 7 | − | Tx/D | 284/6,116 | 60–69/60–692 | 66/58 | 12/22 |
| Teraoka (110) 1995 | Japan | Multiple society registries | 1964–2002 | 9 | − | CTx; LTx/HD; Home; Night; PD | 6,367/123,926 | − | − | − |
| Cowie (21) 1994 | USA | Regional registry | 1974–1983 | 9 | <65 years: | Tx/D | 129/117 | 35 | 62 | 100 |
| ESRD-DM1; | 50/298 | 55 | 49 | 100 | ||||||
| ESRD-DM2 | ||||||||||
| El-Reshaid (111) 1994 | Kuwait | Multiple sites | 1986–1990 | 5 | − | CTx; LTx/HD; PD | 289/358 | 452 | 64 | 154 |
| Ojo (38) 1994 | USA | Multiple regional registries | 1984–1989 | 6 | Black | CTx/D | 236/554 | 40/40 | 66/66 | 25/24 |
| Pugh (42) 1994 | USA | Regional registry | 1975–1985 | − | nHispanic White; | Tx/HD; Home; PD | 649/4,731 | 50–592 | − | 224 |
| Mexican-Amercian; | 264/2,898 | 50–59 | 44 | |||||||
| Black | 261/3,160 | 50–59 | 20 | |||||||
| Del Pilar Garofano Plazas (112) 1993 | Spain | Multiple sites | − | None | − | Tx/HD | 45/111 | 38/54 | 56/50 | 0/74 |
| Port (7) 1993 | USA | Multiple regional registries | 1984–1989 | 6 | <65 years | CTx/WL D [nWL D] | 799/770 [3,451] | − | 60 [−] | |
| Brunori (113) 1992 | Italy | Single site | 1981–1990 | 10 | − | CTx/HD; PD | 228/188; 232 | 36/53 | − | − |
| Alamartine (57) 1991 | France | Single site | 1973–1987 | 15 | − | Tx/D | 120/158 | 45 | 38/51 | − |
| Julius (114) 1989 | USA | Regional registry | 1981–1984 | None | − | CTx; LTx/HD; PD | 83; 80/171; 125 | 41–60; 20–40/41–60; 41–602 | 52; 61/49; 54 | 25; 34/26; 174 |
| Kjellstrand (115) 1989 | USA | Two sites | 1963–1985 | − | − | Tx/D | 2,579/2,004 | − | − | − |
| Petrie (116) 1989 | New Zealand | Single site | − | None | − | CTx/HD; PD | 30/75 | 39/43 | 67/55 | −. |
| Silins (75) 1989 | Canada | National registry | 1981–1986 | 5 | − | Tx/D | 2,436/5,996 | 45–642 | 61 | 184 |
| Fauchald (67) 1988 | Norway | Multiple sites | 1981–1985 | 6 | − | CTx; LTx/WL D [nWL D] | 122/127 [119] | 66/66 [70] | 69 | − |
| Morris (117) 1988 | UK | Single site | − | None | − | Tx/HD; Home; PD | 69/69 | 49/54 | 40/34 | − |
| Burton (51) 1987 | UK | Single site | 1974–1985 | 11 | − | CTx; LTx/HD; PD | 183/276; 158 | 38/42; 51 | 64/63; 58 | 5/4; 124 |
| Churchill (118) 1987 | Canada | Single site | − | None3 | − | Tx/HD; Home; PD | 79/42; 42; 31 | − | − | − |
| Khauli (22) 1986 | USA | Single site | 1976–1982 | 8 | ESRD-DM; DM1 | CTx; LTx/D | 52/48 | 34/41 | − | 100 |
| Khauli (33) 19865 | USA | Single site | 1976–1982 | 8 | DM | CTx; LTx/HD; PD | 48/52 | 33/42 | − | 100 |
| Evans (119) 1985 | USA | Multiple sites | − | None | − | CTx; LTx/HD; Home; PD | 144/347; 287; 81 | 37/52; 47; 50 | 48/50; 64; 46 | 8/10; 8; 16 |
| Garcia-Garcia (58) 1985 | USA | Single site | 1973–1981 | 9 | − | CTx; LTx/D | 103/238 | 47 | 45 | 28/29 |
| Minetti (120) 1985 | Italy | − | 1972–1985 | 14 | − | CTx/HD WL | 284/211 | − | − | − |
| Hellerstedt (121) 1984 | USA | Multiple sites | 1978–1983 | 6 | − | CTx; LTx/HD; Home; PD | 1,187/1,999 | 21–45/46–602 | 62/55 | − |
| Zimmerman (34) 1984 | USA | Two sites | 1971–1983 | − | DM | CTx; LTx/HD; PD | 36; 37/66 | 37; 31/48 | − | 100 |
| Krakauer (69) 1983 | USA | National registry | 1977–1980 | − | − | CTx; LTx/D | 7,595; 3,491/65,270 | 31–40; 21–30/>502 | 63; 60/56 | 7; 8/94 |
| Kramer (23) 1983 | Europe | EDTA-ERA | 1976–1981 | − | ESRD-Lupus; | Tx; GF/D | 120; 48/815 | − | − | − |
| ESRD-PCKD | 1,085; 407/6,293 | |||||||||
| Vollmer (60) 1983 | USA | Multiple sites | 1960–1979 | 19 | − | CTx; LTx/D | 255/594 | 45–542 | 55 | 114 |
| Cestero (122) 1980 | USA | Multiple sites | 1967–1978 | − | − | Tx/HD; Home | 150/299; 109 | 43–48 | − | − |
| Nicholls (123) 1980 | UK | − | 1968–1978 | 10 | − | CTx/D | 48/50 | 37 | − | − |
| Avram (124) 1979 | USA | Single site | 1973–1978 | 5 | − | CTx; LTx/DM D [nDM D] | 69/122 [482] | − | 75/63 [57] | 6/100 [0] |
| Dumler (125) 1979 | USA | Single site | 1972–1978 | 6 | − | CTx: LTx/HD | 140/443 | 40/62 | − | 0 |
| Hull (126) 1979 | USA | Single site | ≥1970 | 5 | − | CTx; LTx/D | 248/605 | − | 56 | − |
| Bonney (127) 1978 | USA | Multiple sites | 1967–1975 | 8 | − | CTx/HD; Home | 84/328; 41 | 30/43; 46 | − | 0/30; 24 |
| Golper (128) 1978 | USA | Single site | 1971–1977 | 7 | >45 years | CTx/D | 30/51 | 51/51 | − | 3 |
| Kreis (71) 1978 | France | Single site | 1962–1976 | 7 | − | CTx/HD; Home | 272/440; 210 | 30/<50 | − | − |
| Price (74) 1978 | Canada | Single site | 1964–1976 | 12 | − | CTx/HD; Home; PD | 305 | 38 | 67 | − |
| Cantaluppi (76) 1977 | Italy | Single site | ≥1972 | 5 | − | CTx; LTx/HD; Home | 66/61 | 34/37 | 71/66 | − |
| Higgins (129) 1977 | Canada | Single site | 1962–1975 | − | − | Tx/HD | 87/55 | 31 | 62 | 24 |
| Rao (29) 1977 | USA | Single site | 1972–1976 | 5 | DM | CTx; LTx/HD | 160/60 | 35/40 | 64/57 | 100 |
| Bonomini (64) 1975 | Italy | Single site | 1966–1974 | 9 | − | CTx; LTx/D | 39/113 | − | − | − |
| Krumlovsky (72) 1975 | USA | Single site | 1970–1974 | 5 | − | CTx; LTx/HD | 85/92 | − | − | − |
| Lowrie (130) 1973 | USA | − | 1964–1972 | 9 | − | CTx; LPTx; LSTx/Home | 112; 93; 79/125 | 38; 24; 36/44 | 62; 71; 58/73 | 1; 1; 0/54 |
- USA = United States of America; GOV = government; UK = United Kingdom; UKRR = United Kingdom Renal Registry; ERA-EDTA = European Renal Association-European Dialysis and Transplant Association; USRDS = United States Renal Data System; ANZDATA = Australia and New Zealand Dialysis and Transplant Registry; CORR = Canadian Organ Replacement Register; WL = waitlisted; nWL = not waitlisted; ECD = extended-criteria donor; HBV+= hepatitus B virus positive; HCV+= hepatitis C virus positive; Hep–= hepatitis negative; DM = diabetes mellitus; nDM = no diabetes mellitus; PTCA = percutaneous transluminal coronary angioplasty; CABG = coronary artery bypass graft; nECD = not expanded criteria donor; HCV+ donor = hepatitis C virus donor; HCV– donor = hepatitis C virus negative donor; HCV–= hepatitus C virus negative; ESRD-DM = end-stage renal disease caused by diabetes mellitus; DM1 = diabetes mellitus type 1; nObese = not obese; nIndigenous = non-indigenous; ATSI = aboriginal and Torres strait islander; ESRD-Lupus end-stage renal disease caused by lupus; ESRD-Other = end-stage renal disease caused by something other than diabetes mellitus or lupus; ESRD-DM2 = end-stage renal disease caused by diabetes mellitus type 2; nHispanic = non-Hispanic; ESRD-PCKD = end-stage renal disease caused by polycystic kidney disease; CTx = cadaveric donor transplantation; LTx = living donor transplantation; HD = hemodiaylsis; PD = peritoneal dialysis; Tx = trasnplantation; GF = graft failure dialysis; Home = home hemodialysis; Self = self hemodialysis; Sat = dialysis at a satellite unit; Night = nocturnal hemodialysis; D = dialysis; LPTx = living parent donor transplantation; LSTx = living sibling donor transplantation. The studies are sorted by year and the first author's last name.
- 1Mean.
- 2Median.
- 3There was a second interview.
- 4Cause of end-stage renal disease.
- 5This study used the same transplantation group as the other Khauli 1986 study.
- 6Quality-of-life studies were not excluded if they had cross-sectional designs.
Populations studied
Dialysis modalities considered in eligible studies included in-center hemodialysis, satellite hemodialysis, home hemodialysis, nocturnal dialysis, peritoneal dialysis and multiple modalities (populations including both hemodialysis and peritoneal dialysis). Transplantation used allografts from deceased and living donors.
Mean age of transplant recipients in the included studies ranged from 30 to 68 years (median 44); the majority of patients were male (median 62%). We collected data on the proportion of patients in each group who had diabetes, coronary artery disease, hypertension, heart failure, stroke, lung disease, peripheral vascular disease, malignancy, infectious disease and smoking status. Unfortunately, these data were reported very infrequently and so are not reported here. Characteristics that were explored by the included studies as potential determinants of the benefit of transplantation were hepatitis B and/or hepatitis C virus serostatus of the recipient (13,14) and donor (15), extended-criteria donor (16,17), primary cause of ESRD (18–23), findings of coronary angiography (24,25), coronary artery bypass surgery (26), a low-protein diet (27), cancer (28), diabetes (22,29–34), obesity (35), employment (36), race (37–42) and age (7,43–47).
Risk of bias assessment
All eligible studies used a cohort design: 26% were prospective, 36% were based on registries with prospectively collected data, 2% were ambispective (both prospective and retrospective data collection), 18% were retrospective and 13% did not specify the relative timing of hypothesis generation and data collection. The risk of bias of included studies is reported in Table S2 and summarized in Figure 2.

Risk of bias of included studies. The responses for each question in this risk of bias tool are represented by different colors, segmented along a horizontal bar. Light gray depicts the percent of studies responding with the smallest risk of bias. Medium gray depicts the percent of studies responding with the greatest risk of bias. Dark gray indicates a moderate or unclear risk of bias. The responses to “Study design?” are prospective, registry and retrospective. The responses to “Waitlisted?” and “Contemporaneous groups?” are yes, unclear and no. The responses to “Population described?” and “Model adjustment?” are yes, partial and no.
Mortality
In all, 163 cohorts (77 studies; 1 800 119 participants) reported unadjusted comparisons of mortality associated with transplantation as compared with dialysis. Of these, 76% found a significantly lower risk of death associated with transplantation and 7% found a significantly lower risk of death associated with dialysis (Figure S1).
Six studies (7,38,46,48–50; 19 945 participants) reported adjusted relative hazards for all-cause mortality in discrete time periods (Figure 3). During the period immediately after transplantation (five studies reported a time period of <30 days and one <3 months [48]), mortality tended to be significantly greater in transplant recipients (HR range 0.9–5.03). These studies also reported the relative hazard of mortality at 1 year after transplantation, which in all cases, was significantly lower among transplant recipients (HR range 0.19–0.49).
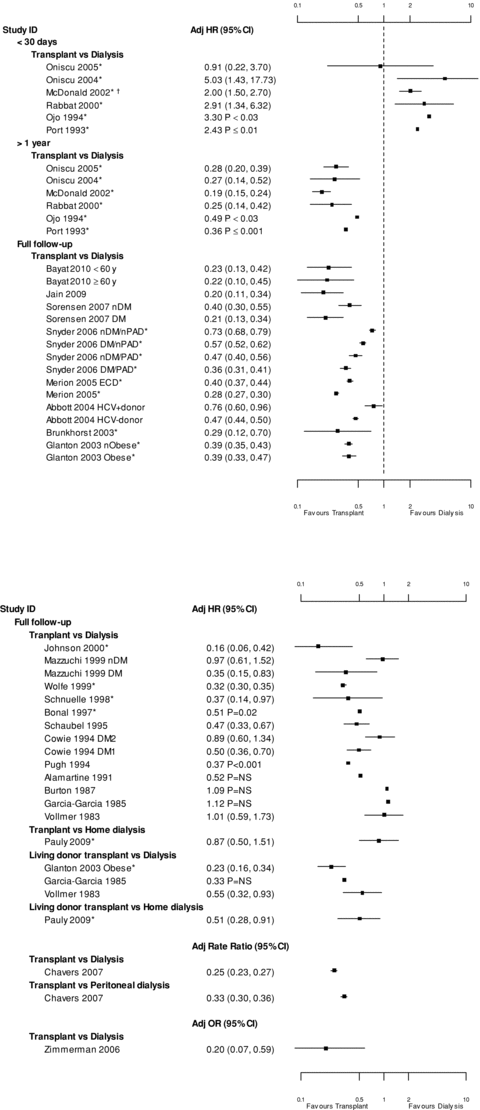
Adjusted ratio of all-cause mortality by time period.*Indicate waitlisted dialysis groups. †0–3 months of follow-up. Tx = transplant; D = dialysis; nDM = no diabetes mellitus; DM2 = type 2 diabetes mellitus; HepCdonor = hepatitis C donor; nDM/nPAD = no diabetes mellitus/no peripheral arterial disease; DM/nPAD = diabetes mellitus/no peripheral arterial disease; DM1 = type 1 diabetes mellitus; nDM/PAD = no diabetes mellitus/peripheral arterial disease; HCV + donor = hepatitis C donor; HCV – donor = hepatitis C negative donor; nDM = no diabetes mellitus; ECD = extended criteria donor; nObese = not obese; DM/PAD = diabetes mellitus/peripheral arterial disease; DM = diabetes mellitus; Adj HR = adjusted hazard ratio; Adj OR = adjusted odds ratio; CI = confidence interval; NS = not significant; ESRD = end-stage renal disease; RRT = renal replacement therapy. Oniscu 2005 adjusted for age, gender, ESRD cause, social deprivation, time on RRT and comorbidities. Oniscu 2004 adjusted for age, gender, ESRD cause, social deprivation, dialysis modality and distance to center. MacDonald 2002 adjusted for age and comorbidities. Rabbat 2000 adjusted for age, gender, race, time on RRT and ESRD cause. Ojo 1994 adjusted for age, gender and ESRD cause. Port 1993 adjusted for age, gender, race, ESRD cause and era. Bayat 2010 adjusted for age, BMI, comorbidities, albumin and urgent first dialysis session. Jain 2009 adjusted for age, race, ESRD cause, comorbidities, dialysis modality and time on RRT. Pauly 2009 adjusted for age, gender, comorbidities, era; matched on race, DM and time on RRT. Sorenson 2007 adjusted for age, gender and era. Synder 2006 adjusted for age, gender, race, ethnicity, BMI, ESRD cause, comorbidities and time on RRT. Merion 2005 adjusted for age, gender, race, ethnicity, blood type, ESRD cause, comorbidities at waitlist, era, panel reactive antibody values, dialysis modality, time from first RRT to waitlist and donation service area for the organ procurement organization. Abbott 2004 adjusted for age, race, BMI, smoking, ESRD cause, comorbidities, era, albumin and access complications. Brunkhorst 2003 matched for age, gender, era, time on RRT and time with DM. Glanton 2003 adjusted for age, race, BMI, cause of ESRD, comorbidities, era and albumin. Johnson 2000 adjusted for time on RRT. Mazzuchi 1999 adjusted for age, gender, alcoholism and comorbidities. Wolfe 1999 adjusted for age, sex, race, ESRD cause, era and time from first RRT to waitlist. Schnuelle 1998 adjusted for age, gender, BMI, comorbidities, residual urine volume, number of previous transplants performed and time on RRT. Bonal 1997 adjusted age, gender and comorbidities. Schaubel 1995 matched on age, ESRD cause and number of comorbidities. Cowie 1994 adjusted for age, gender, weight, era, comorbidities and blood pressure. Pugh 1994 adjusted for age, race, ESRD cause and center size. Garcia-Garcia 1985 adjusted for age, gender, race, ESRD cause, comorbidities, era, age of DM onset, insurance and age at transplantation. Vollmer 1983 adjusted for age, number of comorbidities and era. Burton 1987 did not report what they adjusted for. Chavers 2007 adjusted for age, gender, race, era, ESRD cause and time on RRT. Zimmerman 2006 adjusted for age and gender.
Thirty-eight cohorts (8,15,17,19,21,30,31,35,37,42–44,51–61; 23 studies; 904 610 participants) reported adjusted relative hazards, rates or odds for mortality during total follow-up after transplantation (maximum follow up ranged 6–19 years; Figure 3). Seventy-nine percent of these results significantly favored lower mortality in the transplantation groups (HR range 0.16–0.76) and 21% were nonsignificant (HR range 0.33–1.12). These studies also included perioperative deaths, and thus, demonstrate that the higher short-term risk of death associated with kidney transplantation is more than offset by the lower risk of mortality during subsequent follow up. In the subset of studies including only waitlisted participants (10 studies; 474 522 participants), 94% of comparisons significantly favored lower mortality for transplant recipients (15 of 16 comparisons; HR range 0.16–0.73).
Three studies (35,58,60) reported relative hazards separately for living donor transplantation and for deceased donor transplantation, and found that the relative hazards of transplantation, compared to dialysis, tended to be lower for analyses of living donors (HR 0.23, 0.33 and 0.55) than for analyses of deceased donors (0.39, 1.12 and 1.01, respectively). However, given the small number of studies, no firm conclusions can be drawn from these results.
Evidence of effect modification for mortality
In multivariable meta-regression of 20 studies (13,27,51–55,63–74; 865 208 participants), we found that the unadjusted relative risk of mortality associated with transplantation (compared with dialysis) was lower in more recently performed studies (p < 0.001; Figure 4, panel A). Results were similar when a subset of 10 studies (7,19,46,56,76–79), including only 97 873 dialysis patients who had been placed on the transplant waiting list, were considered (p = 0.12; Figure 4, panel B). Variables assessing risk of bias (i.e. study design [prospective, registry, etc.], adequate description of population, prevalent vs. incident patients, censoring at modality switch and funding) were not significantly associated with the benefit of transplantation in meta-regression. Other variables were not tested as they were not relevant to the unadjusted mortality results (e.g. outcome definitions, adjusted model) or they did not sufficiently differ in value (e.g. contemporaneous groups).

Meta-regression line for year of study publication and mortality risk ratio associated with transplantation, compared with dialysis patients. The mortality risk ratio is plotted against the year of study publication. Circles are the observed estimates; size is based on the inverse of the standard error of each study. The three lines are the fitted and the upper and lower bounds of the 95% confidence intervals. Panel (A) compares transplant patients with dialysis patients who may or may not have been active on the transplant waiting list and panel (B) compares transplant patients with dialysis patients who had been placed on the transplant waiting list. The era of study publication was significantly associated with the risk of mortality associated with transplantation, indicating that the relative magnitude of the benefit increased over time (panel A, p < 0.001). A similar trend was shown in Panel B (p = 0.12). RR, risk ratio.
Limited information was available on characteristics modifying the association between transplantation and mortality. One study (60) tested whether age, the number of associated diseases and dialysis vintage affected the association between transplantation and mortality, but found no significant evidence of effect modification. Another study (61) also tested the association between age and the mortality benefit of transplantation, but found no significant effect modification. A third study (31) found that the reduction in mortality associated with transplantation was greater among patients with peripheral arterial disease than in those without. We found no evidence that the association between transplantation and mortality was modified by dialytic modality.
Overall, there was no consistent association between markers of study risk of bias and the magnitude of the mortality reduction associated with transplantation. However, the benefits of transplantation seemed less pronounced in the subset of studies that were restricted to patients who were waitlisted for transplantation.
Meta-regression did not identify factors that significantly modified the association between transplantation and clinical outcomes other than mortality.
Cardiovascular events and hospitalization
In unadjusted analyses, four of six cohorts (19,20,25,33; four studies; 189 769 participants) found that transplantation significantly reduced the risk of myocardial infarction; two of five cohorts (19,20,56; three studies; 190 109 participants) found that transplantation significantly reduced the risk of stroke and two further studies found that transplantation significantly reduced the risk of heart failure (11 369 participants [18]) and the incidence of ischemic heart disease (552 participants [56]). Only two studies reported adjusted analyses for cardiac events. One (19; 92 participants) found that transplantation from a deceased donor significantly reduced the rate of cardiac events by 76% (relative rate 0.24, 95% CI: 0.07–0.81) and the other (18; 11 369 participants) found no association between transplantation and heart failure. The risk of infection-related hospitalization seemed to be significantly decreased among transplant recipients, with both cohorts (one study; 336 986 participants) favoring transplantation. Results for all-cause hospitalization were more equivocal, with only 5 of 10 cohorts (six studies; 354 256 participants) significantly favoring transplantation. Results of these analyses are summarized in Figure 5.

Ratios of cardiac events and hospitalizations among transplant recipients, compared to dialysis patients. The small solid diamonds represent the ratios (risk, hazard, rate) of cardiac events and hospitalizations between transplant patients and either hemodialysis and/or peritoneal dialysis patients (for each individual study). The black-colored diamonds are adjusted ratios; the gray-colored diamonds are unadjusted ratios. The shaded gray region denotes values between 0.80 and 1.25. Data points outside the gray region represent large differences between transplant patients and dialysis patients (ratio ≥ 20% reduction or improvement). Some studies contributed more than one cohort (datapoint) to analyses.
Quality of life
In unadjusted analyses comparing QoL using the SF-36 between transplant recipients and dialysis patients, 47–100% of cohorts (55,80–87; depending on the domain considered), significantly favored the transplantation groups and none significantly favored the dialysis groups. Three cohorts (80,88; two studies; 497 participants) reported the adjusted association between transplantation and SF-36 scores as compared with dialysis. The vast majority of analyses significantly favored transplantation over hemodialysis. One small cohort (80; 64 participants) comparing transplantation to peritoneal dialysis was not significantly different for any of the domains reported. These findings are summarized in Figure 6. Results were broadly similar for unadjusted and adjusted analyses comparing QoL assessed using other instruments including EQ-5D, Karnofsky Performance Index, SIP, GHQ, WHO-QoL, 15D and TTO (data not shown).
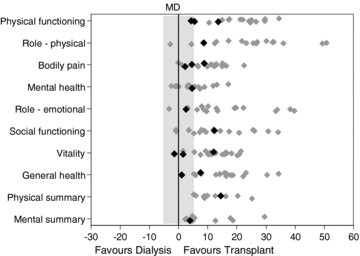
Mean differences in domains of the short form health survey (SF-36) among transplant recipients, compared to dialysis patients. The small solid gray diamonds represent the mean difference in SF-36 domains between transplant patients and either hemodialysis and/or peritoneal dialysis patients (for each individual study). The black-colored diamonds are adjusted mean differences; the gray-colored diamonds are unadjusted mean differences. The shaded gray region denotes the minimal clinical important difference for SF-36 domains; Samsa et al. (131) found that a difference between 3 and 5 was clinically important. Data points outside the gray region represent large differences between transplant patients and dialysis patients (mean difference ≥5). Some studies contributed more than one cohort (datapoint) to analyses. SF-36, short form health survey-36 items; MD, mean difference.
Discussion
In this systematic review of 110 studies including a total of 1 961 904 participants with kidney failure, kidney transplantation was associated with reduced risk of mortality and cardiovascular events as well as better QoL than treatment with chronic dialysis. Results were consistent for different dialysis modalities, for transplantation from both deceased and living donors and across countries with differing health care systems. These results confirm that kidney transplantation is the preferred modality of treatment for chronic kidney failure, and justifies current attempts to increase the number of patients worldwide who benefit from kidney transplantation—by increasing rates of deceased and living kidney donation, expanding the pool of potential donors and recipients and reducing the likelihood that potentially viable organs are discarded.
As expected, mortality among transplant recipients increased sharply as compared with those remaining on dialysis during the perioperative period (62). Thereafter, mortality among transplant recipients significantly declined such that cumulative mortality associated with transplantation was significantly lower than among patients treated with dialysis (62,89). Contemporary dialysis patients have more comorbidity and must wait longer for transplantation than patients awaiting kidney transplantation in previous years (6). Despite this, our results suggest that the relative benefit of transplantation may have significantly increased over time, compared to remaining on dialysis. For example, the unadjusted relative risk of mortality associated with transplantation (compared with dialysis) decreased from 0.44 in 1985 to 0.17 in 2005. This finding may relate to use of more potent immunosuppression, better management of comorbid medical conditions, more careful selection of transplant recipients or perhaps, to highe mortality in more recent years among the comparator pool of patients remaining on dialysis (89–91). However, because our study did not identify the explanation for this finding, these suggestions are speculative. Alternatively, because the apparently greater benefit of transplantation in more recent years was based on unadjusted analyses, it is possible that confounding by comorbidity or other characteristics wholly or partially explains this finding. Finally, we used the median year of each study's accrual period as a proxy for era of transplantation—which may have led to the ecological fallacy. Therefore, although appealing, our conclusion that the relative benefit of transplantation has increased over time should be viewed with caution.
Immunosuppressive medications used in transplant recipients may cause anemia, hypertension, glucose intolerance and dyslipidemia (91). Despite this, in analyses based on a total of 10 studies (333 881 patients), we found that transplantation was associated with significantly lower risk of cardiovascular events compared to treatment with dialysis (18–20,25,33,56). Although this finding might be partially because of selection of healthier patients for transplantation, results were similar in analyses restricted to dialysis patients who were active on the transplant waiting list—which should minimize the effect of such bias. Although immunosuppressive medications can predispose to infection, our results suggest that transplantation is associated with reduced risk of hospitalization for infection—emphasizing the high risk of sepsis associated with vascular and peritoneal access required to perform dialysis. We did not find clear evidence that transplant recipients were at lower risk of all-cause hospitalization than dialysis patients, perhaps because allograft recipients require hospitalization for transplantation itself as well as any surgical complications.
We found consistent and clinically relevant improvements in QoL associated with kidney transplantation (78,86,92). These results were consistent across a variety of settings, were preserved in adjusted analyses, and were observed for a broad range of QoL instruments (78,86,92,93). Because markedly reduced QoL is a hallmark of kidney failure, these improvements may be the most important benefit of kidney transplantation, compared with remaining on dialysis.
Although one of the major objectives of our study was to examine factors that modified the clinical benefits of transplantation (as compared to dialysis), we identified few data addressing this objective. However, findings from a single study (with 96 736 participants) suggested that the mortality reduction associated with transplantation was more pronounced in patients with peripheral arterial disease. Meta-regression was unhelpful for identifying factors that modified the association between transplantation and outcomes other than mortality.
To our knowledge, this is the most comprehensive and up-to-date summary of the potential benefits of kidney transplantation, as compared with dialysis. We used a carefully designed literature search and rigorous methods to capture and synthesize the results of 110 studies, published over a period of more than four decades. Our results clearly illustrate the clinical benefits of kidney transplantation. Together with the dismal outcomes associated with dialysis and the dramatic worldwide increases in projected waiting times for kidney transplantation among patients with kidney failure, these findings emphasize the urgent need to increase rates of deceased and living kidney donation (94).
As with all systematic reviews, the strength of our conclusions is dependent on the analysis of aggregate (study-level) data and the quality and availability of studies. In addition to assessing studies for bias using items from Downs and Black (11), we added specific items of bias pertinent to our question. Second, we found a significant increase in the benefit of kidney transplantation over time, perhaps because of improvements in the care of transplant recipients. Unfortunately, despite our best efforts, we were unable to identify patient-level or study-level factors that were associated with greater or lesser benefit from transplantation. Third, although we sought to determine a summary estimate of the benefit of kidney transplantation for clinically relevant outcomes, there was substantial heterogeneity in all pooled analyses (I2≥ 97%), making the validity of meta-analysis questionable. Despite use of meta-regression, we were unable to explain the source of this heterogeneity—which may be because of the wide range of renal replacement modalities we included, to the observational nature of the various studies, to the ecological fallacy (95) or to the fact that some (but not all) studies included dialysis patients who were not eligible for transplantation (selection bias). However, the magnitude and consistency of the benefit associated with transplantation across analyses leave little doubt that transplantation is beneficial as compared with dialysis, even if this benefit cannot be easily conveyed in a single summary measure. Finally, because multiple studies from certain countries were captured by our search, it is possible that a small minority of patients were included in more than one study. Because we did not statistically pool results across studies and because results were consistent for all analyses, this is unlikely to have affected our results.
Given the scarcity of renal allografts, future studies should explicitly seek to identify patient-level factors associated with the benefit of transplantation for outcomes other than mortality. For some outcomes (such as cardiovascular events or all-cause hospitalization), this could be achieved by patient-level meta-analysis (perhaps of registry data from different countries), which would increase statistical power and also reduce the risk of the ecological fallacy. Other outcomes (such as QoL) will require detailed prospective study, perhaps in conjunction with data collected to determine candidacy for transplantation. Because statistical adjustment for potential confounders will be critical for the success of such initiatives, consensus on the best way to measure and adjust for comorbidity, accruing after wait-listing for transplantation, would be an important next step.
In summary, we found that (as compared with dialysis), kidney transplantation is associated with substantial reductions in the risk of mortality and cardiovascular events, as well as clinically relevant improvements in QoL. Despite increases in the age and comorbidity of contemporary transplant recipients, the relative benefits of transplantation (as compared to remaining on dialysis) seem to be increasing over time. Given the current organ shortage, these findings validate current attempts to increase the number of people worldwide that benefit from kidney transplantation.
Acknowledgments
The authors sincerely thank Ellen Crumley for librarian support, Ghenette Houston for administrative support, Mohammad Karkhaneh, Natasha Krahn and Sophanny Tiv for additional reviewer support.
Funding source: This study was funded by the Canadian Institutes of Health Research. Drs. Tonelli and Klarenbach were supported by salary awards from the Alberta Heritage Foundation for Medical Research. Dr. Tonelli was also supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease. Drs. Tonelli and Klarenbach were supported by a joint initiative between Alberta Health and Wellness and the Universities of Alberta and Calgary. Dr. Gill was supported by a salary award from the Michael Smith Foundation. Dr. Knoll is supported by a University of Ottawa Chair in Clinical Transplantation Research.
Authors’ contributions
M.T., G.K., S.K., J.G. and Ms. N.W. contributed to the design of the study. N.W. performed the statistical analyses and managed the project. M.T. wrote the first draft of the manuscript. All authors contributed to data acquisition, the interpretation of results and critical revision of the article for intellectually important content.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




