Primary CMV Infections Are Common in Kidney Transplant Recipients After 6 Months Valganciclovir Prophylaxis
Abstract
Prolonging cytomegalovirus (CMV) prophylaxis in CMV seronegative recipients of a kidney from CMV seropositive donor (D+/R–) may reduce the incidence of late infections. We analyzed late-onset primary CMV infections after 6 months valganciclovir prophylaxis.
Data from all CMV D+/R– kidney transplant recipients between January 2004 and December 2008 at our center were analyzed. Patients with a functioning graft at 6 months after transplantation who received 6 months of valganciclovir prophylaxis 900 mg once daily were included (N = 127). CMV was diagnosed with quantitative PCR. Prophylaxis was completed in 119 patients. Prophylaxis was stopped at 3–5 months due to leukopenia or gastrointestinal side effects in eight patients. Late-onset primary CMV infection developed in 47/127 (37%) patients median 244 days after transplantation (range 150–655) and median 67 days after the cessation of prophylaxis (range 1–475). Four infections were asymptomatic. In others, symptoms included fever (N = 28), gastrointestinal symptoms (nausea, vomiting, diarrhea) (N = 24), respiratory tract symptoms (N = 12), and hepatopathy (N = 6). Median peak viral load was 13500 copies/mL (range 400–2 831 000). Recurrent CMV infection developed in 9/47 (19%) patients. No significant risk factors for CMV infection were identified.
Symptomatic primary CMV infections were commonly detected also after prolonged valganciclovir prophylaxis.
Introduction
Despite modern prevention and treatment strategies, infections caused by cytomegalovirus (CMV) in immunosuppressed organ transplant recipients still remain a considerable problem. In addition to increased costs, morbidity, and even mortality, CMV infections may also be associated with increased frequency of acute rejections (1) and reduced graft function and survival (2). CMV seronegative recipients of an organ from a seropositive donor (D+/R–) are at greatest risk of CMV infection, and without prophylaxis more than 50% of these high-risk patients will develop symptomatic infection (3). Thus, prophylaxis is recommended for this patient group for at least 3 months after transplantation (4). The most common agent used for prophylaxis nowadays is valganciclovir, a valine ester of ganciclovir with improved oral bioavailability. In addition to side effects, most commonly leukopenia, high costs limit the use of valganciclovir.
Although CMV infections can be effectively prevented by oral ganciclovir or valganciclovir (5,6), late-onset primary CMV disease occurs in approximately 18–31% of kidney transplant recipients after the cessation of 3 months prophylaxis (7–9). Some evidence suggests that prolonging prophylaxis up to 6 months may reduce the incidence of late-onset infections (7,10,11). However, preliminary study of our material showed a high frequency of late-onset primary CMV infections in 48% of patients despite prolonged 6 months prophylaxis with valganciclovir (12). Most of the infections were symptomatic, and most common symptoms were fever and gastrointestinal symptoms. Similar findings of increased frequency of tissue-invasive CMV infections, especially infections of the gastrointestinal tract, have been reported after valganciclovir prophylaxis (5,9).
All adult kidney transplant recipients in Finland with CMV D+/R– serostatus have received 6 months valganciclovir prophylaxis since the beginning of 2004. The aim of this study was to analyze the frequency and clinical course of late-onset primary CMV infections occurring after 6 months valganciclovir prophylaxis in Finnish kidney transplant recipients, and to identify possible risk factors for late-onset primary CMV infections with special emphasis on the intensity of immunosuppression.
Materials and Methods
Data from all Finnish CMV seronegative adult patients who received a kidney transplant from a CMV seropositive donor (D+/R–) after January 2004 were retrospectively analyzed. Patients with a functioning graft at six months after transplantation who received valganciclovir prophylaxis according our current policy of 6 months, and who had completed one year of follow-up were included (N = 127). The total number of adult kidney transplantations performed in Helsinki University Hospital between January 2004 and December 2008 was 868. Before 2004, valaciclovir was used for CMV prophylaxis in D+/R– recipients, and valganciclovir for 3 months was used only for a short period of time in a small number of patients in 2003. No data about the incidence of CMV infections in these historical cohorts were unfortunately available. Of the patients included in the study, 25 patients from Helsinki University Hospital nephrology clinic were also participants in our previous study of late-onset primary CMV infections (12).
All kidney transplantations in Finland are performed in Helsinki University Hospital Transplant Unit. After 3–12 weeks of follow-up at the transplant unit, patients are followed in respective local nephrology centers, from which follow-up data are routinely sent to a nationwide Finnish Kidney Transplant Registry. Patients were followed at the outpatient clinics in respective nephrology centers, which have individual protocols for the follow-up of transplant recipients. Generally, laboratory tests (including measurements of renal function and trough levels of cyclosporine or tacrolimus) were taken with 1–3 weeks intervals for the first six months, with 2–4 weeks interval until 12 months after transplantation, and with 4–8 weeks interval during the second posttransplant year. All patients were on maintenance dialysis before transplantation.
Baseline immunosuppression was usually a triple-drug regimen with Cyclosporine A, mycophenolate mofetil (MMF) and steroid. In immunologically high-risk patients (long waiting time, poor match and retransplantation) cyclosporine was replaced by tacrolimus, and/or induction therapy with basiliximab was administered. In the majority of patients with stable graft function and especially in patients with problems in glycemic control or osteoporosis, steroids were usually withdrawn slowly during the first or second posttransplant year. Biopsy-proven acute rejections of grade I-II (13,14) were treated with high-dose intravenous corticosteroids, and/or conversion of cyclosporine to tacrolimus.
All the patients included in this study were planned to receive oral valganciclovir prophylaxis for 6 months after transplantation (900 mg once daily if normal renal function). Intention to treat- population was included in the analysis. Compliance to valganciclovir was not tested. CMV infections were not routinely monitored during or after prophylaxis; samples for the detection of CMV were taken only in clinical suspicion of CMV infection (fever, respiratory tract symptoms, leukopenia, thrombocytopenia, hepatopathy, gastroenteritis and graft dysfunction), except for the 25 patients from Helsinki University Hospital Nephrology clinic, who were participants of a previous clinical study and in whom CMV-DNAemia was screened with a 2–6 weeks interval after the cessation of prophylaxis (12). CMV-DNAemia was diagnosed with quantitative PCR. Patients with a positive CMV DNAemia were defined as suffering from CMV infection, and symptoms at the time of diagnosis were described. CMV disease was not defined specifically, and the degree of fever, or levels of thrombocytopenia or leukopenia were not recorded. Laboratory diagnosis of CMV was made in three university hospital laboratories. Most of the samples were analyzed in the Helsinki University Hospital Department of Virology with a TaqMan based real-time quantitative plasma PCR (15), and also the two other laboratories used quantitative real-time plasma PCR methods. Samples were all analyzed in respective laboratories, and no samples were sent from other laboratories to Helsinki University Hospital Department of Virology. CMV infections were treated with IV ganciclovir or high-dose valganciclovir (900 mg twice daily if normal renal function), followed by 1–6 months secondary oral valganciclovir prophylaxis in most patients.
Baseline data and follow-up data from all risk patients were reviewed from The Finnish Kidney Transplant Registry, and clinical course and treatment of CMV infections were reviewed from patient charts. Baseline data included: primary renal disease leading to uraemia, length of pretransplant dialysis, cold ischemia time, HLA mismatch, recipient age and sex and delayed graft function as defined by the need of dialysis during the first posttransplant week. Follow-up data included: occurrence of acute rejection, kidney function as measured by plasma creatinine and estimated glomerular filtration rate (GFR) using the Cockcroft-Gault equation (16), trough levels and doses of cyclosporine and tacrolimus, steroid and MMF dose.
All data are expressed as median [interquartile range], unless otherwise indicated. Statistical significances between the groups were measured by the nonparametric Mann–Whitney's U-test and Fisher's exact test. Correlations between variables were analyzed with linear regression. Graft and patient survival probabilities were estimated by the Kaplan–Meier method, and differences between two groups were determined by the log rank test. Univariate and multivariate logistic regression was used to estimate risk factors (OR) for CMV infections. The doses and trough levels of immunosuppression, patient age and gender, renal function, delayed graft function, cold ischemia time, and HLA mismatch were included in the analysis of risk factors for CMV. The calculations were performed with SPSS statistical software (version 17.0, SPSS Inc, Chicago, IL). p-Values of <0.05 were considered significant.
Results
During the study period altogether 142/868 transplantations were performed from CMV seropositive donors to adult CMV seronegative recipients in Finland. Seven patients in the beginning of the study period who received either valaciclovir prophylaxis or valganciclovir prophylaxis for 3 months according to our old policies were excluded. Similarly, five patients who lost their graft before the end of valganciclovir prophylaxis were excluded from the analysis. Insufficient data were available from two patients, and one patient with simultaneous islet cell and kidney transplantation was excluded from the analysis. Altogether 127 patients were included in the study. Of the subjects included in the study, 42 had renal failure and uraemia due to diabetic nephropathy, 24 due to glomerulonephritis, 16 due to polycystic kidney disease and 45 due to other causes. Median length of pretransplant dialysis was 18 [22] months. Median HLA A, B/DR mismatch was 2/1, and median cold ischemia time 22 [5] hours. Altogether 17 patients received tacrolimus as baseline immunosuppression instead of cyclosporine, and 6 patients received induction therapy. Median follow-up was 43 months (range 12–68 months). Six patients died during the study period, and four patients lost their grafts and returned to dialysis. Reasons for deaths were: acute myocardial infarction, metastatic breast cancer, accidental hypothermia and traffic accident, respectively, and intracerebral hematoma in two patients. Reasons for graft losses were chronic rejection in three patients and severe hydronephrosis caused by inoperable hepatocellular carcinoma and peritoneal carcinomatosis in one patient. Deaths or graft losses were not associated with valganciclovir or CMV. Median plasma creatinine at 12 months after transplantation was 107 [46]μmol/L and median estimated GFR 72 [33] mL/min. Acute rejection was diagnosed in 10 patients (8%). All rejections were reversible with treatment with IV steroids or conversion from cyclosporine to tacrolimus.
Altogether 119 patients completed the 6 months valganciclovir prophylaxis. Prophylaxis was stopped prematurely 3–5.5 months after transplantation due to leukopenia in seven patients and due to gastrointestinal side effects in one patient. Prophylaxis doses were adjusted according to renal function, but no other dose reductions were reported. One patient received prolonged valganciclovir prophylaxis for 8 months due to prolonged unexplained fever and bacterial infections, and one patient received prolonged prophylaxis for 9 months due to rejection treatments.
No symptomatic CMV infections were detected during valganciclovir prophylaxis. Late-onset CMV infection developed in 47 (37%) patients median 67 days after the cessation of prophylaxis (range 1–475) and median 244 days after transplantation (range 150–655) (Figure 1). One patient developed CMV infection on the first day after planned cessation of CMV prophylaxis. However, nonadherence to valganciclovir prophylaxis could be suspected, as the infection responded well to valganciclovir therapy and no antiviral resistance was clinically suspected. One patient, in whom valganciclovir prophylaxis was terminated prematurely at 4 months after transplantation due to leukopenia, developed primary CMV infection 150 days after transplantation. Infection was asymptomatic in four patients; symptomatic CMV infection developed in 43 patients (34%). Symptoms included fever (N = 28), gastrointestinal symptoms such as nausea, vomiting, diarrhoea, or abdominal pain (N = 24), respiratory tract symptoms (N = 12), elevated transaminases (N = 6) and graft dysfunction (N = 1). Median viral load at diagnosis in symptomatic patients was 8200 copies/mL (range 490–2831 000), and the median peak viral load was 13 500 copies/mL (range 1100–2831 000). Median viral load of the asymptomatic patients at diagnosis was 3250 (range 400–34 000), and the median peak viral load 19150 copies/mL (range 400–45700) (P = NS, between symptomatic and asymptomatic patients). Symptoms associated with CMV infection resolved with treatment and declining viral load. Tissue-invasive CMV infection was verified in only three patients: one patient suffered from histologically confirmed CMV duodenitis and one patient from histologically confirmed CMV colitis, and one patient showed a positive CMV finding from bronchoalveolar lavage fluid confirming the clinical suspicion of CMV pneumonitis. Patients with and without CMV infection are characterized in Table 1. Patient or graft survival did not differ between patients with or without CMV infection (data not shown). Of the asymptomatic patients, two were participants in the earlier clinical study and were screened for CMV DNAemia, and in two patients no specific reason for screening for CMV could be identified from the patient files.
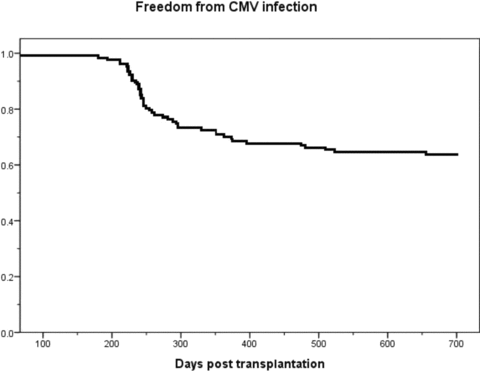
Description of the occurrence of late-onset primary CMV infections after 6 months of valganciclovir prophylaxis.
| No CMV infection (N = 80) | Late CMV infection (N = 47) | |
|---|---|---|
| Recipient age | 45 [18] | 47 [17] |
| Length of follow-up (months) | 47 [27] | 40 [27] |
| Length of pretransplant dialysis (months) | 20 [26] | 17 [20] |
| Median HLA- A,B/DR mismatch | 2/1 | 1/1 |
| Patients with diabetic nephropathy | 28 (35%) | 14 (30%) |
| Delayed graft function (%) | 26 (33%) | 16 (34%) |
| Patients on tacrolimus (%) | 11 (14%) | 6 (13%) |
| Patients with induction therapy (%) | 2 (2.5%) | 4 (8.5%) |
| Patients with acute rejection (%) | 8 (10%) | 2 (4%) |
| Plasma creatinine at 6 months (μmmol/L) | 104 [35] | 114 [21] |
| Plasma creatinine at 12 months (μmmol/L) | 101 [24] | 118 [45] |
| Plasma creatinine at last follow-up (μmmol/L) | 113 [48] | 120 [47] |
| Cyclosporine daily dose (mg/kg)1 | 3.0 [1.4] | 2.8 [1.1] |
| Cyclosporine trough level (μg/L)1 | 149 [41] | 160 [59] |
| Tacrolimus daily dose (mg/kg)1 | 0.06 [0.03] | 0.04 [0.04] |
| Tacrolimus trough level (μg/L)1 | 8.0 [2.5] | 6.2 [4.4] |
| Mycophenolate dose (mg/kg/day)1 | 24.1 [13.4] | 21.5 [8.2] |
| Methylprednisolone dose (mg/kg/day)1 | 0.06 [0.04] | 0.07 [0.05] |
| Valganciclovir prophylaxis terminated prematurely (%) | 4 (5%) | 4 (8.5%) |
- All data expressed as median [interquartile range] unless otherwise indicated.
- 1At the time of diagnosis of CMV infection, or at day 289 after transplantation (the mean time of CMV diagnosis) in patients with no CMV infection.
- All differences are nonsignificant.
Of the 47 CMV primary infections, 24 were treated with IV ganciclovir for 7–26 days followed by valganciclovir treatment or secondary prophylaxis and 18 were treated with only oral valganciclovir (900 mg twice daily if normal renal function). In one patient with a mild infection and a low viral load, infection was treated successfully with reduction of immunosuppression (MMF dose reduction). In four patients, the exact treatment was not evident from the patient files. All treatments resulted in negative CMV-DNAemia. After the treatment of CMV infection, secondary prophylaxis with valganciclovir was given to 39 patients for median 120 days (range 10–210). No clinically suspected cases of ganciclovir resistant viruses were detected, and no laboratory testing for resistance was performed.
In logistic regression, no significant risk factors for CMV infection were identified. The risk of CMV infection was not associated with age or sex of the recipient, cold ischemia time, HLA mismatch, tacrolimus or induction therapy, delayed graft function, acute rejection, renal function, doses or trough levels of immunosuppressive drugs, or the premature termination of valganciclovir prophylaxis (data not shown). CMV antibody development or immunoglogulin levels during or after prophylaxis were not routinely monitored and were not available. No cases of polyomavirus-associated nephropathy were detected, but no data about other opportunistic infections were available.
Recurrent CMV infection developed in 9/47 patients (19%) median 32 days after the end of secondary prophylaxis (range 12–61). No significant difference was recorded in the length of secondary prophylaxis in patients with recurrent infection compared to patients with no relapse (90 [75] vs. 120 [90] days respectively, P = NS). One patient who received no secondary prophylaxis after the treatment of primary infection with IV ganciclovir developed recurrent infection 25 days after the end of treatment. Of the recurrent infections, five were asymptomatic. In others, symptoms included gastrointestinal symptoms (N = 3), fever (N = 2), and respiratory tract symptoms (N = 1). Median peak viral load was 1035 copies/mL (range 610–3420). All recurrent infections were treated with oral valganciclovir for total of 30–120 days, except for one asymptomatic infection with a low viral load, which was successfully treated with MMF dose reduction. Recurrent infections responded well to treatment with valganciclovir, and no cases of ganciclovir resistance were clinically suspected. In logistic regression, the risk of recurrent infection was not associated with tacrolimus therapy or induction therapy, delayed graft function, acute rejection, renal function, doses or trough levels of immunosuppressive drugs, length of secondary prophylaxis or treatment modality of the first infection episode (IV ganciclovir vs. valganciclovir) (data not shown).
A second infection relapse developed in two patients. In one patient, asymptomatic activation of the virus with a low viral load of 690 copies/mL was detected 174 days after the end of last valganciclovir treatment and was treated with valganciclovir. One patient with metastatic breast cancer who had received multiple courses of cytotoxic anticancer treatment, developed an asymptomatic CMV reactivation 1614 days (54 months) after transplantation with a peak viral load of 14 500 copies/mL, and was successfully treated with IV ganciclovir for 2 weeks.
Discussion
Delayed-onset primary CMV infection occurring after prophylaxis is still a significant clinical problem after kidney transplantation. In our retrospective analysis of 127 Finnish kidney transplant recipients at risk for primary CMV infection, the incidence of late infections was relatively high; late-onset CMV infection developed in 37% patients, and symptomatic CMV infection in 34%. Importantly, in 24 patients primary infection was clinically significant and required hospitalization and treatment with IV ganciclovir. However, the severity of CMV infections was not specifically recorded, and in the first years of the study, treatment of CMV infections with valganciclovir was not routine clinical practice. Risk of late-onset primary CMV infections was not associated with the intensity of immunosuppression, acute rejections, or renal function. No significant risk factors for these late-onset infections of were identified, although the retrospective design and small total number of infections limits our analysis.
Valganciclovir, an orally bioavailable valine ester of valganciclovir, is used in most transplant centres for CMV prophylaxis in CMV seronegative recipients of an organ from a seropositive donor (17). Current guidelines recommend 3–6 months prophylaxis for these high-risk patients (4,18). After 3 months of antiviral prophylaxis, late-onset primary CMV infections are reported to occur in 18–31% of kidney transplant recipients (7–9). Evidence suggests that prolonging prophylaxis for 6 months reduces the incidence of late-onset infections (7,10,11). In a study by Doyle et al., 24 weeks of oral ganciclovir prophylaxis in 31 patients was associated with a reduced incidence of symptomatic CMV infections when compared to a historical cohort of 39 patients with 12 weeks of oral ganciclovir prophylaxis (7% vs. 31%) (7). In a recent randomized controlled trial of 326 kidney transplant recipients (IMPACT study), 200 days of valganciclovir prophylaxis was associated with a reduced incidence of late-onset CMV disease compared to 100 days of prophylaxis (16.1 vs. 36.8% respectively), and similarly a reduced incidence of CMV viremia (37.4% vs. 50.9%) (10). In a retrospective analysis of 222 patients from two consecutive time-periods by Luan et al., 6 months valganciclovir prophylaxis was associated with reduced incidence of CMV disease when compared to 3 months prophylaxis (12.1% vs. 20.9%), whereas no significant difference was recorded in the incidence of CMV infections (24.4% vs. 26.7%) (11). In our preliminary analysis of 25 patients, late-onset CMV infection developed in 48% patients, most of whom had symptoms of CMV infection (12). The high incidence of late-onset primary CMV infections in our population was confirmed in this present nationwide analysis. However, findings of our relatively small retrospective single centre analysis from our homogenous transplant population may not be comparable to other populations or studies. In addition, the lack of control group is a major limitation of our study. Our study also is limited by the lack of systematic screening for asymptomatic primary CMV infections (except for the 25 patients included in our earlier study of late-onset primary CMV infections), which explains the high percentage of symptomatic CMV infections in our cohort. However, careful screening for CMV DNAemia probably could have revealed an even higher frequency of subclinical primary infections after the cessation of prophylaxis. The strength of our study is our long experience with 6 months valganciclovir prophylaxis, enabling a long follow-up time for a relatively large population of high-risk patients, as primary infections may occur also late after transplantation.
Some evidence suggests that prophylaxis with an effective antiviral agent may impair the development of CMV-specific T-cell responses (19–21) or the maturation of antibodies (22), possibly increasing the risk of late-onset infections occurring after prophylaxis. Preemptive therapy as an alternative to universal prophylaxis could possibly reduce this problem (23). However, in a recent randomized controlled study comparing universal prophylaxis and pre-emptive therapy, prophylaxis was associated with better kidney graft survival (8), supporting the use of universal prophylaxis. Current guidelines also recommend prophylaxis for high-risk D+/R– patients (4,18). Valganciclovir was well tolerated in our population, and only eight patients had to terminate prophylaxis prematurely due to side effects.
The Finnish kidney transplant population differs from other populations, in which primary CMV infections have previously been studied. In our cohort most of the kidneys are from well-matched deceased donors from our genetically isolated population, and the frequency of acute rejections is low despite cyclosporine-based immunosuppression in most patients. In addition, the incidence of delayed graft function is relatively high, partly explained by increased cold ischemia times due to the long geographic distances in our country. This may also contribute to the risk of CMV infections. In our material, immunosuppression is relatively conservative, and induction therapy is rarely used in contrast to other studies of CMV prophylaxis, in which most of the patients received quadruple immunosuppression including induction therapy with either ATG or IL-2- receptor antagonists (6,7,9).
Risk factors for late-onset primary CMV infections in high-risk recipients are not well described. In a large study comparing the efficacy of oral ganciclovir and valganciclovir prophylaxis in CMV D+/R- solid organ transplant recipients (PV 16 000), reduced renal function and female sex were the only significant risk factors for late-onset CMV infection (5). In other studies, delayed graft function, other infections (bacterial and fungal), and induction therapy with thymoglobulin have been associated with increased risk of late-onset CMV infections (7,9,11). Viral load screening after prophylaxis or seroconversion occurring during or after prophylaxis, on the other hand, are shown to be poor predictors of late-onset CMV disease in these high-risk patients (24,25). We failed to identify any risk factors for late-onset CMV infection. However, the small sample size and low total number of infections limit our analysis of risk factors and possible confounding factors. In addition, CMV serology was not routinely monitored after transplantation and was not available for analyses. The intensity of immunosuppressive medication seemed not to be associated with increased risk of CMV infections, as could be predicted by the overall high incidence of infections in our patient population with relatively conservative immunosuppression. As the control of CMV replication and infection after transplantation is achieved mainly by CD8+ and CD4+ T-cell mediated immune responses (26,27), a novel strategy to predict CMV infections could be to measure CMV-specific T-cell responses (20,28–30). In contrast to a recent study by Arthurs et al., in which delayed-onset CMV infection was associated with allograft loss and mortality (9), primary CMV infections were not associated with poorer graft function or graft loss in our material. However, small sample size and short follow-up for detecting differences in graft or patient outcomes limits our analysis.
The most common symptoms of CMV infection in our study were fever and gastrointestinal symptoms. Similar findings of changed clinical picture of delayed-onset primary CMV infection towards gastrointestinal disease after solid-organ transplantation have been reported (9,31,32), and CMV prophylaxis with valganciclovir may be associated with increased incidence of tissue-invasive CMV disease (5,9,23). In our study, 24 patients suffered from gastrointestinal symptoms, suggestive of gastrointestinal CMV disease. However, in a majority of patients in our study, diagnosis of gastrointestinal CMV disease was not confirmed by endoscopy or biopsy findings, but was limited to the description of symptoms. Similarly only one patient with respiratory tract symptoms had a confirmed CMV pneumonitis. Although most patients in our study had symptoms attributable to CMV infection, CMV disease was not specifically defined in our material, and not all symptoms and findings (especially the degree of fever or leukopenia) were exactly defined in our material, and some of the symptomatic infections probably did not meet the criteria of CMV syndrome in consensus guidelines (33) or the criteria of CMV disease used in multicenter studies (5). Thus, the comparison of the frequency of symptomatic CMV infections in our material to the frequencies of CMV disease in other studies may not be justified. Despite these limitations, we believe our findings bring new insights into primary infections occurring after prophylaxis. Considerable variation was recorded in the viral loads of patients with primary CMV infection. The lack of screening in most patients probably explains the high viral loads at diagnosis and the large variation in viral loads. Viral loads were slightly lower with asymptomatic patients, although the difference did not reach statistical significance. CMV quantitative PCRs were performed in three different laboratories with somewhat different methods, limiting our interpretation of viral load analyses.
In conclusion, the incidence of late-onset primary CMV infections in our population was relatively high, and infections occurred also relatively late after transplantation. Most of the infections were symptomatic, and most common symptoms were from the gastrointestinal tract. Late-onset primary CMV infections remain a clinically important problem also after 6 months of valganciclovir prophylaxis.
Funding Sources
This study was funded by Helsinki University Hospital Research Funds (EVO to P.K.) and Academy of Finland (to I.H.)




