An Early Regional Experience with Expansion of Milan Criteria for Liver Transplant Recipients
Abstract
The Milan Criteria (MC) showed that orthotopic liver transplantation (OLT) was an effective treatment for patients with nonresectable, nonmetastatic HCC. There is growing evidence that expanding the MC does not adversely affect patient or allograft survival following OLT.
The adult OLT programs in UNOS Region 4 reached an agreement allowing lesions outside MC (one lesion <6 cm, ≤3 lesions, none >5 cm and total diameter <9 cm—[R4 T3]) to receive the same exception points as MC lesions. Kaplan–Meier curves and log-rank tests were used to compare survival data. Chi-squared and Mann–Whitney U tests were used to compare patient data. A p-value of <0.05 was considered significant. All statistical analyses were performed on SPSS 15 (SPSS, Chicago, IL).
Four hundred and forty-five patients were transplanted for HCC (363-MC and 82-R4 T3). Patient demographics were found to be similar between the two groups. Three year patient, allograft and recurrence free survival between MC and R4 T3 were found to be 72.9% and 77.1%, 71% and 70.2% and 90.5% and 86.9%, respectively (all p > 0.05).
We report the first regionalized multicenter, prospective study showing benefit of OLT in patients exceeding MC based on preoperative imaging.
Abbreviations:
-
- COD
-
- cause of death
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- LRT
-
- loco-regional HCC therapy
-
- MC
-
- Milan Criteria
-
- MC T2
-
- Milan Criteria T2 HCCs
-
- OLT
-
- orthotopic liver transplant
-
- R4 T3
-
- region 4 T3 HCCs
-
- TACE
-
- transarterial chemoembolization
-
- UCSF
-
- University of California – San Francisco
-
- UNOS
-
- United Network of Organ Sharing
Introduction
Currently, hepatocellular carcinoma (HCC) is the 5th most common cancer in the United States but is the 3rd leading cause of cancer death (1). The incidence of HCC has doubled in the past 2 decades with an estimated 8500–11 000 new cases per year (2,3). This coupled with the increase in hepatitis C (HCV) related cirrhosis and nonalcoholic steatohepatitis (NASH) has led to the prediction that the incidence of HCC may further double over the next 20 years (2). These increasing numbers, unfortunately, have not been matched by advances in our ability to treat and cure this often fatal disease.
Historically, our treatment modalities for HCC have been met with poor outcomes. Overall 5 year survival rates regardless of treatment modality were 20–40% (2). Following basic oncologic principles, orthotopic liver transplant (OLT) has emerged as the ideal treatment of HCC by providing complete oncologic resection (2). Although OLT for HCC has a solid foundation in surgical principles, early experiences produced less than optimal outcomes partially due to poor patient selection (4–7). This changed, however, in 1996 when Mazzafero et al. popularized new selection criteria for patients undergoing OLT for HCC. These criteria became known as the Milan Criteria (MC T2) and demonstrated that OLT could be performed on patients with a single tumor ≤5 cm or no more than three tumors with the largest diameter ≤3 cm and achieve significant survival benefit. Using these criteria, the authors demonstrated a 4 year overall survival and recurrence free survival of 85% and 92%, respectively (8). These results have been validated in numerous studies since 1996 and have been adopted by the Organ Procurement and Transplantation Network (OPTN) thereby playing a role in cadaveric liver allocation in the United States.
Despite these results, many argue that the MC are too restrictive (2,9–18). Attempts to expand the MC have gained momentum and there is an enlarging body of literature supporting these claims. In 2001, Yao et al. proposed the University of California – San Francisco (UCSF) criteria and demonstrated that a modest expansion (a single lesion ≤6.5 cm in diameter or two to three lesions ≤4.5 cm with total tumor diameter ≤8 cm) in the MC did not adversely affect long-term patient or allograft survival (19). The original UCSF study demonstrated 1 and 5 year patient survival rates of 90% and 75%, respectively. When compared to MC T2, the difference in patient survival was not found to be statistically significant (19). Other studies expanding MC T2 have shown similar results with survival comparable to that seen in the original Mazzafero study (2,10,19). However, the major drawback of these and other studies is that they are single center based on retrospective data and use a combination of preoperative imaging and explant data.
In previously published data using the International Registry of Hepatic Tumors in Liver Transplantation, Onaca et al. compared survival rates among 1206 patients who underwent OLT between 1992 and 2005. They found that recurrence free survival for single lesions 3.1–5.0 cm were equivalent to single lesions >5 cm but <6 cm. They also found that when comparing tumor size and tumor number, patients outside MC T2 derived survival benefit (13). Therefore, based on this data, the liver transplant centers in Region 4 proposed a new inclusion criteria for OLT in HCC patients exceeding MC T2 and sought to prospectively evaluate the results of this expansion. Patients with a single tumor ≤6 cm or two to three tumors with the largest diameter ≤5 cm and total diameter ≤9 cm (R4 T3) were granted a MELD exception score of 22, just as patients with MC T2 tumors. All patient data was prospectively collected across 11 Region 4 adult liver transplant centers from 2004 to 2007 making this the first regionalized, multicenter, prospective study examining the effect of expanding the inclusion criteria for OLT in HCC patients.
Materials and Methods
All patients being evaluated for OLT for HCC received cross sectional imaging of the chest, abdomen and pelvis (MRI with gadolinium or triple phase contrast-enhanced CT scan) and a bone scan to delineate tumor size/number and rule out extrahepatic disease. This radiographic data determined inclusion suitability. Region 4 developed an agreement to allow adult patients (>17 years of age) with HCC outside MC T2 criteria to receive OLT. This accepted variance allowed for a single lesion ≤6 cm or up to three lesions with the largest ≤5cm and the total tumor diameter not to exceed 9 cm. In addition, the potential recipients could not have evidence of extrahepatic disease, macrovascular invasion on imaging and/or be candidates for surgical resection. When all of these criteria were met, the R4 T3 candidate was submitted to the Region 4 Regional Review Board for HCC MELD score exception (initial MELD score of 22, as is granted for MC T2 recipients). Between October 1, 2004 and December 31, 2007, all OLT candidates diagnosed with HCC in Region 4 were followed longitudinally and re-evaluated every 3 months to insure that they still met inclusion criteria. The majority of patients while awaiting OLT underwent loco-regional HCC therapy (LRT) which consisted of transarterial chemoembolization (TACE) every 3 months with 50 mg of adriamycin and radiofrequency ablation for HCCs ≤3 cm. While HCCs >3 cm received TACE alone. Candidate eligibility for LRT was left to each individual center. These treatments continued until the patient was either transplanted or dropped out of the study. Recurrence was defined as cause of death (COD) due to HCC or posttransplant malignancy. Dropout was defined as being too sick for transplantation or progression of disease outside the accepted inclusion criteria. COD was separated into the following categories: recurrence, cardiac, pulmonary complications, infection/sepsis, multi-system organ failure, allograft failure, technical complication and other.
All statistical analyses were computed using SPSS version 15 (SPSS, Chicago, IL). Kaplan–Meier curves and log-rank tests were used to calculate and compare patient, allograft and recurrence-free survival. Chi-squared tests were used to compare categorical variables including history of LRT, gender, dropout at 30 days, dropout at 1 year and race. Mann–Whitney U tests were used to compare continuous variables including lab MELD and age at transplantation. A p-value of <0.05 was considered statistically significant in all cases.
Results
Demographics
During the study period, a total of 515 adult HCC patients were enrolled. Sixty-four patients did not undergo OLT by the end of the study period. Six patients did not meet MC T2 or R4 T3 criteria and were excluded during analysis. In total, 445 patients (363 patients—MC T2, 82 patients—R4 T3) underwent OLT for HCC. As shown in Table 1, 74% of patients transplanted with MC T2 lesions were male as compared to 84% of patients with R4 T3 lesions. This difference was found to be statistically significant between the two groups (p = 0.044). Mean age at the time of transplantation was similar between the two study groups, 56.6 and 57.6 years for the MC T2 and R4 T3 groups, respectively. The distribution of OLT recipients within the groups across race was also found to be similar. Mean laboratory MELD at time of transplant for patients with MC T2 lesions was 13 and 13.9 for patients with R4 T3 lesions. Comparison of the mean match MELD (MELD including exceptions) demonstrated that match MELD values were again similar. MC T2 patients had a match MELD of 22.9 while the R4 T3 patients had a match MELD of 23.3. Waiting times also did not statistically differ between the two study groups, MC T2 patients waited 231 days while R4 T3 patients waited 208 days. There was a statistically significant difference between the groups in regards to history of LRT. Approximately 60% of patients with MC T2 lesions received LRT while 73% of patients with R4 T3 lesions underwent LRT (p = 0.021). LRT was not used as a part of a downstaging protocol and only patients who met criteria for transplantation at presentation were included. Upon review of the native liver pathologic explant reports, 17.64% of patients transplanted exceeded their radiographic listing criteria.
| MC T2 (n = 363) | R4 T3 (n = 82) | p-Value | |
|---|---|---|---|
| Sex | |||
| ♂Male | 73.6% | 84.1% | 0.044 |
| ♂Female | 26.4% | 15.9% | |
| Age (mean) | 56.6 ± 7.5 | 57.6 ± 7.2 | 0.206 |
| Race | |||
| ♂White | 59.5% | 64.6% | 0.719 |
| ♂Black | 5.8% | 3.7% | |
| ♂Hispanic | 19.3% | 18.3% | |
| ♂Asian | 6.3% | 8.5% | |
| ♂Multiracial | 8.8% | 4.9% | |
| ♂Other | 0.3% | 0% | |
| Lab MELD (mean) | 13 ± 5.1 | 13.9 ± 6.9 | 0.374 |
| Match MELD (mean) | 22.9 ± 2.1 | 23.3 ± 2.9 | 0.109 |
| Hx of Local HCC Therapy | 59.5% | 73.2% | 0.021 |
| Days on Waitlist (mean) | 231 | 208 | 0.443 |
| Dropout at 30 days | 2.2% | 2.4% | 0.577 |
| Dropout at 1 year | 7.7% | 9.6% | 0.337 |
| Recurrence | 6.6% | 9.8% | 0.319 |
Patient survival
To determine if expanding the inclusion criteria for OLT in patients with HCC adversely affected patient outcome, we evaluated posttransplant patient survival between MC T2 and R4 T3 patients. As shown in Table 2, similar rates of overall patient survival were demonstrated over the first 3 years of follow up. Patients undergoing OLT for MC T2 lesions demonstrated 1 and 3 year survivals of 89.6% and 72.9% while R4 T3 lesions revealed patient survivals of 91.1% and 77.1% (Figure 1). This difference was not found to be statistically significant (p = 0.75). Further subdividing the two patient study groups to evaluate the effect of pretransplant LRT revealed the same patient outcome trends. Specifically, patients with MC T2 lesions that did not undergo LRT had 1 and 3 year patient survivals of 88.9% and 74.6% compared to 1 and 3 year survivals of patient survivals of 90% and 71.9% in patients who received LRT prior to OLT. Patients with R4 T3 lesions that did not undergo LRT had 1 and 3 year patient survivals of 95.5% and 71.5% compared to 89.7% and 78.8% in patients who underwent LRT prior to liver transplantation. These differences did not reach statistical significance.
| 1 year | 3 year | p-Value | |
|---|---|---|---|
| Patient survival | |||
| MC T2 | 89.6% | 72.9% | 0.73 |
| R4 T3 | 91.1% | 77.1% | |
| MC T21 | 90% | 71.9% | 0.561 |
| R4 T31 | 89.7% | 78.8% | |
| MC T22 | 88.9% | 74.6% | 0.723 |
| R4 T32 | 95.5% | 71.5% | |
| Allograft survival | |||
| MC T2 | 88.1% | 71% | 0.743 |
| R4 T3 | 87.7% | 70.2% | |
| MC T21 | 88.3% | 69.5% | 0.774 |
| R4 T31 | 88.2% | 72% | |
| MC T22 | 87.6% | 73.5% | 0.286 |
| R4 T32 | 86.4% | 64.7% | |
| Recurrence-free survival | |||
| MC T2 | 97% | 90.5% | 0.499 |
| R4 T3 | 96% | 86.9% | |
| MC T21 | 97% | 91% | 0.389 |
| R4 T31 | 94.7% | 85.1% | |
| MC T22 | 97% | 89.7% | 0.763 |
| R4 T32 | 100% | 91.7% | |
- 1Denotes preoperative LRT.
- 2Denotes no preoperative LRT.
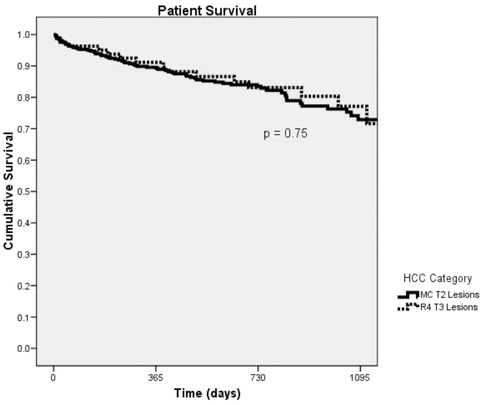
Overall patient survival.
Allograft survival
Allograft survival between the two groups does not vary significantly in this early portion of our analysis (Table 2). One and 3 year allograft survival for MC T2 lesions was found to be 88.1% and 71%. Similarly, 1 and 3 year allograft survival for patients with R4 T3 lesions was 87.7% and 70.2%. This difference did not reach statistical significance. Again, only looking at patients that received local HCC therapy revealed similar results. Patients with MC T2 lesions had 1 and 3 year survivals of 88.3% and 69.5%, respectively. The patients with R4 T3 lesions had similar allograft survivals at 1 and 3 years of 88.2% and 72%, respectively. Comparisons of patients that did not receive preoperative LRT also demonstrated the same trend. Patients with MC T2 lesions had 1 and 3 year allograft survivals of 87.6% and 73.5%. R4 T3 patients had 1 and 3 year allograft survivals of 86.4% and 64.7%. These differences were not found to be statistically significant.
Recurrence free survival
Recurrence free survival was calculated as patient death due to recurrent disease or posttransplant recurrence at follow up. Recurrence data were collected either as COD or reported on the posttransplant malignancy form. We recognize that recurrence is likely underreported to the OPTN as transplant centers may not learn of a recipient's recurrence as the COD. One and 3 year recurrence free survival in patients with MC T2 lesions were 97% and 90.5%. Similarly, patients with R4 T3 lesions demonstrated 1 and 3 year recurrence free survivals of 96.9% and 86.9% (Figure 2A). As shown in Table 2, the differences among the two study group did not reach statistical significance. Patients who received LRT also demonstrated similar recurrence free survival results. MC T2 lesions that received LRT had 1 and 3 year survivals of 97% and 91% compared to patients with R4 T3 lesions which had recurrence free survivals of 94.7% and 85.1%, respectively (Figure 2B). These differences also did not reach statistical significance (Table 2). The same holds true when comparing recurrence free survival for those that did not receive LRT (p = 0.763). Further examination of patients in regard to preoperative LRT and recurrence free survival by diagnosis reveals that preoperative LRT did not improve recurrence rates. Patients both in the MC T2 group and the R4 T3 group had similar recurrence free survival rates when compared according to history of preoperative LRT (MC T2: p = 0.755 and R4 T3: p = 0.409).
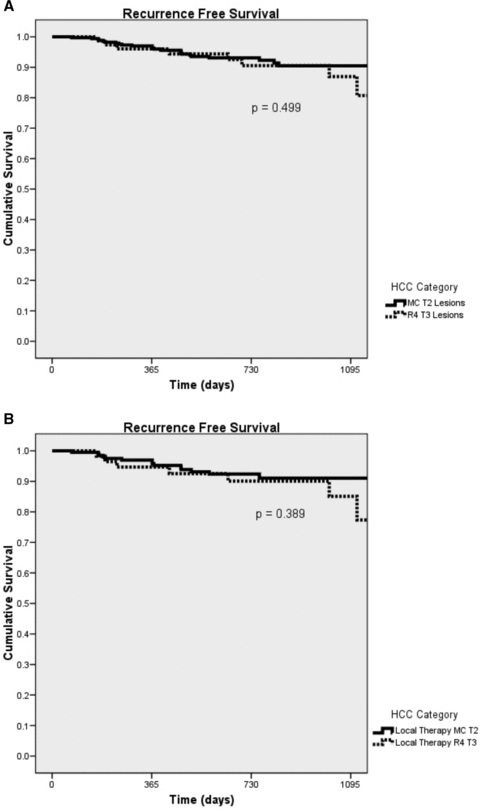
(A) Overall recurrence free survival. (B) Recurrence free survival for patients receiving pre-OLT local HCC therapy.
Cause of death
Comparisons of COD between groups revealed that they were similar. As seen in Table 3, COD due to recurrence was more prominent in R4 T3 lesions (MC T2 – 30.9% and R4 T3 – 43.8%), however this difference did not reach statistical significance (p = 0.193). In the COD group classified as other, MC T2 recipients died due to 1 case of trauma, 1 cerebrovascular insult, 1 case of graft versus host disease and 14 deaths were due to an unknown cause. In the R4 T3 other group, the COD consisted of one episode of acute pancreatitis and four unknowns.
| p = 0.193 | MC T2 (n = 68) | R4 T3 (n = 16) |
|---|---|---|
| Recurrence | 21 (31%) | 7 (44%) |
| Cardiac | 0 | 1 (6%) |
| Pulmonary | 5 (7%) | 0 |
| Infection/sepsis | 10 (15%) | 3 (19%) |
| MOF | 6 (9%) | 0 |
| Graft failure | 5 (7%) | 0 |
| Technical complication | 4 (6%) | 0 |
| Other | 17 (25%) | 5 (31%) |
Discussion
The introduction of MC T2 improved 5 year survival post-OLT for HCC from below 50% to greater than 70% (4,5,20). Over the past decade, questions have been raised as to whether patients with an increased tumor burden outside the MC could potentially benefit from OLT. Most notably, the UCSF criteria has demonstrated on both explant pathology and preoperative imaging that results similar to what has been demonstrated in MC T2 can be achieved (9). Even more recently, Onaca et al. demonstrated that tumors larger than what was proposed in the UCSF criteria still benefited from OLT. These single center data demonstrate that patients with HCC derive survival benefit outside MC T2.
Our study represents the first prospective, regionalized, multicenter experience designed to determine benefit of OLT in patients exceeding MC T2 in a prospective manner using preoperative imaging to determine inclusion criteria. Many of the preceding attempts to look at expanded criteria based their analysis on explant data rather than preoperative imaging. In doing so, an assumption is made that imaging studies accurately reflect pathologic tumor burden and this may not be true.(13,19). Preoperative imaging may underestimate tumor stage up to 16% of the time and overestimate it in up to 9% of cases (19). This potential problem is circumvented in prospective data collection. Other studies examining outcomes for patients with HCC post-OLT have relied on pathological information including nodal invasion, grade and vascular invasion that are not often available preoperatively (3,21,22). Currently, preoperative imaging is the most widely used and accepted method of tumor staging and validating criteria utilizing modern imaging techniques provides the most clinically relevant information in these patients.
Comparisons of patients included in the study between groups show that the two groups are similar. No differences exist when looking across age, race, calculated MELD or match MELD. This shows that patients satisfying the MC T2 did not differ demographically or in disease severity from patients outside the MC T2 but meeting R4 T3 criteria. In examining history of LRT, the patients satisfying the expanded R4 T3 criteria have a higher probability of receiving preoperative LRT. However, the presence of LRT pretransplant did not adversely or positively affect overall patient survival during the follow-up period of the current study.
Overall patient and allograft survival differences between groups did not reach statistical significance but perhaps more importantly, recurrence free survival did not differ between groups. Tumor burden as seen on preoperative imaging is used as a surrogate to tumor biology to predict aggression and recurrence. These data have shown that patients undergoing liver transplantation for R4 T3 lesions draw survival benefit up to 3 years post-OLT. When evaluating patients for recurrence free survival that received preoperative LRT it is demonstrated that while more R4 T3 patients receive LRT than MC T2, disease-free survival benefits were essentially equal. In prospectively showing that patient, allograft and recurrence free survival are comparable between MC T2 and R4 T3 patients, the argument that MC T2 is too restrictive is further supported for the first time by a regionalized, prospective, multicenter study. When examining COD between groups, we see that R4 T3 patients perform as well as MC T2 patients in regards to recurrence. All other COD are similar between groups as well. Analysis of the data in regards to preoperative LRT shows that recurrence free survival between groups that received preoperative LRT and those that did not also were equivalent. This element of our statistical analysis questions the role of preoperative LRT in patients being considered for transplantation. However, this interpretation of the data must be done carefully because this study was not designed to answer this question. To further elucidate the role of preoperative LRT, protocols regarding its institution, longer follow up and larger numbers would have to be put in place.
A possible confounding factor in the study is the non standardized use of immunosuppression between centers. The use of tacrolimus versus sirolimus varies and there is no clear advantage to either. Some data suggests that sirolimus may improve outcomes post-OLT for patients with HCC, these findings have not been evaluated in a randomized, prospective fashion and thus are not standard of care (23–25). In future studies, the use of immunosuppression should be standardized or follow a research protocol across centers allowing comparison of patients across different treatment arms.
With the continued shortage of available cadaveric donor organs and increasing numbers of potential recipients, an expansion of the MC would mean more patients without an increase in donors. In doing so, an argument must be made that the benefit to these patients would exceed the harm to patients currently on the list. Volk et al. created a Markov model that examined this question. They found that a 5 year survival of 61% was needed to justify expansion of MC T2 (26). Although our follow up has not reached 5 years, our 3 year data suggests that even this more stringent criteria will be met.
Conclusion
HCC is a disease amenable to treatment by OLT. Early experiences were met with dismal results. The introduction of MC T2 transformed HCC from a contraindication to OLT to a priority diagnosis. Since that time however, new studies have shown that patients outside of MC T2 derive comparable survival benefit post-OLT. Our study represents the first regionalized, multicenter, prospectively collected liver transplant experience for HCC experience involving expansion of MC T2. Our preliminary results show that patients with a single tumor ≤6 cm or two to three tumors with the largest diameter ≤5 cm and total tumor diameter ≤9 cm have comparable patient, allograft, and recurrence free survival as patients who meet the more stringent MC T2. It must be noted that our data pertains to Region 4 and that these outcomes may not translate to regions with different waiting times. The report of the HCC consensus conference does not recommend a national policy for expanding MC T2 at present but encourages regional agreement (27). The data presented should encourage other regions to perform similar studies to determine the applicability of expanded criteria for OLT in HCC within their region.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




