Extracorporeal Membrane Oxygenation in Nonintubated Patients as Bridge to Lung Transplantation
Abstract
We report on the use of veno-arterial extracorporeal membrane oxygenation (ECMO) as a bridging strategy to lung transplantation in awake and spontaneously breathing patients. All five patients described in this series presented with cardiopulmonary failure due to pulmonary hypertension with or without concomitant lung disease. ECMO insertion was performed under local anesthesia without sedation and resulted in immediate stabilization of hemodynamics and gas exchange as well as recovery from secondary organ dysfunction. Two patients later required endotracheal intubation because of bleeding complications and both of them eventually died. The other three patients remained awake on ECMO support for 18–35 days until the time of transplantation. These patients were able to breathe spontaneously, to eat and drink, and they received passive and active physiotherapy as well as psychological support. All of them made a full recovery after transplantation, which demonstrates the feasibility of using ECMO support in nonintubated patients with cardiopulmonary failure as a bridging strategy to lung transplantation.
Introduction
Lung transplantation is a potentially life-saving therapeutic option for patients with refractory cardiopulmonary failure due to pulmonary hypertension. Although the number of transplantations realized worldwide is expanding, mortality on the waiting list remains relevant as the number of patients in need of a transplant exceeds the number of suitable donor organs. The Eurotransplant system allows allocation of patients according to medical urgency (1). Still, even patients who are ranked top priority often have waiting times of several weeks or even months until a donor organ becomes available (1).
Bridging patients with end-stage heart or lung disease to transplantation remains one of the most difficult challenges of modern medicine. This is particularly true for patients with pulmonary hypertension refractory to medical therapy. In these patients, endotracheal intubation and mechanical ventilation is often not a viable option as this strategy carries a high risk of right heart failure. Extracorporeal membrane oxygenation (ECMO) has been used for bridging to transplantation in patients with right heart failure (2,3), but, to the best of our knowledge, almost exclusively in patients receiving mechanical ventilation. These patients are therefore exposed to the risks of ECMO as well as the risks and complications of prolonged sedation and mechanical ventilation, especially pneumonia and septicemia (4). A potential alternative to ECMO is the use of a pumpless lung assist device inserted between the pulmonary artery (PA) and the left atrium (LA) as reported earlier (5). This technique allows bridging to transplantation but requires at least temporary sedation, intubation and cardiac surgery, all posing a threat for the successful outcome after transplantation, especially when the waiting time exceeds several weeks.
In order to circumvent these problems, we hypothesized that the use of veno-arterial ECMO in awake and spontaneously breathing patients might be an option for long-term cardiopulmonary support, avoiding the drawbacks and complications associated with intubation and prolonged mechanical ventilation. Here, we report our experience with the first five patients who were treated with this novel strategy at our institution between July 2008 and July 2009.
Case 1
A 29-year-old female (Table 1) with chronic thromboembolic pulmonary hypertension associated with a ventricular-atrial shunt underwent pulmonary endarterectomy but had persistent pulmonary hypertension after surgery. She was successfully weaned from the ventilator 3 days after the operation, but during the following few days she developed progressive right heart failure nonresponsive to medical therapy including sildenafil, intravenous iloprost and catecholamines. Nine days after surgery, the patient developed right heart failure with anuria and lactic acidosis (Table 2).
| Pat | Age | Gender | Diagnosis | Days on ECMO | Type of Tx | Outcome |
|---|---|---|---|---|---|---|
| 1 | 29 | Female | CTEPH | 35 | BLTx | Discharged, alive, follow-up 14 months after Tx |
| 2 | 53 | Male | PAH, LF, SSc | 11 | BLTx | Deceased after Tx |
| 3 | 41 | Male | PH, LF due to sarcoidosis | 18 | BLTx | Discharged, alive, follow-up 6 months after Tx |
| 4 | 54 | Female | IPF | 35 | BLTx | Discharged, alive, follow-up 4 months after Tx |
| 5 | 55 | Female | IPAH | 8 | - | Deceased prior to Tx |
- CTEPH = chronic thromboembolic pulmonary hypertension; PAH = pulmonary arterial hypertension; LF = lung fibrosis; SSc = systemic sclerosis; PH = pulmonary hypertension; IPF = idiopathic pulmonary fibrosis; IPAH = idiopathic pulmonary arterial hypertension; BLTx = bilateral lung transplantation.
| Pat 1 | Pat 2 | Pat 3 | Pat 4 | Pat 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ECMO | 24 h ECMO | Pre-ECMO | 24 h ECMO | Pre-ECMO | 24 h ECMO | Pre ECMO | 24 h ECMO | Pre ECMO | 24 h ECMO | |
| Oxygen supply | NRM 12 L/min | NRM/NC 4–6 L/min | NIV FiO2 0.8 | NRM 6–8 L/min | NIV FiO2 1.0 | NRM/NC 4–6 L/min | NIV FiO2 0.8 | NRM 4–6 L/min | NRM 10 L/min | NRM 8 L/min |
| SaO2 | 70–80% | 85–90% | 80–90% | >90% | 70–77% | >90% | 80–85% | >90% | 70–80% | 86–90% |
| Mean SAP (mmHg) | 58 | 107 | 60 | 80 | 70 | 93 | 60 | 80 | 47 | 80 |
| CVP (mmHg) | 26 | - | 26 | - | 24 | - | 25 | - | 23 | - |
| ScvO2 | 40% | 65% | 33% | 75% | 33% | 67% | 42% | 75% | 43% | 63% |
| Creatinine umol/L | 85 | 59 | 79 | 61 | 95 | 42 | 212 | 130 | 194 | 96 |
- NRM = nonrebreathing mask; NC = nasal cannula; NIV = noninvasive ventilation; FiO2= inspired oxygen fraction; SAP = systemic arterial pressure; CVP = central venous pressure; ScvO2= central-venous oxygen saturation.
At that time, it was decided to insert veno-arterial ECMO while the patient was fully awake. A 20-French Heartport cannula was placed in the left femoral vein and advanced into the right atrium. A 15-French Novalung cannula was placed in the left femoral artery. Another 7-French introducer sheath was inserted in the femoral artery distal from the Novalung cannula and connected to the arterial branch of the ECMO circuit to ensure sufficient blood flow in the limb. Systemic oxygenation and blood pressure were measured via a line inserted in the right radial artery.
The whole procedure was performed under local anesthesia without sedation. The mean device flow was maintained between 3.2 and 3.5 L/min resulting in immediate hemodynamic stabilization (Tables 2 and 3). Pulmonary arterial hypertension (PAH) medications and catecholamines were discontinued. Renal function recovered immediately and so did lactic acidosis. The patient remained on ECMO support for 35 days. During the whole time, she was nonsedated and cooperative, able to eat and drink and she received passive and active physiotherapy. A febrile episode was successfully treated with levofloxacin and daptomycin (no cause of infection was identified and blood cultures remained negative). There were no signs of hemolysis and the red cell and platelet counts remained normal throughout the ICU stay. Bilateral lung transplantation was performed 44 days after pulmonary endarterectomy. The patient left the operating theatre without extracorporeal support and had a slow but uneventful postoperative recovery. She was discharged from the hospital 44 days after transplantation and has been alive and at home for more than 1 year at the time of writing.
| Pat | Pump | Oxygenator | ECMO blood flow L/min | ECMO O2% | ECMO gas flow L/min | Oxygenator replacement day |
|---|---|---|---|---|---|---|
| 1 | Maquet Rotaflow RF 32 | Maquet Quadrox PLS | 3.2–3.5 | 100 | 2.5–3.0 | 19 |
| 2 | Medtronic—Biomedicus | Maquet Quadrox PLS | 3.0–3.5 | 100 | 3.0–3.5 | None |
| 3 | Maquet Rotaflow RF 32 | Maquet Quadrox PLS | 4.0–4.5 | 100 | 4.0–5.0 | None |
| 4 | Maquet Rotaflow RF 32 | Maquet Quadrox PLS | 2.8–3.3 | 80–100 | 4.0–5.0 | None |
| 5 | Maquet Rotaflow RF 32 | Maquet Quadrox PLS | 3.0–3.5 | 80–100 | 2.0–3.0 | None |
- ECMO = extracorporeal membrane oxygenation.
Case 2
This 53-year-old male (Table 1) had a history of pulmonary fibrosis and severe PAH associated with progressive systemic sclerosis for which he was treated with bosentan and sildenafil. He was admitted to the hospital with pneumonia, which responded to antibiotic therapy. Nevertheless, he developed progressive right heart failure refractory to treatment with intravenous iloprost and dobutamine. On ICU admission, the patient was profoundly hypotensive and hypoxemic requiring noninvasive ventilation (NIV) with an FiO2 of 0.8 to achieve peripheral oxygen saturations of 80–90% (Table 2).
Veno-arterial ECMO was implanted on an emergency basis using the same technique as described in the first patient. The ECMO blood flow ranged between 3.0 and 3.5 L/min, which resulted in immediate stabilization of hemodynamics and gas exchange (Table 2). However, after 4 days on ECMO support, the patient developed spontaneous but massive epistaxis and required endotracheal intubation (at that time, the activating clotting time, ACT, was 200 s). Epistaxis was managed successfully with electrocoagulation therapy. After that episode, the patient remained on ventilator support but was stable during the following days, and a suitable donor organ became available after 10 days on ECMO. Bilateral lung transplantation was performed successfully but the postoperative course was complicated by several episodes of rejection as well as infectious complications and the patients died 2 months after transplantation from septic multiorgan failure.
Case 3
A 41-year-old male (Table 1) with lung fibrosis und severe pulmonary hypertension due to sarcoidosis was transferred to our ICU with combined heart and lung failure. At the time of admission, he received high-flow oxygen supplementation (15 L/min via a nonrebreathing mask) but remained profoundly hypoxemic (Table 2). Right heart catheterization showed right heart failure with a right atrial pressure of 24 mmHg, a mean PA pressure of 44 mmHg, a cardiac index of 1.2 L/min/m2 and a mixed venous oxygen saturation of 33%. Mean systemic blood pressures did not exceed 70 mmHg. The patient was treated with noninvasive ventilation (FiO2 1.0), sildenafil and increasing doses of dobutamine. However, 5 days after admission he developed cardiogenic shock with profound systemic hypotension, lactic acidosis and anuria.
Veno-arterial ECMO was inserted on an emergency basis as described above. ECMO flow ranged between 4.0 and 4.5 L/min resulting in substantial improvements of blood pressure and blood gases, respectively (Table 2). Renal function recovered immediately once ECMO was implanted and blood lactate levels returned to normal within 12 h. The patient remained fully awake (Figure 1), was cooperative and underwent physiotherapy with active training. After 18 days on ECMO support, a donor organ became available and the patient underwent successful bilateral lung transplantation. He left the ICU on the 6th postoperative day and was discharged from the hospital 20 days after transplantation.
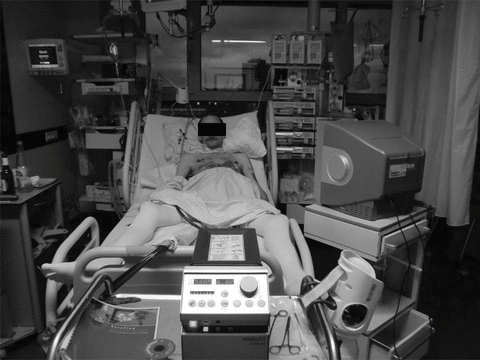
This male patient is nonsedated, spontaneously breathing and watching TV while receiving cardiopulmonary support from extracorporeal membrane oxygenation. The venous cannula is inserted in the right femoral vein and advanced into the right atrium; the oxygenated blood is returned via the arterial line, which is placed in the left femoral artery.
Case 4
This 54-year-old female (Table 1) suffered from advanced idiopathic pulmonary fibrosis with severe pulmonary hypertension. Her treatment at home included sildenafil, oxygen and noninvasive ventilation. The patient was admitted to our hospital with cardiopulmonary failure. On arrival she was comatose, hypotensive and in anuric renal failure despite treatment with norepinephrine and dobutamine (Table 2). Arterial blood gases confirmed advanced and progressive respiratory failure (on admission, pH 7.16, paCO2 114 mmHg; 1 h later pH 7.05, paCO2 158 mmHg, despite the use of NIV). Markedly elevated central venous pressures and low central venous oxygen saturations (Table 2) indicated right heart failure. In accordance, echocardiography showed pulmonary hypertension with an estimated systolic PA pressure of 80 mmHg and severe right ventricular dilatation.
Veno-arterial ECMO was inserted on an emergency basis. The patient was unconscious at that time due to the high carbon dioxide levels. The ECMO blood flow ranged between 2.8 and 3.3 L/min. Within 12 h, the arterial paCO2 fell to 45 mmHg and the patient regained consciousness. NIV was terminated and the peripheral oxygen saturation remained >90% while the patient was breathing supplemental oxygen at flow rates between 4 and 6 L/min. Catecholamines were discontinued, diuresis returned immediately after ECMO insertion and serum creatinine values normalized within 3 days.
The patient was fully awake, orientated and communicative and was seen daily by a physiotherapist for exercise and respiratory training. There were no signs of hemolysis but the patient had intermittent bleeding from the site of the arterial cannulation requiring repeated blood transfusion. After 35 days of ECMO support, a suitable donor organ was available and the patient underwent bilateral lung transplantation. Because of the presence of positive HLA antibodies the patient was treated with plasma exchange and rituximab after surgery. The postoperative course was complicated by primary graft dysfunction with prolonged mechanical ventilation. The patient was eventually weaned from the ventilator and was discharged from the ICU 18 days after transplantation. On postoperative day 20, she was able to walk and on day 44, she was transferred to a rehabilitation facility.
Case 5
This 55-year-old female (Table 1) with idiopathic PAH was transferred to our hospital with right heart failure and renal failure nonresponsive to treatment with sildenafil, bosentan, intravenous iloprost and dobutamine. On admission, the patient was hypotensive and anuric. She had low central venous oxygen saturations and high right-sided filling pressures. At the same time she was profoundly hypoxemic despite high-flow oxygen supplementation (Table 2).
Veno-arterial ECMO was implanted on an emergency basis under local anesthesia. ECMO flow rates between 3.0 and 3.5 L/min resulted in immediate hemodynamic and respiratory stabilization as well as recovery of renal function. However, on the 3rd day on ECMO, massive pulmonary bleeding occurred, and the patient required endotracheal intubation. The ACT at that time was 193 s. The source of bleeding could not be identified but bleeding stopped after heparin was paused for 4 h. However, the patient developed severe sepsis and died from multiorgan failure on the 8th day of ECMO support.
Discussion
The present case series show that veno-arterial ECMO can be used in awake patients presenting with cardiopulmonary failure to ensure stabilization of hemodynamics and gas exchange even when optimized medical therapy proves unsuccessful. In most of our patients, ECMO was inserted on an emergency basis and it was remarkable to see these patients stabilizing within minutes. Prolonged use (up to 35 days in this series) of this technique was feasible and provided a successful bridging strategy to lung transplantation. None of the patients in this series had signs of limb ischemia, hemolysis, platelet activation or a systemic inflammatory response while being on ECMO support and there were no clinically apparent systemic embolisms. We believe that the absence of these complications in the present series was related to several factors including relatively low blood flow rates, the use of low-resistance, heparin-coated silicone membrane oxygenators as well as the use of modern pumps combining highly effective blood flow with low blood damage (6). One patient developed infection of unknown origin responsive to broad-spectrum antibiotics. Intractable septicemia developed in one patient after she required endotracheal intubation following massive pulmonary bleeding. The remaining patients had no infectious complications. Overall, of the five patients treated with this strategy, four survived until transplantation and three made a full recovery.
As in other ECMO studies (7–9), the most important complications in this small case series were related to bleeding. One patient required endotracheal intubation after developing severe epistaxis and another patient required endotracheal intubation because of pulmonary bleeding. One additional patient had recurrent bleeding episodes from the site of the arterial cannula that were manageable but required repeated blood transfusions. The use of ECMO requires continuous anticoagulation. In our center, we use intravenous heparin to achieve ACTs between 180 and 200 s. Thus, bleeding complications may not be fully avoided with the devices currently in use. Research is ongoing to develop systems and membranes that allow the use of extracorporeal devices without aggressive anticoagulation.
The prolonged use of ECMO support in patients waiting for lung transplantation cannot and should not be considered standard procedure (2,10). Such patients may have a higher risk of peri- and postoperative complications and a much longer recovery time than patients who are transplanted in a ‘normal’ setting (2). Nevertheless, our results show that prolonged use of ECMO is feasible and can result in good outcomes. This is remarkable as all patients in the present series were moribund and none of them would have survived without ECMO support. Although case reports have already demonstrated the feasibility of prolonged ECMO use (11,12), we were still surprised to see that patients would tolerate ECMO times up to 35 days without major complications. ECMO blood flow rates of 3–4 L/min were sufficient to provide adequate perfusion and oxygenation of the whole body. Blood flow through the lungs was not monitored but was probably low as the patients were in right heart failure, venous blood from the right atrium was withdrawn into the ECMO circuit and all PAH medications were stopped at the time of ECMO insertion.
The main novel aspect of our strategy was the intention to keep the patients awake and spontaneously breathing during the time of ECMO insertion, and thereafter. This strategy allowed us to avoid the potentially disastrous hemodynamic consequences of general anesthesia in patients with right heart failure as well as the numerous drawbacks of endotracheal intubation, sedation and long-term mechanical ventilation (4,13–15). We assume that abstaining from intubation and mechanical ventilation was crucial in preventing septic complications. Three of the five patients in this series remained nonsedated until the time of transplantation. None of these patients had a serious infectious problem. All of these patients kept their ability to breathe spontaneously and to eat and drink without the need of artificial nutrition. In addition, they all received passive and active physiotherapy as well as psychological support throughout the waiting time. It is likely that these factors contributed to the good outcome and the relatively rapid recovery of these patients.
The ECMO approach described here is an alternative to the use of a pumpless lung assist device inserted between the PA and the LA (PA-LA) as described recently (5). The advantage of the awake veno-arterial technique is that no sedation, intubation and mechanical ventilation are required at any time, whereas the PA-LA approach necessitates general anesthesia and surgery for device placement. In contrast, a major advantage of the PA LA approach is that no blood pump is needed. Extubation and mobilization of these patients seems to be possible (5). It is also unclear, which technique results in a more effective unloading of the right ventricle. The PA-LA approach results in an immediate reduction of the right ventricular afterload whereas veno-arterial ECMO unloads the right ventricle mainly by reducing preload (decreasing right ventricular diameter and thereby also reducing afterload). With both techniques systemic perfusion pressure and systemic oxygenation improve, which is important for breaking the vicious circle of right heart failure. However, we did not systematically assess right ventricular function in our patients while they were on extracorporeal support.
These data have all the limitations of small case series but they may be viewed as a proof-of-concept study demonstrating the feasibility of using ECMO support in nonintubated patients. Only selected patients will be suitable candidates for this strategy. It certainly requires a huge amount of self-discipline and motivation to tolerate several weeks of being restrained to a hospital bed and connected to cardiopulmonary devices. Ongoing support from family members, friends, physicians, therapists and nurses is a prerequisite of success. Bed-side TV, DVD players, music, newspapers, books, laptops, etc. proved to be helpful. We prefer placing both the arterial and the venous cannula in one leg to allow the patients to move as much as possible. Using other cannulation sites such as jugular veins and subclavian arteries may allow further ambulation but so far, we felt that the femoral approach was superior in terms of safety. All three patients who survived until hospital discharge are without need for assistance and report an excellent quality of life. They all said that they would not hesitate to undergo the same procedure again.
So far, we have used ECMO support only as a last resort in patients with terminal illness as bridge-to-transplantation. Based on our hitherto experience, we now consider using this strategy earlier, that is, prior to the development of terminal right heart failure or in order to avoid endotracheal intubation in patients with end-stage respiratory failure. The concept of using ECMO support in awake patients may also be an option in other clinical settings, for instance, in patients with acute lung failure of various etiologies, where ECMO might be used as bridge-to-recovery to avoid endotracheal intubation and mechanical ventilation.
In conclusion, these preliminary data support the concept that the use of ECMO in awake patients may be a viable bridging strategy for selected patients with cardiopulmonary failure that can be applied successfully over prolonged periods of time.
Acknowledgment
We are indebted to the staff of the medical ICU for their ongoing skillful care of their patients.
Funding: None




