The Molecular Phenotype of Heart Transplant Biopsies: Relationship to Histopathological and Clinical Variables
Abstract
Histopathology of endomyocardial biopsies (EMB) is the standard rejection surveillance for heart transplants. However, ISHLT consensus criteria for interpreting biopsies are arbitrarily defined. Gene expression offers an independent re-evaluation of existing diagnostic systems. We performed histologic and microarray analysis on 105 EMB from 45 heart allograft recipients. Histologic lesions, diagnosis and transcripts were compared to one another, time posttransplantation, indication for biopsy and left ventricular ejection fraction (LVEF). Histologic lesions presented in two groups: myocyte–interstitial and microcirculation lesions. Expression of transcript sets reflecting T cell and macrophage infiltration, and γ-interferon effects correlated strongly with each other and with transcripts indicating tissue/myocardium injury. This molecular phenotype correlated with Quilty (p < 0.005), microcirculation lesions (p < 0.05) and decreased LVEF (p < 0.007), but not with the histologic diagnosis of rejection. In multivariate analysis, LVEF was associated (p < 0.03) with γ-interferon inducible transcripts, time posttransplantation, ischemic injury and clinically indicated biopsies, but not the diagnosis of rejection. The results indicate that (a) the current ISHLT system for diagnosing rejection does not reflect the molecular phenotype in EMB and lacks clinical relevance; (b) the interpretation of Quilty lesions has to be revisited; (c) the assessment of molecules in heart biopsy can guide improvements of current diagnostics.
Introduction
The monitoring of heart transplant recipients requires histologic examination of endomyocardial biopsies (EMB), obtained either as routine surveillance protocol biopsies (PB) or as biopsies for cause (BFC) when rejection is clinically suspected. The major goal is to identify acute cellular rejection (ACR) and/or antibody-mediated rejection (ABMR). Histopathological assessment of EMB follows consensus criteria generated by pathologists and clinicians—the International Society for Heart and Lung Transplantation (ISHLT) classification (1). The histological lesions (e.g. infiltrates, myocyte damage) were empirically defined and assembled into diagnoses under arbitrary rules. The classification system—lesions, rules and diagnoses—evolved as new findings and diagnostic tests emerged (e.g. C4d staining for diagnosing ABMR) (2–4). Every 2 years the Banff working group for heart allograft pathology has reviewed the classification based on new data (4–6). Establishing consensus around how lesions are defined and translated into diagnoses was essential for improving clinical practice and permitting data to be compared between centers and pathologists. However, consensus does not mean correctness; indeed, consensus is the first step in defining which beliefs are incorrect (5).
There are many concerns about the current ISHLT system. First, it has never been independently validated against another system for biopsy assessment, due to the absence of independent measurements of pathology in the allograft tissue. Second, there is considerable disagreement among observers: this was highlighted in the Cargo study (7,8). Third, the definition of lesions, rules, and diagnoses is often counterintuitive: for example, two foci of myocyte damage with an accompanying infiltrate indicate a diagnosis of a moderate acute cellular rejection (ACR Grade 2R), a diagnosis which triggers therapy (9) but the same extent of infiltration with only one focus of myocyte damage is called ACR Grade 1R, a diagnosis without clinical significance. In both situations (ACR 1R or 2R) it is irrelevant whether the infiltrates consist of 10 cells or 10 000 cells. However, neither inflammation nor myocyte damage is specific for rejection: both lesions can be observed in myocarditis in native hearts. In contrast, a lesion observed only in transplanted hearts, the Quilty effect, is considered a diagnosis but clinically irrelevant (2), and it remains difficult to distinguish Quilty from ACR (8).
In this study, we hypothesized that the parallel assessment of heart transplant EMBs by histological and molecular features compared to clinical features would offer an external comparison for the ISHLT classification and show whether molecules can add insight. Thus, we took a purely data-driven approach, assessing 105 EMB using Affymetrix microarrays, histopathology and clinical features. We included BFC as well as PBs: by comparing these two conditions we can define which features in the biopsy are related to a clinical relevant phenotype representing an indication for a biopsy. We summarized the microarray results using a system of nonoverlapping pathogenesis-based transcript (PBTs) sets. The PBT system annotates transcripts as representing discrete biological events relevant in organ transplants: infiltration by T cells or macrophages; γ-interferon effects and effects of injury on the parenchyma, that is myocytes and endothelium. We independently developed this approach in experimental systems and tested it in kidney transplant biopsies, where PBTs correlate with lesions, diagnoses, allograft function and outcomes (10–13). It is critical to note that PBTs are not derived or influenced by the present data, and thus represent a simple unit of measurement of the molecular changes. The aims of this study were to (i) define the molecular phenotype in EMB by assessing the relationships among PBTs; (ii) correlate the molecular phenotype with histological lesions and ISHLT-diagnoses; and (iii) relate the molecular phenotype to clinical variables, that is left ventricular ejection fraction (LVEF), indication and time of biopsy.
Materials and Methods
Patients and biopsies
The study was approved by the University of Alberta Research Ethics Board (Issue #5299). After receiving written informed consent from 45 patients, 105 right ventricular EMB (82 PB and 23 BFC) were obtained between June 2007 and December 2008. In addition to, at least four bioptome bites for standard histopathological work up, an extra bite was immediately immersed in RNALater (Ambion, Austin, TX) and stored at −20°C (11). Histological slides were re-evaluated by one pathologist (M.M.) following international consensus (Table 1) (2). For all cases where additional frozen material was available (n = 88) stains for C4d and C3d were done by indirect immunofluorescence (13). LVEF at biopsy was assessed by echocardiography. Values greater than 50% were considered normal. Biopsies were labeled BFC by a trained transplant cardiologist (D.K.) if acute rejection was clinically suspected, either due to a significant drop in LVEF or clinical symptoms suggestive of rejection, that is presence of congestive or low output symptoms: fever, malaise, myalgias, joint discomfort, flu-like symptoms, sinus tachycardia, pericardial rub, new onset of frequent artrial premature contractions, atrial fibrillation or flutter, new or enlarging pericardial effusion by echocardiography, history of sever fatigue, listlessness, sudden onset of progressive dyspnea at low levels of activity, syncope or presyncope, orthopnea or paroxysmal nocturnal dyspnea, rales on auscultation, raised jugular venous pressure, S3 or S4 gallop, prominent P2, hepatomegaly, cardiomegaly on chest X-ray, acute pulmonary edema, peripheral edema, diminished peripheral pulses, hypotension. All other biopsies were considered as PB done for surveillance purposes.
| Histological lesion | Description for evaluation |
|---|---|
| Interstitial edema | % Surface area of available myocardium showing interstitial edema, that is visible spaces between myocytes. |
| Perivascular inflammation% | % Surface area of available myocardium with perivascular inflammation. The absolute number of perivascular inflammatory foci seen in all available biopsy fragments was also recorded and correlated significantly (r = 0.89, p < 0.001) with the extent of the total perivascular infiltrate. |
| Interstitial inflammation% | % Surface area of available myocardium with interstitial inflammation. The absolute number of interstitial inflammatory foci seen in all available biopsy fragments was also recorded and correlated significantly (r = 0.97, p < 0.001) with the extent of the total interstitial infiltrate. |
| Hemorrhage | % Surface area of available myocardium with interstitial hemorrhage/aggregates of extravasated erythrocytes |
| Capillary microthrombi/fibrin aggregates | 0 = absent; 1 = single/few; 2 = multiple |
| Capillaritis | % Capillaries with intra-luminal accumulation of inflammatory cells |
| Capillary endothelium injury | Swelling or denudation: 0 = absent; 1 = present |
| C4d% | % Intramural capillaries with circumferential, linear C4d stain |
| C3d% | % Intramural capillaries with circumferential, linear C3d stain |
| Foci of myocyte damage | Absolute number of foci showing myocyte damage/myocytolysis in all available biopsy fragments |
| Histological diagnosis | Description of criteria |
|---|---|
| Acute cellular rejection (ACR) | According to ISHLT 2004: Grade 0–3R |
| Antibody-mediated rejection (AMR) | According to ISHLT 2004: 0 = absent; 1 = present |
| Quilty-lesion | According to ISHLT 1990: 0 = absent; 1 = Quilty A; 2 = Quilty B: |
| Previous biopsy site | According to ISHLT 1990: 0 = absent, 1 = present |
| Ischemic injury | According to ISHLT 1990: 0 = absent, 1 = present |
Microarray experiments
RNA extraction, labeling and hybridization to HG_U133_Plus_2.0 GeneChips (Affymetrix, Santa Clara, CA) were carried out according to protocols published at http://www.affymetrix.com and as previously described (11). Microarray data was preprocessed by RMA. Fold change for each gene was calculated as the ratio of expression values in each heart allograft biopsy versus the average value from three histological normal PB showing the lowest expression of cytotoxic T-cell-associated transcripts.
Pathogenesis-based transcript sets
Microarray gene expression results for each of the 105 biopsies were summarized as PBT scores: the geometric mean of fold changes across all probesets in that PBT. By the PBT approach, large scale and cumbersome microarray gene expression results are collapsed into single PBT scores representing a measurement of the respective biological process in the tissue (11,14–19). The PBTs were derived from experimental models (mouse kidney and heart allografts and human cell lines) and represent a priori defined major biological processes in organ allografts: infiltration of cytotoxic T cells (QCATs, n = 25 probesets) (19), infiltration of macrophages (QCMATs, n = 71 probesets) (20), γ-interferon (GRIT1, n = 68 probesets) (16) or injury- and repair-induced transcripts (cIRIT3, n = 620 probesets) (17), endothelial transcripts (ENDATs, n = 119 probesets) (13) and heart parenchymal transcripts with decreased expression during injury- and rejection-like solute carriers (15) and heart-specific transcripts (e.g. troponins) (HT, n = 1248 probesets). Table 2 provides a detailed description of the PBTs analyzed in this study. The probesets in each PBT as well as the related algorithms showing how the PBTs were derived are available at (http://transplants.med.ualberta.ca/Nephlab/data/gene_lists.html).
| PBT abbreviation | PBT name | Biological description | Reference |
|---|---|---|---|
| HT | Human cardiac, heart transcripts | Cardiac selective transcripts showing high expression in normal control mouse heart but low expression in inflammatory cells | Adapted from kidney Refs. (15) and (29) to heart |
| cIRIT3 | Human cardiac injury and repair induced transcripts—set 3 | Transcripts induced by a nonimmune heart injury, highly increased in response to alloinjury | Derived for this study from mouse heart transplants in analogy to mouse kidney transplants (17) |
| ENDAT | Endothelium-associated transcripts | Literature-based transcript set, in which genes were identified based on their selective expression in cultured human endothelial cells when compared with non-endothelial cells | Described in detail in Ref. (13) |
| QCAT | Quantitative cytotoxic T-cell-associated transcripts | Quantitative burden of T-cell-associated transcripts in rejecting organ, assessed by a refined set of most robustly expressed cytotoxic T-cell-associated transcripts | Described in detail in Ref. (19) |
| GRIT1 | γ-Interferon and rejection induced transcripts—set 1 | γ-Interferon inducible transcripts in inflammatory cells | Described in detail in Ref. (11) |
| QCMATs | Quantitative constitutive macrophage associated transcripts | Quantitative burden of transcripts in the tissue associated with constitutively activated macrophages | Described in detail in Ref. (26) |
- 1The probesets in each PBT as well as the algorithms how the PBTs were derived are available at http://transplants.med.ualberta.ca/Nephlab/data/gene_lists.html.
Statistical analysis
Data analyses were performed using SPSS 15.0 statistical software package (SPSS Inc., Chicago, IL), Bioconductor version 2.4, and R version 2.9.1. Comparisons between continuous variables (PBTs, histology lesions (Table 1), LVEF) and ordinal variables (Quilty, ACR) were assessed as Spearman correlations. Comparison between continuous and binary variables (ABMR, ischemic injury, previous biopsy site, biopsy indication, time posttransplantation) was done by logistic regressions. Comparison between binary and ordinal variables used the Chi-square test or Fisher's exact test. Results are given as p-values while the level of significance was set at p < 0.05. Univariate analysis was used to determine which PBTs, diagnosis, histologic lesions and clinical variables would be considered for the multivariate model. A p-value tolerance of 0.05 was used to determine the adequacy of the data. For multivariate analysis, a forward stepwise method was used to process the model.
Results
Donor, recipient and biopsy demographics
Demographics for the 105 biopsies examined in 45 patients are shown in Table 3. Donor demographics were similar for the 23 BFCs and 82 PBs. Of the 23 biopsies for cause, 8 were due to a significant drop in LVEF and 15 due to clinical symptoms suggesting rejection. BFC were taken in younger recipients and later posttransplant. The LVEF at biopsy was lower for BFC than for PB as expected (51% vs. 57%, p = 0.013). More Quilty type B lesions were seen in biopsies with clinical suspicion for rejection (BFCs); while surprisingly ACR was not more frequent in biopsies with clinical problems (BFC) compared to those without (PB). We observed only two cases of ABMR, both in BFC (p = 0.046).
| All biopsies (n = 105) | Protocol biopsies1 (n = 82) | Biopsies for cause2 (n = 23) | p-Value2 | |
|---|---|---|---|---|
| Number of patients | 45 | 36 | 12 | |
| Donor gender: female/male | 13/32 | 10/26 | 4/8 | ns |
| Mean donor age in years | 32.2 (14–60) | 33.1 (14–60) | 30.6 (16–47) | ns |
| Donor ethnicity | ||||
| Caucasian | 32 (71.1%) | 29 (80.6%) | 5 (41.7%) | ns |
| Asian | 3 (6.7%) | 2 (5.5%) | 1 (8.3%) | ns |
| Hispanic | 2 (4.4%) | 1 (2.8%) | 1 (8.3%) | ns |
| Unknown | 8 (17.8%) | 4 (11.1%) | 5 (41.7%) | ns |
| Recipients gender: female/male | 12/33 | 10/26 | 2/10 | ns |
| Mean recipient age at transplantation in years | 51.4 (17–69) | 54.6 (17–69) | 44.6 (24–65) | 0.009 |
| Recipient ethnicity | ||||
| Caucasian | 43 (95.6%) | 34 (94.4%) | 12 (100%) | ns |
| Asian | 1 (2.2%) | 1 (2.8%) | 0 | ns |
| Multiracial | 1 (2.2%) | 1 (2.8%) | 0 | ns |
| Number of biopsies per patient | ||||
| Patients with one biopsy | 29 (64.4%) | 25 (69.4%) | 6 (50.0%) | ns |
| Patients with multiple biopsies | 16 (35.6%) | 11 (30.6%) | 6 (50.0%) | ns |
| Mean number of biopsies per patient | 2.3 (1–8) | 2.3 (1–8) | 1.9 (1–7) | ns |
| Mean time between transplantation and biopsy in days | 542.8 (8–4451) | 434.1 (8–2676) | 931.7 (26–4451) | 0.014 |
| Number of biopsies ≤12 months posttransplantation | 70 (66.7%) | 56 (68.3%) | 14 (60.9%) | ns |
| Number of biopsies >12 months posttransplantation | 35 (33.3%) | 26 (31.7%) | 9 (39.1%) | ns |
| Maintenance immunosuppression regimens at biopsy | ||||
| MMF, tacrolimus, steroids | 16 (15.2%) | 13 (15.9%) | 3 (13.1%) | ns |
| MMF, tacrolimus | 11 (10.5%) | 9 (11.0%) | 2 (8.7%) | ns |
| MMF, cyclosporine, steroids | 38 (36.2%) | 27 (32.9%) | 11 (47.8%) | ns |
| MMF, cyclosporine | 13 (12.4%) | 13 (15.9%) | 0 | ns |
| mTOR inhibitor, cyclosporine, steroids | 17 (16.2%) | 14 (43.8%) | 3 (13.1%) | ns |
| Others | 10 (9.5%) | 6 (7.3%) | 4 (17.4%) | ns |
| Mean% LVEF at biopsy | 55.8 (15–70) | 57.3 (40–70) | 50.7 (15–60) | 0.013 |
| ISHLT-Diagnosis by histopathology1 | ||||
| ACR 0 | 40 (38.1%) | 32 (39.0%) | 8 (34.8%) | ns |
| ACR 1R | 58 (55.2%) | 46 (56.1%) | 12 (52.8%) | ns |
| ACR 2R | 7 (6.7%) | 4 (4.9%) | 3 (13.1%) | ns |
| ACR 3R | 0 | 0 | 0 | ns |
| ABMR 0 | 103 (98.1%) | 82 (100%) | 21 (91.3%) | 0.046 |
| ABMR 1 | 2‡ (1.9%) | 0 | 2 (8.7%) | 0.046 |
| Quilty absent | 38 (36.2%) | 33 (40.2%) | 5 (21.7%) | ns |
| Quilty A | 35 (33.3%) | 28 (34.1%) | 7 (30.4%) | ns |
| Quilty B | 32 (30.5%) | 21 (25.6%) | 11 (47.8%) | 0.041 |
| Previous biopsy site | 53 (50.5%) | 40 (48.8%) | 13 (56.5%) | ns |
| Ischemic injury | 20 (19.1%) | 16 (19.5%) | 4 (17.4%) | ns |
- LVEF = left ventricular ejection fraction; ACR = acute cellular rejection; ABMR = antibody-mediated rejection; ns = not significant.
- 1Comparing protocol biopsies versus biopsies for cause.
- 2More than one diagnostic label could be assigned to the same biopsy and one patient can have protocol and/or biopsies for cause.
Histological lesions in EMB present as two groups
Correlation analysis revealed two groups of histologic lesions (Table 4): microcirculation changes (capillaritis, capillary endothelial injury/swelling, microthrombi, interstitial hemorrhage and edema) and myocyte–interstitial changes (perivascular and interstitial infiltrates, myocyte damage).
| Microcirculation changes | Myocyte–interstitial changes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage | Interstitial edema | Capillary microthrombi | Capillary endothelium injury | % Capillaritis | C3d% | C4d% | Foci of myocyte damage | Perivascular inflammation% | Interstitial inflammation% |
| Hemorrhage | 0.282 | 0.11 | 0.292 | 0.333 | −0.01 | 0.272 | −0.05 | −0.09 | 0.15 |
| Interstitial edema | 0.12 | 0.333 | 0.363 | −0.01 | 0.282 | 0.16 | 0.17 | 0.12 | |
| Capillary microthrombi | 0.493 | 0.373 | 0.04 | 0.08 | −0.14 | 0.02 | 0.14 | ||
| Capillary endothelium injury | 0.803 | −0.09 | 0.08 | 0.06 | 0.03 | 0.16 | |||
| % Capillaritis | −0.10 | 0.06 | 0.12 | 0.10 | 0.224 | ||||
| C3d% | 0.463 | 0.18 | 0.16 | 0.08 | |||||
| C4d% | 0.01 | 0.10 | 0.19 | ||||||
| Foci of | 0.393 | 0.403 | |||||||
| myocyte damage | |||||||||
| Perivascular inflammation% | 0.473 | ||||||||
| Interstitial inflammation% | |||||||||
- 1Spearman correlation, r-values are given in the table.
- 2p < 0.01.
- 3p < 0.001.
- 4p < 0.05.
The percentage of capillary C4d staining correlated with percent C3d staining, and with interstitial edema and hemorrhage. When BFC were analyzed separately (both ABMR cases were in BFC), C4d correlated with microcirculatory lesions (edema: r = 0.53, p = 0.02; hemorrhage: r = 0.47, p = 0.05). (The phenotypes associated with HLA antibodies will be presented in a separate manuscript; Sis et al., manuscript in preparation.)
In a separate analysis, we included Quilty effect as a lesion rather than as a diagnosis (since it is not assembled from lesions as the other diagnoses). As a lesion, Quilty correlated with myocyte–interstitial changes: interstitial infiltrates (r = 0.22, p = 0.02); perivascular infiltrates (r = 0.21, p = 0.03) and myocyte damage (r = 0.42, p < 0.0001).
The molecular phenotype of EMB
Unlike lesions, all PBTs correlated strongly with one another. Cluster analysis of their correlation coefficients revealed two biological patterns: inflammation and injury/dedifferentiation. Inflammation was represented by γ-interferon inducible transcripts (GRITs), cytotoxic T cell transcripts (QCATs), and macrophage-associated transcripts (QCMATs). Injury/dedifferentiation was reflected by increased expression of cardiac injury-induced (cIRIT3), and endothelial transcripts (ENDATs), as well as decreased expression of heart transcripts (HT) (Figure 1). The inflammation-related transcripts strongly correlated with each other and with an increased expression of the injury transcripts. Increased inflammatory and injury PBTs correlated with increased expression of transcripts associated with endothelial cell activation. Injury transcripts also correlated with decreased expression of HTs which include transcripts usually highly expressed in normal hearts and here associated with the function of myocytes, that is solute carriers, energy metabolism and myocyte-specific transcripts such as myosin and troponins (for complete lists of probesets go to: http://transplants.med.ualberta.ca/Nephlab/data/gene_lists.html).
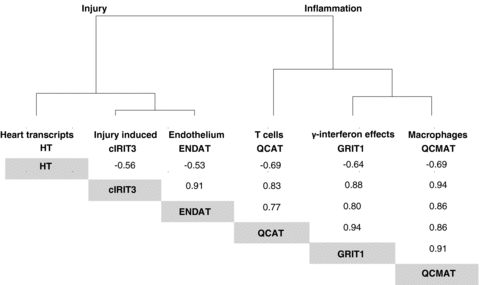
Associations* between PBTs. Dendogram showing the associations in between PBTs: increased expression of inflammation associated transcripts correlates with increased expression of injury transcripts and decreased expression of transcripts otherwise expressed in normal heart. Corresponding Spearman* correlation coefficients are given at the bottom, which are all significant at p < 0.001.
The molecular phenotype in EMB does not correlate with ISHLT diagnosis of ACR but is associated with Quilty and microcirculation lesions
Increased expression of inflammation/injury PBTs and decreased expression of HTs were poorly associated with the ISHLT diagnoses (Table 5). Surprisingly, only Quilty lesions were associated with increased expression of inflammation/injury PBTs and decreased expression of HTs. The diagnosis of ACR was not associated with expression of PBTs. Moreover, ACR was not associated with individual genes. Using 8206 IQR filtered probesets across all biopsies, there were no significantly changed transcripts in ACR 2R rejection biopsies (n = 7) or all ACR (n = 65) biopsies compared to nonrejecting biopsies (grade 0R with no Quilty or other pathology, p > 0.05, false discovery rate = 0.05).
| Significant associations are indicated by their p-Value inverse relationship are given in parentheses | ||||||
|---|---|---|---|---|---|---|
| Heart transcripts (HTs) | Injury induced (cIRIT3) | Endothelium (ENDAT) | T cells (QCAT) | γ-Interferon effects (GRIT1) | Macrophages (QCMAT) | |
| Histology diagnoses | ||||||
| Quilty (0 = no, 1 = A, 2 = B) | (0.005) | 0.002 | 0.004 | <0.001 | <0.001 | 0.001 |
| ACR | ns | ns | ns | ns | ns | ns |
| ABMR2 | na2 | na2 | na2 | na2 | na2 | na2 |
| Previous biopsy site | ns | ns | ns | ns | ns | ns |
| Ischemic injury | ns | ns | ns | ns | ns | ns |
| Histology lesions | ||||||
| Hemorrhage | ns | 0.003 | 0.015 | ns | 0.017 | 0.009 |
| Interstitial edema | (0.036) | <0.001 | <0.001 | 0.011 | 0.009 | <0.001 |
| Capillary microthrombi | ns | ns | ns | ns | ns | ns |
| Capillary endothelium injury | ns | 0.031 | ns | ns | ns | ns |
| % Capillaritis | ns | 0.001 | 0.033 | 0.050 | 0.012 | 0.006 |
| C3d% | ns | ns | ns | ns | ns | ns |
| C4d% | ns | ns | ns | ns | ns | ns |
| Foci of myocyte damage | ns | ns | ns | 0.019 | ns | ns |
| Perivascular inflammation | ns | ns | ns | ns | ns | ns |
| Interstitial inflammation | ns | ns | ns | ns | ns | ns |
- 1Interrelationships were assessed between continuous (PBTs, histology lesions) and ordinal (Quilty, ACR) variables as Spearman correlations, and between continuous and binary variables (ABMR, ischemic injury, previous biopsy site) as logistic regressions. Results are given as p-values if < 0.05.
- 2Because only two cases with ABMR were available no statistical analysis was performed (na = not applicable).
PBTs correlated more strongly with selected lesions, particularly microcirculation lesions, than with diagnostic labels (Table 5). Interstitial edema, hemorrhage and capillaritis correlated with increased expression of injury transcripts, inflammation PBTs, endothelial transcripts and decreased expression of HTs. Interstitial inflammation did not correlate with PBTs. Myocyte damage showed a weak correlation with the expression of cytotoxic T-cell-associated transcripts.
The relationships between increased expression of PBTs with one another and with clinical and histologic features are summarized in Figure 2. When individual biopsies (X-axis) were lined up by increasing scores for cytotoxic T-cell transcripts, other gene sets also lined up, reflecting the correlations among the PBTs. The ribbon above Figure 2 illustrates how cases with reduced LVEF, Quilty and BFC were associated with more disturbed PBTs but ACR diagnosis was not. This figure underscores a stereotyped disturbance in expression of inflammation and injury-related transcripts, and the relationship of this disturbance to the clinical and histologic features.
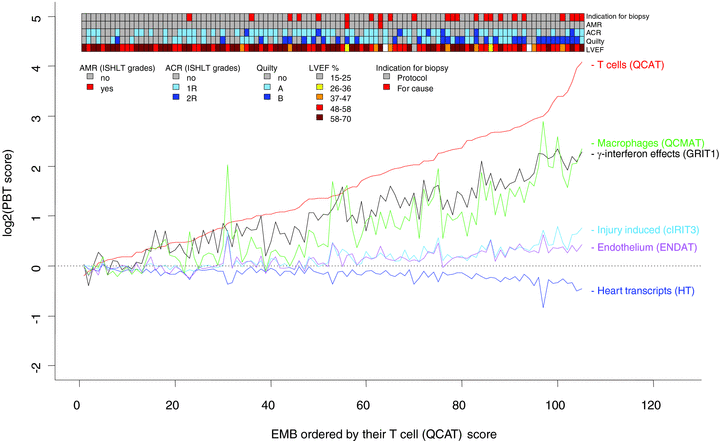
Interrelationships between PBTs, LVEF, indication for biopsy and ISHLT diagnosis of rejection. On the X-axis individual biopsies are lined up by increasing scores for cytotoxic T-cell transcripts. Reflecting the strong correlations among the PBTs, the other gene sets also lined up. The ribbon at the top illustrates how cases with reduced LVEF, Quilty, and BFC are associated with more disturbed PBTs but ACR diagnosis was not. This figure underscores a stereotyped disturbance in expression of inflammation and injury-related transcripts, and the relationship of this disturbance to the clinical and histologic features.
Interrelationships between clinical variables, PBTs, diagnoses and lesions
The molecular changes were more strongly related to the clinical features (LVEF and biopsy indication) than to the histologic lesions or diagnoses. Thus, increased expression of injury (cIRITs), endothelial (ENDATs) and the inflammation PBTs (GRITs, QCATs, QCMATs) correlated with decreased LVEF at the time of biopsy and with BFCs (Table 6). In addition, injury-induced transcripts were inversely related to time posttransplant, probably reflecting the injury of the donation–implantation process and its subsequent resolution over time.
| Significant associations are indicated by their p-Value, inverse relationship are given in parentheses | |||
|---|---|---|---|
| Time posttransplantation (prior 1 year = 0, post 1 year = 1) | Indication for biopsy (PB = 0, BFC = 1) | LVEF | |
| PBTs | |||
| Heart transcripts (HTs) | ns | ns | ns |
| Injury induced (cIRIT3) | (0.05) | 0.006 | (0.001) |
| Endothelium (ENDAT) | ns | 0.001 | (0.006) |
| T cells (QCAT) | ns | <0.001 | (0.003) |
| γ-Interferon effects (GRIT1) | ns | 0.004 | (0.001) |
| Macrophages (QCMAT) | ns | 0.003 | (0.007) |
| Histological diagnoses | |||
| Quilty (0 = no, 1 = A, 2 = B) | ns | ns | (0.028) |
| ACR | ns | ns | ns |
| ABMR2 | na2 | na2 | na2 |
| Previous biopsy site | ns | ns | ns |
| Ischemic injury | ns | ns | (0.041) |
| Histology lesions | |||
| Hemorrhage | ns | ns | ns |
| Interstitial edema | ns | 0.001 | (0.002) |
| Capillary microthrombi | ns | ns | (0.003) |
| Capillary endothelium injury | ns | ns | (0.019) |
| % Capillaritis | ns | ns | ns |
| C3d% | ns | ns | ns |
| C4d% | ns | ns | ns |
| Foci of myocyte damage | ns | 0.007 | ns |
| Perivascular inflammation | ns | 0.015 | ns |
| Interstitial inflammation | ns | 0.022 | ns |
| Clinical variables | |||
| Time posttransplantation | 0.02 | (0.032) | |
| Indication for biopsy (BFC = 1, PBx = 0) | ns | (0.003) | |
- 1Interrelationships were assessed between continuous (PBTs, histology lesions, time posttransplantation, LVEF) and ordinal (Quilty, ACR) variables as Spearman correlations, continuous and binary variables (ABMR, ischemic injury, previous biopsy site, indication for biopsy) as logistic regressions, and binary and ordinal variables as Chi-square test. Results are given as p-values if < 0.05.
- 2Because only two cases with ABMR were available no statistical analysis was performed (na = not applicable).
Among histologic diagnoses (Table 6), there was no association of ISHLT ACR grade with LVEF or biopsy indication. Quilty was associated with a reduced LVEF at biopsy. The diagnosis of ‘ischemic injury’ was weakly associated with reduced LVEF, but other diagnoses were not. (Having a BFC was associated with the diagnosis of ABMR, but having only two cases precludes conclusions.)
Among histologic lesions, microcirculation lesions (edema, capillary microthrombi, endothelial injury) were associated with lower LVEF, whereas interstitial inflammation and myocyte damage were not (Table 6). Interstitial inflammation, myocyte damage and interstitial edema were more frequent in BFC.
Patient-based analysis
To exclude bias by similarities between sequential biopsies from the same patient, we repeated the analysis with only one biopsy per patient (the latest biopsy after transplantation from each patient). This analysis essentially confirmed the associations described earlier (Tables S1–S4), with the exception of the associations with LVEF. This may reflect the reduced sample size and a smaller variability in the LEVF late posttransplantation.
Multivariable analysis of variables related to LVEF
In a forward stepwise multivariable model, we studied those variables that are associated with LVEF at the time of biopsy. All variables (lesions, diagnosis, PBTs, clinical variables) were analyzed separately and nine that were univariately significant at p < 0.05 were included into the multivariate model. As expected and thus corroborating the validity of the model, a BFC or a late biopsy was associated with a reduced LVEF. In addition, a diagnosis of ischemic injury and transcriptional changes of increased γ-interferon effects were associated with an impaired LVEF (Table 7).
| Multivariate analysis | Adjusted r2= 0.2235 | Odds ratio | Upper bound | ||
|---|---|---|---|---|---|
| Lower bound | |||||
| γ-Interferon effects (GRIT1) | −2.412 | 0.031 | 1.037 | 1.514 | 2.210 |
| Ischemic injury | −4.687 | 0.010 | 1.053 | 1.238 | 1.455 |
| Time posttransplantation (prior 1 year = 0, post 1 year = 1) | −4.060 | 0.008 | 1.067 | 1.279 | 1.534 |
| Indication for biopsy (PB = 0, BFC = 1) | −5.319 | 0.004 | 1.064 | 1.207 | 1.369 |
The effect of treatment on the molecular phenotype
In six patients who received antirejection treatment, follow-up biopsies within less than 4 weeks after treatment were available. Similar to findings in kidney allografts (12), we observed a trend toward a decrease in expression in inflammatory and injury PBTs and an increase (i.e. normalization) in expression of HTs (Figure 3). However, due to the very small number of matching follow-up biopsies, the differences did not reach statistical significance, with the exception for the QCATs (p = 0.02).
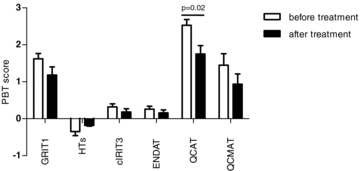
Effect of rejection treatment on PBT expression. In a subset of six patients with available follow-up biopsies, a trend toward an ameliorated molecular phenotype after rejection treatment can be observed.
Discussion
This study undertook a data-driven examination of the histologic and molecular features in EMB compared to clinical variables: LVEF, time posttransplant and whether the biopsy was triggered by clinical suspicion for rejection. To prevent bias, the population was inclusive, not selected to represent extreme phenotypes, because that approach would be based on the existing classification and exclude difficult and ambiguous cases (21). The histological features were assessed using the ISHLT system, and the molecular phenotype by measuring PBTs sets, which were previously defined using experimental material, and not trained on the present data (11,13,16,17,19). The microarray analysis of the EMB revealed a striking stereotyping in the molecular phenotype, with all PBT scores highly correlated with each other. There were two main subgroups of PBTs: inflammation related and injury-dedifferentiation related (including endothelial transcripts). The molecular changes were more pronounced when the biopsy was done for clinically suspected rejection, and correlated with impaired LVEF. But the histologic features generally did not distinguish biopsies for clinical cause or correlate with LVEF. The molecular changes were most strongly correlated with Quilty and microcirculation lesions, but did not correlate with ACR diagnosed by the current ISHLT rules. In summary, this study indicates that (1) the molecular phenotype offers biological and diagnostic insights not available through histopathology; (2) lesions currently regarded as diagnostic for ACR (e.g. interstitial, perivascular cell infiltration, myocyte damage) show little correlation with the clinical and molecular phenotype; and (3) in a significant proportion of cases, the inflammatory changes currently classified as Quilty probably represent a form of ACR.
The transcript changes in EMBs from heart allografts revealed a stereotyped molecular disturbance in which increased expression of transcript sets reflecting inflammation (T cells, macrophages, γ-interferon effects) were associated with injury-induced transcripts, endothelial transcripts and loss of transcripts expressed in normal myocardium. This iconic disturbance is very similar to that described in kidney transplant biopsies (11), an anticipated observation since many transcripts sets have no tissue specificity, e.g. T cells, macrophages, γ-interferon effects. The correlations among these nonoverlapping transcript sets reveal a complex linkage between inflammation, injury and tissue de-differentiation that is probably a general principal of tissue responses to stress, rather than specific for a disease state. Heart-specific transcripts such as the troponins showed decreased expression in the injured and inflamed biopsies. This is in contrast to the increase of troponin proteins in the circulation observed after myocardial injury. It can be hypothesized that existing troponin proteins are shed from injured cells into the circulation while their transcription ceases as part of the general dedifferentiation and injury response of the myocardium. Nevertheless, this stereotyped molecular disturbance had clinical correlations superior to the histopathology, was more pronounced in BFC than in PBs, and correlated with impaired allograft function, even in multivariate analysis.
The finding that Quilty correlates with the molecular phenotype, BFC and impaired LVEF invites a reassessment of Quilty. Quilty originated in the pure descriptive recognition of subendocardial inflammatory follicular aggregates in human heart allografts (22). Efforts to establish whether or not Quilty lesions predict impaired outcomes have been limited by the fortunate paucity of failures under modern immunosuppression and the complexity of the covariates related to long-term outcome (23,24). In our series Quilty, that is the ordinal analysis of no Quilty–Quilty A–Quilty B, correlated strongly with the molecular phenotype. Because the same molecular phenotype in kidney transplants is associated with acute T-cell-mediated rejection and here best correlates with the total interstitial inflammatory burden (11,12,25), this suggests that at least some of the inflammation currently called Quilty represents rejection. It can be hypothesized that acute cellular (T-cell-mediated) rejection is relatively similar in all organs and tissues (26). Excluding all Quilty lesions from the diagnosis of ACR is therefore not supported by this study, and has been challenged repeatedly in the past by other studies (23,27). Currently, a heterogeneous morphological spectrum of lesions is summarized under Quilty, reaching from very small subendocardial aggregates of a few lymphocytes to large invasive inflammatory compartments with accompanying myocyte damage. Such an approach implies that the pure subendocardial localization of inflammation is associated with a certain biological specificity. But the conflicting data regarding the significance of Quilty might be explained by heterogeneity within these lesions. Against this background and guided by the molecular features, we suggest an alternative system for scoring subendocardial inflammatory lesions: instead of a simple absent/present call, we propose a descriptive approach to assess the actual extent (percent biopsy surface involved, uni- or multifocal) and pattern (invasive, noninvasive, with or without myocyte damage) of subendocardial inflammation. In addition, specific assessment of the molecular phenotype of such variants of Quilty lesions after laser microdissection is currently underway in our laboratory. Together this would allow for a data-driven approach to clarify when this type of inflammation resembles acute T-cell-mediated rejection and thus is clinically significant.
Our finding that current criteria for ACR show no correlation with the molecular phenotype or clinical parameters (clinical suspicion for rejection, LVEF) underscores the need to revisit the current ISHLT classification. The observed histological lesions are divided as two groups: lesions related to inflammatory infiltrates (including Quilty) and lesions related to injury in the microcirculation. Current ISHLT criteria for ACR are based on the combination of interstitial inflammation (excluding Quilty) with coinciding myocyte damage. With this approach, the quality of the lesion is more crucial than the quantity. Large interstitial infiltrates can be present, and as long as no myocyte damage is observed in two foci a clinically relevant (i.e. ACR 2R) diagnosis cannot be rendered. On the other hand, cases with two small infiltrates and accompanying myocyte damage are sufficient for this usually treated diagnosis. This approach implies diagnostic specificity for myocyte injury, but just outside Quilty. The observed molecular phenotype does not support such an approach. A class comparison between ACR grade 0 and ACR 1R and 2R cases did not reveal any significant difference in terms of gene expression. The strongest histological correlate to the molecular phenotype was the total inflammatory burden in the tissue (including Quilty lesions), a finding similar to kidney transplants (25). Furthermore, the inflammatory histological and molecular phenotype was strongly associated with an increased expression of injury associated transcripts and a decrease in transcripts expressed in normal hearts, indicating that any inflammation is associated with injury and dedifferentiation of the myocardium.
Microcirculation changes (capillaritis, interstitial hemorrhage, edema) correlated with an abnormal molecular phenotype, but also with BFC and decreased LVEF. Microcirculatory injury lesions also correlated with transcripts associated with endothelial cell changes. This association was recently observed by our group in kidney transplants with signs of antibody-mediated rejection (13). Furthermore, Quilty lesions have been associated with microvasculopathy (24) and in our series was associated with an increased endothelial transcript score. Studies analyzing the relationship of the anti-HLA antibody to the inflammation patterns (including Quilty) and to microcirculation injury are currently underway.
The EMB could be misleading because it is an ‘edge’, which could manifest changes that are not diffuse. ‘Edges’ are avoided in other types of organ transplants for diagnostics, for example the subcapsular region of a kidney allograft biopsy. Thus, the interpretation of EMBs can be challenged by whether they represent the entire heart. Our results indicate that the molecules measured in EMBs potentially better represent the state of the transplanted heart. The fact that not the same biopsy pieces were analyzed by histology and microarrays could be a potential source for the observed poor correlations between certain, more focal morphological lesions and the molecular phenotype. However, it can be assumed that sampling error is to a similar extent impeding on histology as it is on molecular measurement, that is cases where histology misses the relevant lesion while they are captured for gene expression analysis might be as frequent as vice versa. But, molecular changes such as increased γ-interferon effects are uniform and diffuse in the tissue, and could be fairly represented in EMB. Furthermore, histopathology is not very sensitive in assessing the response to tissue injury (i.e. decreased myocyte function) in the absence of inflammation. This and the potentially more sensitive assessment of any response to treatment could be the advantages of transcriptome analysis in EMB, potentially offsetting current limitations in histopathology. The molecular phenotype of the tissue can be used as an independent test to refine and improve the definition of histopathological criteria for assessing EMB (28). Moreover, once the molecular and histological phenotypes of the EMB are refined and validated, non-biopsy-based diagnostic tests can be reliably validated against them. Thus, the present results can be a first step in a progressive development of an improved diagnostic system in heart transplantation.
Acknowledgments
Funding sources: This research has been supported by Alberta Health Services, Genome Canada, Genome Alberta, the University of Alberta Hospital Foundation, Roche Molecular Systems, Roche Organ Transplantation Research Foundation and Alberta Advanced Education and Technology.
Conflicts of Interest
The authors declare no conflicts of interest.




