A comparison of swimming capacity and energy use in seven European freshwater fish species
Abstract
Abstract – Migrating fish species with different swimming capacities and energy use show different capacities for passing obstacles between habitats, such as culverts and fish ladders. Here, we present an integrated study on swimming capacity and energetic use in seven European freshwater fish species with different ranges of migration (brown trout Salmo trutta L., European perch Perca fluviatilis L., roach Rutilus rutilus L., common carp Cyprinus carpio L., gudgeon Gobio gobio L., bullhead Cottus gobio L. and stone loach Barbatula barbatula L.). Critical (Ucrit), optimal (Uopt) and maximum (Umax) swimming speed and oxygen consumption (MO2) were analysed and showed values correlated to migration capacity with highest swimming capacities in trout and roach and lowest in stone loach and bullhead. The resulting data can be used to make estimates of maximum passable water speeds in culverts. In conclusion, long-distance migrators show higher swimming capacities and can potentially clear obstacles easier than short distance migrators with lower swimming capacities. Even small obstacles (<25 cm) could be a barrier for genetic exchange between populations in short-distance migrators.
Introduction
Swimming performance is one of the crucial factors determining survival within the aquatic environment in fish. Predator–prey interactions, reproduction, migration and dispersal are of great ecological importance and depend on the capacity for locomotion of the individual (Kolok 1999; Reidy et al. 2000). Therefore, fish species, adapted to different lifestyles and habitats, can be compared in terms of swimming capacity and energy metabolism. With increasing velocity, several swimming speeds are defined: for long distance swimming, fish can swim at optimal swimming speed (Uopt), i.e., the speed at which the energetic cost of transport (CoT) is the lowest, powered by aerobic muscles (Brett 1964; Webb 1971, 1975; Videler 1993). Gait transition (Utrans) marks the transition from aerobically powered steady swimming to aerobically and anaerobically powered unsteady swimming (Beamish 1978; Videler 1993). When swimming faster, fish reach critical swimming speed (Ucrit), the speed at which it is generally assumed that maximum oxygen uptake occurs (Webb 1971; Farrell & Steffensen 1987; Keen & Farrell 1994; Gregory & Wood 1999). Although arguably less informative then other speed definitions in terms of swimming physiology and swimming speeds in the wild, Ucrit gives a good estimate for swimming capacity in general, as it includes aerobic and anaerobic swimming (Videler 1993; Hammer 1995). The maximum speed (Umax), finally, is the white muscle powered speed that can be kept constant only for some milliseconds to seconds and is used for fast and short bursts. This speed is mostly used when escaping from predation or catching prey, but also for short bursts (Howland 1974; Webb 1984; Domenici & Blake 1997; Bergstrom 2002). With increasing swimming speeds, the sustainability of the swimming modes decreases from Uopt, which can be kept almost indefinitely, to Umax (Brett 1964; Webb 1975; Videler 1993).
When migrating, fish have to pass obstacles on their migration ways. Both long-distance and short-distance migratory fish species use waterways for migration (Lucas & Baras 2001). Physical barriers can obstruct free migration and can cause fragmentation and finally extinction of fish population (Warren & Pardew 1998;Toepfer et al. 1999; Warren et al. 2000). Often, fish ladders and culverts designed to facilitate the migration are introduced to migratory paths.
Diadromous migration (McDowall 1997) has ruled until now our perception of mobility in fishes, i.e., nondiadromous species have been deemed to be resident. However, there is growing evidence that most so-called resident freshwater fish species undertake potamodromous migrations, either systematically or in a series of river systems (Lucas & Baras 2001; Knaepkens et al. 2004, 2005). To our knowledge, swimming capacity and energetics have not been studied in these species, yet. Therefore, it is important to gather data on swimming performance and energetics of these species, too, to facilitate management of fish ways.
It has been shown that data obtained in forced swimming experiments cannot be applied without caution in the wild (Castro-Santos 2004, 2005; Peake & Farrell 2006). Peake & Farrell (2004) analysed the swimming behaviour of smallmouth bass (Micropterus dolomieu, Lacepede) and showed that at swimming speeds up to Ucrit bass attained a ground speed of about 0.25 times Ucrit. Thus, if the water speed in a culvert is 0.75 times Ucrit or less, the fish should be able to pass it over a longer distance. Also, at high swimming speeds ground speed can be close to or equal to the maximum speed less the 0.75 times Ucrit.
The present study compared the swimming capacity and aerobic energy consumption of seven freshwater fish species. The species tested were brown trout Salmo trutta L., European perch Perca fluviatilis L., roach Rutilus rutilus L., common carp Cyprinus carpio L., gudgeon Gobio gobio L., bullhead Cottus gobio L. and stone loach Barbatula barbatula L. Also, an estimate of acceptable water velocities for fish passages was given, based on the results of swimming speeds.
Materials and methods
Animal holding
Fish (bullhead, gudgeon, stone loach, roach, brown trout, carp and perch) of ca. 4–11 cm body length (bl) were kept at the University of Antwerp in 200 l tanks in softened Antwerp City tap water [ca.: 100.8 ± 3.0 mg·l−1; Mg: 11.0 ± 0.2 mg·l−1; Na: 36.7 ± 1.2 mg·l−1; K: 4.6 ± 0.1 mg·l−1; resulting in a water hardness of 292.4 ± 8.1 mg·l−1 (N = 30); pH 7.3 ± 0.1; (O2) >90% saturation; NH3 < 0.1 mg·l−1 (N = 15)] at a constant temperature of 15 ± 1 °C for at least 3 weeks before experiments started. A flow-through water exchange set up partially renewed the water in the tanks with a turn over rate of 100 l per day. Additional filtering occurred by means of a circular triple filter consisting of a cotton filter, an active carbon filter and a lava stone filter. Fish were fed with defrosted blood-worms (gudgeon, stone loach and bullhead), pond sticks (Tetra Pond, Germany; common carp, roach) or Nutra Fish Food (Skretting, France; brown trout, perch) three times a week (2% total body mass) and starved at 24–48 h prior to experimentation. Fish of 10–30 cm (common carp, roach and perch) were kept at the Flanders Hydraulics Research in Antwerp in 300 l tanks in rain water at a constant temperature of 15 ± 2 °C for at least 3 weeks before experiments started. The water was permanently filtered by means of a mechanical filter and a closed circuit with a turn over rate of 100 l per day. Fish were fed with Pond Sticks (common carp, roach) and Nutra Fish Food (perch) three times a week (2% total body mass) and starved at 24–48 h prior to experimentation.
Determination of Ucrit
Eight fish from the size groups of approximately 5–10 cm (gudgeon, stone loach, common carp, perch, roach and brown trout) were placed in individual separate Blazka-style swimming respirometers with a volume of 3.9 l (Blazka et al. 1960). Bullhead was not included as they successfully anchor themselves with their pectoral fins at the bottom of the respirometers, and do not swim even at high water speeds. The sizes for the inner tube are 35 × 6 cm and 50 × 11 cm for the outer tube (see Beamish et al. 1998 for comparison). Velocity was set to 5 cm·s−1. At this speed, the fish orient themselves towards the current and swim gently. For temperature control, respirometers were submerged on a wet table in a room acclimated to the same temperature as the water in the tanks, and a head tank provided a continuous flow of water saturated with oxygen through each respirometer at a rate of 4 l·min−1 (total volume of the recirculating system approximately 2 × 225 l). These conditions were kept overnight to allow the fish to acclimate to the respirometers. The next day water velocity was then increased in increments of 5 cm·s−1 at intervals of 20 min, until fish fatigued. Fatigue was determined as the situation where the fish could no longer maintain position against the current and were swept downstream. They were held against a mesh screen at the downstream end of the tunnel. Speed was then lowered for a short time to allow fish to restart swimming, and when fish were swept downstream for a third time, they were considered really fatigued and the performance test was terminated.
Eight large fish per species (11–20 cm·bl; common carp, roach and perch) were tested individually in a Brett-style swimming tunnel (Brett 1964) with a cross-section of 0.4 × 0.4 m, a total of length 20 m and a swimming section of 3 m. The total circulating volume of filtered freshwater was 8000 l. Fish were provided with a 2-h acclimation period swimming at 5 cm·s−1. After this period, water velocity was increased automatically in increments of 5 cm·s−1 at intervals of 20 min, until fish fatigued. Fatigue was determined using a photoreceptor-based automated system measuring fish contact with a metal grid delineating the downstream end of the flume. Fatigue was defined as occurring when a fish touched the grid three times in 3 s.
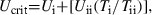
MO2 determination
Fish (stone loach, gudgeon, bullhead, small carp, small brown trout, small and middle-sized roach), were allowed to recover from the Ucrit determination overnight by swimming constantly at a gentle velocity of 5 cm·s−1 and respiration measurements were performed the next morning. Respirometry measurements were started by closing the respirometers for a 1-h period. Oxygen levels never dropped below 70% saturation. During measurements, fish were swimming at different percentages of Ucrit to determine the MO2 at different swimming speeds using WTW-O2-electrodes (oxi340i, WTW, Weilheim, Germany) connected to the computer program Windmill (Jill Studholme; Windmill Software Ltd, Manchester, UK 1996). Oxygen concentrations were then recalculated in oxygen consumption rates in μmol·g−1·h−1. After 1 h of measurement at the lowest speed, respirometers were reconnected to the continuous flow of water saturated with oxygen mentioned above and fish were given 1 h to recover. Subsequently the procedure of MO2 measurement was repeated at a higher velocity.
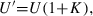
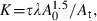
Uopt determination
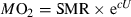
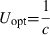
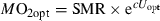
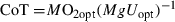
Umax determination
U max determination was carried out for all fish species, including bullhead, under similar physiochemical conditions as for the Ucrit determination in small fish. For small fish (5–10 cm) experiments were carried out in a round white plastic tank with a diameter of 40 cm and a height of 55 cm. For larger fish (10–30 cm), experiments were carried out in a Plexiglas tank of 150 by 150 cm and a height of 50 cm. Water depths was 15 cm for small and 25 cm for large fish, supplied with the same flowing filtered freshwater as in the holding tanks. A reference grid (5 × 5 cm) was drawn on the bottom of the tank for the accurate determination of fish position during escape sequences. Escape responses were induced with a mechanical stimulus, a cubic weight, which was released from a height of 1.5 m above the water surface. Fish were allowed to move freely for at last 15 min before the release of the stimulus. Experiments were carried out at 10, 15 and 20 °C water temperature after a 1-week acclimation period to the respective temperatures.
A PAL video camera (DCR-HC39E Sony Corporation, Tokyo, Japan) was positioned directly above the experimental tank to film the burst swim event. All individuals were filmed three times. Video in PAL consists of 25 frames per second. Each frame can be split into two fields hence the video sequence can be converted into 50 fields per second – 20 ms apart. For this purpose the sequences of escape responses of individual fish were imported into Adobe premiere 6.0 (as AVI files, Audio Video Interleave) and deinterlaced. All recordings were analysed, and the three sequences producing the fastest velocities were chosen for further analysis and averaged (Jordan et al. 2005). Analysis was carried out on 20 fields for each individual and commenced on one field prior to the stimulus contacting. The resulting 400 ms were considered sufficient to record maximum velocity (Domenici & Blake 1997). Each sequence was imported into Vernier Logger Pro 3.3 (Vernier Cooperation, Beaverton, OR, USA) and the XY coordinates of the centre of mass (CoM) were determined. The CoM was measured by hanging frozen fish from two different points (in front of the dorsal fin and at the cloaca) and determining the crossing point of the vertical extension of the two lines. Velocity was determined by calculating the movement of CoM from each field over time. Length-specific velocity was calculated in bl·s−1.
Estimates for water speeds in culverts
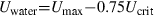
Results
U crit, Uopt, CoT at Uopt and Umax (at three temperatures) of G. gobio, B. barbatula, R. rutilus, C. carpio and S. trutta fario of different sizes are presented in Table 1. For C. gobio, only Umax was determined. Within each species, Ucrit increased with increasing fish size. The highest values for Ucrit were found in large perch and large roach, which reached speeds above 1 m per second. The lowest value was recorded for stone loach. When comparing fish of similar sizes (±7 to 10 cm), perch still performed best, followed by trout, carp and roach.
| Species | bl (cm) | U crit (cm·s−1) | U opt (cm·s−1) | COT at Uopt (J·N−1·m−1) | U max (cm·s−1) | Maximum water speed (cm·s−1) | ||
|---|---|---|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | 15 °C | |||||
| C. gobio | 7.4 ± 0.9 | -- | -- | -- | 112.46 ± 6.72 | 90.43 ± 5.74 | 82.63 ± 3.24 | 61 |
| G. gobio | 10.0 ± 0.3 | 54.16 ± 2.02 | 47.09 ± 2.41 | 0.32 ± 0.02 | -- | -- | -- | -- |
| 12.3 ± 0.3 | 60.17 ± 1.17 | 51.00 ± 2.05 | 0.32 ± 0.03 | 117.61 ± 1.34 | 136.78 ± 1.53 | 116.74 ± 1.87 | 92 | |
| B. barbatula | 7.2 ± 0.5 | 28.25 ± 0.32 | 18.46 ± 4.47 | 0.28 ± 0.03 | 108.04 ± 1.52 | 83.54 ± 1.46 | 72.73 ± 1.57 | 62 |
| R. rutilus | 4.6 ± 0.2 | 45.78 ± 2.10 | 30.93 ± 6.61 | 0.42 ± 0.03 | 55.12 ± 1.37 | 62.37 ± 0.43 | 64.87 ± 0.26 | 28 |
| 7.3 ± 0.3 | 59.45 ± 1.27 | 41.49 ± 13.07 | 0.25 ± 0.02 | -- | -- | -- | -- | |
| 15.7 ± 1.5 | 110.75 ± 6.71 | -- | -- | 139.5 ± 1.64 | 133.25 ± 1.53 | 126.00 ± 1.36 | 50 | |
| S. trutta fario | 7.8 ± 0.2 | 65.43 ± 0.54 | 31.64 ± 0.53 | 0.26 ± 0.02 | 125.86 ± 0.58 | 93.74 ± 0.38 | -- | 45 |
| C. carpio | 4.9 ± 0.1 | 43.31 ± 2.15 | 30.59 ± 4.36 | 0.35 ± 0.03 | -- | -- | -- | -- |
| 10.7 ± 0.2 | 62.30 ± 4.15 | -- | -- | 98.43 ± 0.42 | 103.42 ± 0.34 | 97.34 ± 0.63 | 57 | |
| 22.8 ± 3.9 | 87.09 ± 5.24 | -- | -- | 126.25 ± 1.45 | 134.23 ± 1.52 | 125.42 ± 1.25 | 69 | |
| P. fluviatilis | 10.1 ± 0.2 | 80.56 ± 1.50 | -- | -- | -- | -- | -- | -- |
| 17.8 ± 0.4 | 113.04 ± 1.37 | -- | -- | -- | -- | -- | -- | |
Highest values for Uopt were found in gudgeon and lowest values again in stone loach, suggesting that these are not very good swimmers. Values for CoT at Uopt were all relatively close together. Trout, together with medium-sized roach, showed to be amongst the most efficient swimmers at Uopt, with a CoT of 0.26 ± 0.02 J·N−1·m−1. Finally, highest values for Umax were found in large roach at 10 °C. At 15 °C, gudgeon, large carp and large roach all performed equally well. When comparing equal fish sizes (±7 to 10 cm), trout performed better than carp and gudgeon at 10 °C but not at 15 °C. Except for the very small roach, all species performed suboptimal at 20 °C.
Oxygen uptake at different swimming speeds is given in Fig. 1. Swimming speeds are presented as percentage critical swimming speed and oxygen uptake (MO2) is given in μmol·g−1·h−1 to make values comparable across species. Doing so, the data represent oxygen uptake at a comparable work load, but not necessarily at a comparable speed. In fish that swim well, such as trout, carp and large roach, the curve is relatively steep, indicating that they can power their increasing swimming speeds by extra oxygen uptake. In other species such as the gudeon, which also reached relatively high Ucrit values for its small size, the curve remains relatively flat.
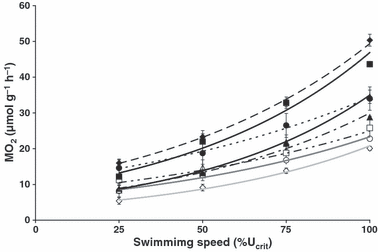
Oxygen uptake at 25%, 50%, 75% and 100% critical swimming speed (Ucrit) for gudgeon Gobio gobio (7.43 ± 0.93 cm, □, r2 = 0.99; 9.96 ± 0.29 cm, ○, r2 = 0.96), stone loach Barbatula barbatula (7.16 ± 0.48 cm, ⋄, r2 = 0.99), roach Rutilus rutilus (4.60 ± 0.16 cm, , r2 = 0.97; 7.36 ± 0.33, •, r2 = 0.99), common carp Cyprinus carpio (4.875 ± 0.08 cm, , r2 = 0.99) and brown trout Salmo trutta fario (7.84 ± 0.20 cm, ♦, r2 = 0.99) (mean ± SD, N = 8).
The active metabolic rate (AMR) is represented by the oxygen uptake at Ucrit and values are given in Table 2, as are the extrapolations to SMR and the scope for activity. SMR is lowest for stone loach; in fact values are only a third of the values for roach and trout. Also AMR is lowest in stone loach, indicating a limited capacity for oxygen uptake in this species. The scope for activity is low in both gudgeon and stone loach. In combination with the high swimming speeds reached by gudgeon, this low scope for activity suggests that perhaps not all of these swimming efforts are powered aerobically. The highest scope for activity was observed in trout and small roach.
| G. gobio | B. barbatula | R. rutilus | C. carpio | S. trutta | |||
|---|---|---|---|---|---|---|---|
| bl (cm) | 7.4 ± 0.9 | 10.0 ± 0.3 | 7.2 ± 0.5 | 4.6 ± 0.2 | 7.4 ± 0.3 | 4.9 ± 0.1 | 7.8 ± 0.2 |
| SMR (μmol·g−1·h−1) | 7.88 ± 1.53 | 6.1 ± 0.72 | 3.64 ± 0.94 | 9.58 ± 0.21 | 10.84 ± 2.46 | 5.98 ± 0.79 | 10.83 ± 2.37 |
| AMR (μmol·g−1·h−1) | 25.79 ± 3.31 | 22.8 ± 0.66 | 20.1 ± 0.58 | 43.6 ± 0.77 | 34 ± 3.26 | 28.8 ± 1.04 | 50.33 ± 3.21 |
| Scope (μmol·g−1·h−1) | 17.9 ± 2.42 | 16.69 ± 0.69 | 16.45 ± 0.76 | 34.01 ± 0.49 | 23.15 ± 2.86 | 22.81 ± 0.91 | 39.99 ± 1.56 |
| c | 0.012 ± 0.003 | 0.013 ± 0.001 | 0.017 ± 0.004 | 0.017 ± 0.003 | 0.012 ± 0.005 | 0.016 ± 0.003 | 0.015 ± 0.007 |
In the present study, MO2 and U are assumed to be related in an exponential way as used by Brett (1964) before. Other studies have found that MO2 is related to U as other than exponential functions (e.g., Videler 1993). In nature, there is probably not a single mathematical model to describe the relationship of MO2 to U in different species, sizes or ages of swimming fish. However, to compare the energetics of the species studied here, a simple exponential equation was appropriate. The r2 remained above 0.96, therefore the model fitted the data well.
Estimates for water speeds in culverts
Maximum water velocities for culverts were calculated according to Peake & Farrell (2004). Results are only calculated at 15 °C as both Ucrit and Umax are required in the calculation. The results are presented in Table 1.
Discussion
The results of the present study present swimming capacities and energy use of different migrating and nonmigrating European fish species. As shown, some migratory fish are ‘better’ swimmers than others and display higher swimming speeds or a lower-energy consumption while swimming. Trout, roach and carp performed the best in terms of speed and endurance, while gudgeon, loach and bullhead were ‘weak’ swimmers. Unfortunately, we were not able to test large trout as the temperature in the large flume could not be controlled in order for this cold-water species to perform under optimal circumstances. This is also the reason for the low maximum passable water flows calculated for this species (Table 1). Jain & Farrell (2003) showed in a repeated swimming performance test that rainbow trout (Oncorhynchus mykiss, Walbaum) performed better when acclimated at low temperatures (ca. 5 °C) than at high temperatures (ca. 17 °C). However, data on swim performance of salmonids are abundant (Brett 1964; Webb 1975, 1978; Harper & Blake 1991), and they are excellent swimmers. This was also confirmed in our study, where swimming performances of the small trout were only preceded by small perch. Roach and perch performed excellent as well, especially the larger individuals. This is not surprising as they are typical long distance migratory species (Vandelanoote et al. 1998; Gerstmeier & Romig 2000, Lucas & Baras 2001). Unexpectedly, also gudgeon showed high Ucrit and Umax values. Gudgeon is a bottom dwelling species with limited migration behaviour, therefore we did not expect such high values. However, the scope for activity in this species was low. This suggests that a relatively large part of the Ucrit could have been fuelled by anaerobic metabolism. Therefore, long migrations at these high swimming speeds are unlikely for this species. The high Ucrit and Umax indicate that gudgeon has the physiological capacity to pass barriers with fast flowing water, at least when jumping is not required. This has recently been confirmed by observations of gudgeon using a fish pass in the field (Kotusz et al. 2006).
In comparison, stone loach, another bottom dwelling species, performed poorly on critical swimming speed and maximum swimming speed. For technical reasons, Ucrit could not be measured for C. gobio. When set into the swimming tunnel, this species positions its pectoral fins to resist the water flow, an adaptation to its natural habitat, where C. gobio lives between rocks and stones in fast streaming creeks and small rivers. However, Johnston et al. (1995) showed that the main part of Cottidae muscles is glycolytical and this might be an explanation for the typical hopping movement they display. C. gobio also lacks a swimming bladder, another adaptation to its environment. Therefore, C. gobio does not show any cruise swimming behaviour but always bursts for locomotion. Although C. gobio is not generally considered to be a migrating species, there is evidence for migrations of several hundreds of meters by some active individuals (Knaepkens et al. 2004, 2005). Like C. gobio, B. barbatula also displaces itself by bursts rather than by continuous swimming. New data indicate that some individuals also perform migrations over several hundreds of meters (Knaepkens, unpublished data). Therefore, species as C. gobio and B. barbatula are included in our study, and their swimming capacities should be taken into consideration when barriers are remediate by the use of fish passes.
As reported by Hammer (1995),Plaut (2001) or Peake et al. (1997), critical swimming speed obtained in the laboratory is not a measurement that can be extrapolated directly to populations swimming in the wild but it does give an indication of the swimming capacities of the species tested. In confined spaces such as swimming tunnels, fish alter their behaviour and show very different maximum sustained speeds compared with fish in the wild (Haro et al. 2004; Peake & Farrell 2006). However, using the methodology of Peake & Farrell (2004) it is still possible to make fairly reliable predictions about swimming speeds in the wild and passable maximum water speeds, based on Ucrit and Umax. The present study aims to give a rough indication for the management of waterways. The Ucrit data cannot be used to estimate maximum allowable speeds in culverts but based on the obtained data from forced swimming tests, estimation could be made for maximum acceptable velocities in fish passages.
As a fish accelerates to pass difficult passages with high water speeds, but also to predate upon prey or escape predators, maximum swimming speeds are used. The results show the capability of some species to accelerate quicker than others. However, species with a higher burst capacity are not necessary more successful in passing difficult passages. For example, C. gobio reaches very high burst speeds but is not known to leap out of the water. Without a swimming bladder it is negatively buoyant and remains on the ground. C. gobio is not expected to pass obstructions on its migration or dispersion paths. Other species as S. trutta, R. rutilus or C. carpio are good leapers and can reach long distances and large heights. Those also show good cruising capacities and in general are more actively moving in the water column. The measurement of Umax was carried out according to traditional methods applied to fast start analysis (Webb 1975; Webb, 1978; Harper & Blake 1991; Domenici & Blake 1997) and was not taken from a natural situation in the field, implying linear bursts. However, the data obtained from such measurements were used before to make estimates of bursting and leaping capacities in freshwater fish (e.g., Wolter & Arlinghaus 2003; Videler 1993). The duration of 400 ms might not be sufficient to reach maximum speeds when comparing with situations in the field, but the maximum velocities obtained from such analysis can give a good estimate of maximum possible speeds when bursts are implied in clearing obstacles on migration paths, and in general for an estimation of anaerobic swimming capacity.
Optimal swimming speed is the speed at which the cost of transport is lowest. It is a theoretical value, and there is no evidence for the actual use of this swimming speed in nature. Moreover, as fish pass difficult areas on migration routes, speeds are often altered and energy saving strategies like burst-and-coast swimming is adopted, as this swimming behaviour has been proven to reduce aerobic energy demands for up to 60% (Weihs 1974). Hinch & Bratty (2000) showed that migrating Sockeye Salmon (Oncorhynchus nerca, Walbaum) never swam at expected, i.e., energy saving speeds. Especially while passing difficult passages on their migration route, burst-and-coast swimming was adopted. Nevertheless, the use of Uopt allows a comparison of the speed and cost of steady swimming between and within species. The data show that smaller fish swimming at Uopt swim slower than bigger fish of the same species, while they use a higher percentage of their scope for activity. This means that they have a higher CoT at Uopt and use more energy per metre swum. Thus, being a larger fish is energetically more advantageous.
When comparing swimming velocities of different fish species and sizes speed per unit bl is often applied (Videler & Wardle 1991; Drucker 1996; Van Damme & Van Doren 1999; Drucker & Lauder 2000; Plaut 2001). However, Drucker (1996) found ‘per unit bl speed’ caused errors in the kinematic and physiological comparison of exercise between different fishes. Furthermore, studies by Kolok (1999) and Reidy et al. (2000) demonstrated significant variability of locomotor performance between individual fish. However, to evaluate swimming performance of fish with regard to the physical clearance of obstructions on migration paths, absolute values of swimming speed seem appropriate, because water velocities in culverts and fish ladders are absolute values. Thus, each individual fish has to withstand a certain threshold value, independent of the kinematic and physiological comparability of its exercise performance.
As swimming performance and energetics are different in different fish species, all fish species should be considered when evaluating possible effects of barriers. Man-made obstacles on the pathways of migrating fish species can be one of the factors reducing migration behaviour and thus leading to fragmentation and finally extinction of fish species. This study shows that typically migratory species such as trout, perch and roach perform best in the swim performance tests. Additionally, also gudgeon perform surprisingly well. It is shown that besides bullhead, which is already threatened in Flanders, stone loach is a species of concern when barriers, even small ones, are present.
Acknowledgements
The authors want to thank Nemo Maes and Charlotte Kleen for technical support. Johan Coeck, Caroline Geeraerts and Hilde Verbiest are acknowledged for support in catching fish in the field. This work was carried out under a grant of the Belgian Science Policy Project EV/05/31A.




