TYK2:p.Pro1104Ala Variant Protects Against Autoimmunity by Modulating Immune Cell Levels
Funding: This work was supported by Intramural Research Program of the National Institute of Aging (HHSN271201100005C, 75N95021C00012), Italian Foundation for Multiple Sclerosis—FISM—Fondazione Italiana Sclerosi Multipla (Grant cod. 2019/S/03) and financed or co-financed with the ‘5 per mille’ public funding. Sardinia Autonomous Region and its regional agency for research and development in Sardinia—Sardegna Ricerche and local Lanusei government for continues support.
ABSTRACT
The TYK2:p.Pro1104Ala (rs34536443) hypomorph variant has been associated with protection against numerous autoimmune disorders. Thus, its mechanism of action becomes of great interest. Here, consistent with the participation of activated immune cells in autoimmunity, we show that the variant regulates the levels of immune cells at a human, general population level and is associated particularly with higher levels of T and B lymphocytes, especially the naïve (non-activated) compartment. Also, consistent with a protective function in autoimmunity, the level of regulatory CD4+ T cells was increased. Thus, this variant decreases immune activation thereby protecting from autoimmunity. Our work links the cellular mechanism regulated by the TYK2:p.Pro1104Ala variant to autoimmunity protection and supports TYK2 as a therapeutic target in autoimmunity.
Abbreviations
-
- FACS
-
- fluorescence-activated cell sorting
-
- GWAS
-
- Genome Wide Association Studies
-
- IFN
-
- interferon
-
- JAK2
-
- Janus kinase 2
-
- MAF
-
- minor allele frequency
-
- MS
-
- multiple sclerosis
-
- RA
-
- rheumatoid arthritis
-
- STAT4
-
- signal transducer and activator transcription 4
-
- T1D
-
- type 1 diabetes
-
- TYK2
-
- tyrosine kinase 2
1 Introduction
The immune response is characterised by extreme diversity. Part of that variability was established during the evolutionary history of populations and is genetically encoded.
GWAS studies have in fact revealed many variants affecting levels of immunity and/or autoimmunity. However, the mechanistic importance of such variability remains unknown. One such variant, TYK2:p.Pro1104Ala is associated with a lowered risk of several autoimmune disorders, and we have analysed its effects on immune cell levels [1-9].
Tyrosine kinase 2, encoded by the TYK2 gene, is expressed ubiquitously and participates in the signalling of various antiviral and immunoregulatory cytokines (type I and type III IFNs, IL10, IL12, IL22 and IL23) with an impact both on immune and non-immune cells [10, 11]. The targeted cytokine pathways are directly involved in immune-mediated disorders.
Expressed in immune cells, TYK2 pairs with JAK2 and engages mainly with STAT4 to mediate signal transduction from IL12. This leads to T-bet expression that promotes differentiation of naïve CD4+T cells into Th1 cells [12]. The TYK2/JAK2 heterodimer, acting through STAT1-3, is further required for IL23 signalling, which regulates the differentiation and survival of Th17 cells [13]. In addition, TYK2 also combines with JAK1 and engages STAT1-2 to mediate signalling of the potent antiviral type 1 IFNs and enhance various proinflammatory immune processes involving dendritic cells, macrophages, regulatory T cell differentiation and function, B cell activation and antibody production [14].
Several TYK2 variants have been associated with autoimmunity and are generally protective [15]. In particular, loss of function mutations and inhibition of TYK2 suppress autoimmunity in mice and humans [9, 16-18]. The TYK2:p.Pro1104Ala variant notably confers protection against autoinflammation in more than 10 autoimmune diseases including autoimmune thyroid disease, ankylosing spondylitis, Crohn's disease, psoriasis, systemic lupus erythematosus, rheumatoid arthritis (RA), sarcoidosis, systemic lupus erythematosus, type 1 diabetes (T1D), multiple sclerosis (MS), inflammatory bowel disease and ulcerative colitis [1-8, 16]. The variant reduces the enzymatic activity of the corresponding protein [9, 16, 17]. Studies have suggested that low enzymatic activity is affected by stabilisation of an inactive conformation [18].
In agreement with the protection from autoimmunity provided by the TYK2:p.Pro1104Ala loss-of-function variant, selective TYK2 inhibition has been intensively explored for the treatment of autoimmune diseases and several inhibitors have been developed [16, 19]. Although the mechanism of cellular regulation for this variant has been studied in patients with mycobacterial disease [20], the effect of TYK2 variants on immune cell subpopulations has not been reported at a general, human population level. Here, we explore the regulatory effects of TYK2:p.Pro1104Ala variant on the levels of immune cells that underlie its regulation of the immune response.
2 Methodology
2.1 Population Cohort
We cross-compared the TYK2:p.Pro1104Ala variant association with different autoimmune disorders with immunophenotyping associations in the SardiNIA cohort [21].
The SardiNIA general population cohort from the Lanusei valley area in Sardinia, Italy, has been previously described [22]. The cohort was phenotyped for over 1500 quantitative traits, including immune-related phenotypes. Details of phenotype and genotype assessments for these samples have been published previously [23].
2.2 GWAS on Immune Phenotypes
For this study, we used GWAS data on 731 immune phenotypes measured in 3757 Sardinian individuals. The traits were measured by extended flow cytometry (FACS) profiling [23, 24]. FACS profiling on fresh blood samples was performed within 2 h of blood collection, as described previously [25]. Summary statistics for 731 immune traits in the region of the TYK2 gene were assessed in the SardiNIA study. These traits include 118 absolute cell counts, 389 MFIs of surface antigens and 32 morphological parameters. The significance threshold has been calculated by correcting the nominal p of 0.05 for these 539 statistically independent traits, applying a multiple-independent test (Bonferroni correction) and reaching a significant threshold of 9.28 × 10−5. The remaining 192 relative counts, corresponding to percentages with respect to hierarchically higher cell population, are instead statistically dependent on the absolute cell counts tested and were not considered for Bonferroni correction.
2.3 Colocalization Analysis
To determine whether a disease and an immune trait share the same genetic causal variant in the TYK2 gene region, we performed a colocalization test in a region of about 50 kb around the TYK2:p.Pro1104Ala variant using the summary statistics for the counts of lymphocyte subpopulations in the SardiNIA cohort and publicly available summary statistics for T1D, RA and MS [26-28]. Two association signals were considered colocalizing if their posterior probability of a shared variant (PP.H4) was ≥ 0.8 [21]. Colocalization analysis was performed using the R ‘coloc’ package [29].
3 Results
3.1 TYK2 :p.Pro1104Ala Variant Worldwide Frequencies
The TYK2:p.Pro1104Ala variant is frequent in European populations (MAF = 4.26%), less common in Sardinia (MAF = 2.79%), rare in African populations (MAF = 0.96%) and absent in East Asia. These observations are in agreement with the selective sweep described for this variant [30]. The TYK2:p.Pro1104Ala variant has a Combined Annotation Dependent Depletion score of 25.5, and is associated with several traits and conditions Figure 1, confirming its functional importance.
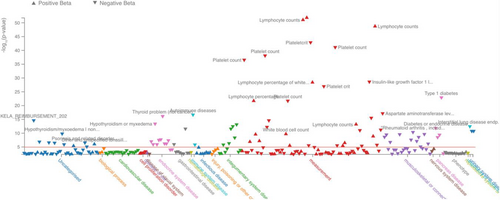
3.2 Effect of TYK2 :p.Pro1104Ala on T Lymphocytes
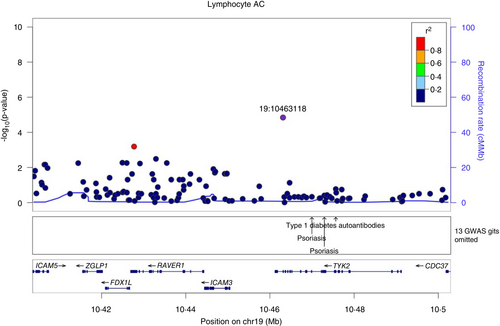
We show that rs34536443 (TYK2:p.Pro1104Ala), allele C which confers protection from different autoimmune disorders has been associated with several immunophenotypes. The most significantly associated cell population in our study is lymphocytes, which substantially increase (p = 1.43 × 10−5, beta = 0.325), Figure 2, Table 1. This result is in agreement with published observations for this variant [31, 32]. In addition, as representative examples of associated traits, we found a very high colocalization probability between the association of total lymphocyte counts in the SardiNIA cohort and T1D (PP.H4 = 96.6%), RA (97.5%) and MS (98.4%) (Figures S1 and S2).
| Trait | N | p | Effect (beta) | STERR |
|---|---|---|---|---|
| Lymphocyte AC | 3653 | 1.43E-05 | 0.325 | 0.074 |
| Lymphocyte %leukocyte | 3669 | 1.75E-04 | 0.282 | 0.075 |
| T cell AC | 3653 | 1.44E-04 | 0.280 | 0.074 |
| CD4+ AC | 3652 | 5.87E-04 | 0.257 | 0.075 |
| Naive CD4+ AC | 3395 | 2.26E-04 | 0.270 | 0.073 |
| CD4 Treg AC | 3405 | 6.48E-04 | 0.265 | 0.078 |
| CM CD4+ AC | 3395 | 9.21E-04 | 0.259 | 0.078 |
| Naive CD8br AC | 3395 | 1.36E-04 | 0.200 | 0.052 |
| CD45RA+ CD8br AC | 3395 | 4.19E-04 | 0.236 | 0.067 |
| CD28+ CD45RA+ CD8br AC | 3408 | 2.38E-05 | 0.229 | 0.054 |
| CD28+ DN (CD4−CD8−) AC | 3408 | 3.18E-04 | 0.240 | 0.067 |
| CD3− lymphocyte %leukocyte | 3669 | 5.10E-04 | 0.264 | 0.076 |
| CD3- lymphocyte AC | 3653 | 3.78E-05 | 0.309 | 0.075 |
| B cell AC | 3653 | 6.60E-05 | 0.293 | 0.073 |
| IgD+ CD24− AC | 3656 | 6.12E-04 | 0.264 | 0.077 |
| Granulocyte %leukocyte | 3669 | 4.96E-04 | −0.264 | 0.076 |
- Note: Columns from left to right give the trait name, p of association, the effect size expressed in standard deviation units (effect), its standard error (STERR). The significant threshold of association p threshold is (p < 9.28E-05).
- Abbreviations: AC—absolute count; CM—central memory; DN—double negative; MAF—minor allele frequency.
In further analyses of lymphocyte compartment, T cell absolute count increases (p = 1.44 × 10−4, beta = 0.280), Table 1, and within the T cell compartment, CD4+ absolute count increases (p = 5.87 × 10−4, beta = 0.257), within which CD4+ naïve T cells increase (p = 2.26 × 10−4, beta = 0.27), Table 1. CD4+ regulatory T cells also suggestively increase (p = 6.48 × 10−4, beta = 0.265), which is in line with the protective function of regulatory T cells in autoimmunity, Table 1.
We also noted that the central memory CD4 reservoir for effector memory T cells increases (p = 9.21 × 10−4, beta = 0.259). Central memory CD4 T cells show enhanced proliferation. These cells synthesise IL2 and have less potential for rapid IFN-gamma or IL4 secretion [33].
In the CD8 T cell compartment, the naïve CD28+ CD45RA+CD8br cell level significantly increased (p = 2.38 × 10−5, beta = 0.229). These cells have not yet encountered antigen and are therefore poorly differentiated. After activation, for example during chronic virus exposure, naïve CD8+ bystander cells differentiate into a memory-like phenotype. Exposure of naïve CD8 T cells to type I interferons drive the rapid acquisition of effector function after antigenic stimulation [34].
Interestingly, we found a suggestive association with the CD28+ double negative (DN, CD4−CD8−) T cell population (p = 3.18 × 10−4, beta = 0.240), Table 1. DN T cells are a rare subset of peripheral T cells whose important role in inflammation, immune-related diseases and cancer has been recently discussed [35]. Our data suggest a positive effect of CD28+DN T cells on autoimmunity, in line with a previous study in which these cells were described to have a regulatory function in non-obese diabetic mouse (NOD) model [36]. DN regulatory T cells have the capacity to prevent graft-versus-host disease and have therapeutic value for autoimmune diseases depending on their regulatory effects on CD8+, CD4+ and B cells [35].
Additionally, CD3- lymphocytes are significantly increased (p = 3.78 × 10−5, beta = 0.309) suggesting an expansion of B cell and/or NK cell levels; indeed, we directly observed an increase in B cells (next paragraph and Table 1).
3.3 Effect of TYK2 :p.Pro1104Ala on B Cells
One of the largest effects of the TYK2:p.Pro1104Ala mutation on immune cell levels is an increase in the absolute count of B cells (p = 6.60 × 10−5, beta = 0.293), Table 1. Within that population, the most associated are IgD+CD24− (p = 6.12 × 10−4, beta = 0.264) that mainly include mature naïve B cells [37].
3.4 Effect of TYK2 :p.Pro1104Ala on Granulocytes
The granulocyte frequency with respect to leukocytes is the only subpopulation suggestively downregulated by the TYK2:p.Pro1104Ala variant (p = 4.96 × 10−4, beta = −0.264), Table 1. This may be a secondary effect, resulting from an alteration of the relative frequencies of cell subsets attendant on the increase of lymphocytes.
4 Discussion
The fundamental role of TYK2 in autoimmunity and immunodeficiencies is well-established. Autosomal recessive, complete TYK2 deficiency leads to immunodeficiency, while variants that reduce TYK2 signalling, including TYK2:p.Pro1104Ala, are associated with protection from autoimmunity. Consistent with those findings, Tyk2−/− mice do not develop experimental autoimmune encephalomyelitis, exhibit impaired polarisation of Th1 and Th2 and a reduction of IL-17+IFN-gamma T cells [38, 39]. Furthermore, the role of TYK2 inhibition on pro-inflammatory Th1 and Th2 has been described [20]. In this regard, the TYK2:p.Pro1104Ala loss-of-function variant has been associated with protection against autoimmunity [16]. Furthermore, we found that the TYK2:p.Pro1104Ala is associated with increased levels of T and B lymphocytes, especially the naïve compartment. These results are in agreement with the previously described findings that in autoimmunity naïve compartment contracts while effector memory cells expand [40, 41].
Although, the increased levels of lymphocytes have been observed in other studies [31, 32], and the effect of the TYK2:p.Pro1104Ala variant on the expansion of naïve T cells has been demonstrated in homozygous individuals with mycobacterial disease [20], to our knowledge no one has dissected, in detail the association of leukocyte subsets with this variant at a general, human population level. Additionally, our study reports the effects of the variant on the other previously unreported shifts in immune cell subpopulations, for example regulatory T cells and the double-negative population, CD28+DN (CD4− CD8−). However, these novel findings require confirmation in other cohorts.
Our results explain why paradoxically, the TYK2:p.Pro1104Ala variant, in the context of its conferred protection against autoimmunity, is at the same time associated with an increased level of lymphocytes.
We observed an increase of central memory helper T cells that have little or no effector function, with a low-activation threshold and high proliferation and differentiation in response to antigenic stimulation. Thus, they are cells arrested at intermediate stages of differentiation preceding effector memory T cells status. They may function as stem cells under homeostatic conditions [42]. This finding suggests that this variant exerts a different effect on individuals in the general population compared to the previously described decrease in this cell population in individuals with mycobacterial disease [20]. Thus, the immune milieu created by the TYK2:p.Pro1104Ala variant is not completely unrelated to a proper immune response and we suggest that the shift in memory helper T cells may have a role in protection from autoimmunity, but this observation requires replication in independent studies.
Furthermore, we observed that TYK2:p.Pro1104Ala variant mediates an increase in CD3-lymphocytes. Previous studies have demonstrated that, in wild-type mice, CD3-negative cells (comprising NK and B cells) produce small amounts of IFN-gamma, in contrast CD3-negative cells from Tyk2-deficient mice did not produce IFN-gamma [43], a mechanism that may ulteriorly contribute to the protection.
Additionally, for first time we show that TYK2:p.Pro1104Ala variant increases the regulatory CD4+ T cells, in line with the protective function of this variant in autoimmunity [44]. Consistently with this finding BMS-986202 selective TYK2 inhibitor redirected CD4+T cells toward a regulatory T cell phenotype [45].
Moreover, considering the cytotoxic T cell compartment, the level of CD28+CD45RA+CD8br naïve cells significantly increase. These are naïve CD8+ bystander cells that have not encountered antigens and, after activation by pathogens like viruses, can differentiate into a memory-like phenotype [34]. Exposure of naïve CD8 T cells to type I interferons causes fast transformation of these cells to effector cells, and still provides a proper response to viral infection even in carriers of the TYK2:p.Pro1104Ala variant [16, 38, 46].
A recent study demonstrated that TYK2 signalling promotes the development of autoreactive CD8+ cytotoxic lymphocytes in autoimmune diabetes. The loss of Tyk2 inhibits the development of the autoreactive CD8+T-BET+ cytotoxic lymphocytes and treatment with BMS-986165 a selective TYK2 inhibitor, inhibits the expansion of T-BET+CTLs [46].
We found another factor in the protection by TYK2:p.Pro1104Ala variant: the suggestive association of DN population CD28+DN (CD4− CD8−). DN T cells are considered disease-causing and reduced or absent expression of CD28 appears to be characteristic for most of the peripheral blood DN cells; they are considered antigen-experienced and differentiated [47]. CD28 is involved in proper differentiation of DN and B7-CD28 interaction promotes proliferation and survival but suppresses differentiation of DN T cells in thymus [48]. It has been known that the majority of DN T cells that contribute to the autoimmune and autoinflammatory disorders exhibit effector phenotypes and thus, we hypothesized that the TYK2:p.Pro1104Ala mutation may suppress DN differentiation, instead promotes differentiation of DN thymocytes into double positive and then regulatory T cells, thus protects from the disease guided by CD28 [49]. Our data suggest a positive effect of CD28+ subpopulations in protecting from autoimmunity, indeed CD28 co-stimulation provides a proper T cell activation promoting cell cycle entry and the production of various cytokines [50].
One of the other largest effects of TYK2:p.Pro1104Ala mutation on immune cell levels was an increase in the absolute count of B cells, most notably the IgD+CD24− mature naïve B cells population increased, confirming the protective effect of naïve cells on autoimmunity.
Our data are in agreement with a recent study that demonstrated that JAK–STAT signalling, apart from its role in immune responses, is a major regulator of immune cell homeostasis, preparing cells for a rapid response to immune stimuli [51].
Conceivably, autosomal recessive complete TYK2 deficiency predisposes to severe recurrent infections [52]. Additionally, the TYK2:p.Pro1104Ala variant selectively impairs cellular responses to IL23, but not to IFN-alpha or IL10, in agreement with its predisposing effect for tuberculosis [20, 53]. Furthermore, healthy individuals with the protective variant decrease IFN I signalling and have a decreased frequency of circulating Tfh (T follicular helper) cells and switched memory B cells [38].
Additionally, the Tyk2 knock-in murine model showed less IL12 receptor signalling and diminished in vitro Th1 skewing. Finally, T cells had reduced IL23-dependent signalling and diminished ability to skew toward Th17 in vitro [38]. However, we do not exclude other effects of the TYK2:p.Pro1104Ala variant on immune system regulation.
Targeting TYK2 has already been exploited clinically. One of the autoimmune diseases, psoriasis is largely an IL23-driven disease, and thus inhibitors of TYK2 are effective therapy. Indeed, BMS-986165 (Deucravacitinib) is a selective potent oral TYK2 inhibitor currently used for psoriasis treatment [54], and two other TYK2 inhibitors (TAK-279, VTX958) are under development for psoriasis treatment. Deucravacitinib's mechanism of action is distinct from JAK inhibitors; it binds to an allosteric site rather than an ATP-binding site, and its selectivity for TYK2 could potentially limit side effects [55]. Oral treatment with the selective TYK2 inhibitor, BMS-986165 was also efficacious and ameliorated progression in murine models of spondylarthritis, lupus nephritis, inflammatory bowel disease, and autoimmune diabetes [46, 56].
Treatment with BMS-986165 has been shown to reduce the expansion of cytotoxic T lymphocytes. In line with that action, Tyk2 deficiency inhibits the development of autoreactive CD8+ T cells and increases inflammatory responses in beta-cells during aging [46].
TYK2 variants are associated with critical COVID-19, and the TYK2:p.Pro1104Ala variant has been shown to be causal [57]. Interestingly, protection from severe COVID-19 has been described in Sardinia [58]. Hypothetically, given the increased risk of severe COVID-19 conferred by the TYK2 variant, its lower frequency on the island could in part, together with other COVID-19 protecting variants, contribute to the protection against severe disease but decrease the general protection from autoimmunity, known to be common in Sardinia.
However, there is no satisfactory explanation for how such a variant, which favours a less aggressive posture of the immune system, arose and persisted in the populations. In a general, variants that protect against the infection are under selective pressure and widespread in a population but at the cost of risk of autoimmunity.
In fact, the TYK2:p.Pro1104Ala variant is the main common risk factor for tuberculosis in endemic regions and increases the risk of severe COVID-19 [16, 20, 59]. Carriers of the TYK2:p.Pro1104Ala variant are predisposed to tuberculosis, acting possibly through reduced IL23 and IL12 signalling-dependent IFN-gamma production [16, 20]. Heterozygous carriers have decreased numbers of follicular helper T cells and lower IFN-signalling in naïve but not in effector cells [38]. Indeed, it has been estimated that this variant arose around 30 000 years ago and then underwent strong negative selection in Europeans over the past 2000 years, which has been inferred from the co-occurrence of the emergence of Mycobacterium tuberculosis [60].
In attempting to understand the complex yin and yang of predisposition to infectious disease and protection from autoimmunity of the TYK2:p.Pro1104Ala variant, our study is a first step toward understanding of cellular mechanisms regulated by this variant.
5 Conclusion
For the first time, we describe that the TYK2:p.Pro1104Ala general autoimmunity protective variant exerts its function through effects on multiple immune cell subpopulations at a human, general population level. The effects of this variant are complex and includes changes additional to cytokine response. It increases the level of lymphocytes in all compartments (CD4+, CD8+ and B), but we confirmed that these cells are mainly the naïve populations that are particularly useful in fighting against autoimmunity. The protective character of the variant is further rationalised by an increase of T regulatory cells with anti-autoimmune potential. Our work underlines the current utility of TYK2 as a therapeutic target in autoimmunity.
Acknowledgements
We would like to acknowledge all the volunteers of SardiNIA study and Davide Murrau (IRGB-CNR) for technical support. Open access publishing facilitated by Consiglio Nazionale delle Ricerche, as part of the Wiley - CRUI-CARE agreement.
Conflicts of Interest
C.S. is currently the employee of Regeneron Pharmaceuticals and beneficiary of stock options and grants in Regeneron. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




