Endocannabinoids in immune regulation and immunopathologies
Funding information
This work was supported by funding from Swarnajayanti Fellowship to DG and Senior Research Fellowship from University Grants Commission, India, to OR
Abstract
Endocannabinoids are key bioactive components of the endocannabinoid system, and the profound influence of endocannabinoids on the modulation of the immune system is being increasingly appreciated. The knowledge of endocannabinoid–immune cell crosstalk will pave the way to therapeutic implications of modulators of this pathway in autoimmune and chronic inflammatory disorders. Endocannabinoids seem to exert both anti-inflammatory and pro-inflammatory effects in specific contexts, based on specific receptor engagement and the downstream signalling pathways involved. In this review, we summarized the biosynthesis, signalling and degradation of two well-studied endocannabinoids—anandamide and 2-arachidonylglycerol in immune cells. Then, we discussed the effects of these two endocannabinoids on the functioning of major innate and adaptive immune cells, along with the choice of receptors employed in such interactions. Finally, we outline our current knowledge on the involvement of anandamide and 2-arachidonylglycerol in context of inflammation, allergies, autoimmunity and metabolic disorders.
Abbreviations
-
- 2-AG
-
- 2-arachidonylglycerol
-
- AA
-
- arachidonic acid
-
- ABHD12
-
- α/β-hydrolase domain-containing protein-12
-
- ABHD6
-
- α/β-hydrolase domain-containing protein-6
-
- AEA
-
- N-arachidonylethanolamine
-
- CB1
-
- cannabinoid receptor 1
-
- CB2
-
- cannabinoid receptor 2
-
- CNS
-
- central nervous system
-
- COX-2
-
- cyclooxygenase-2
-
- DAGL
-
- diacylglycerol lipase
-
- DCs
-
- dendritic cells
-
- DTH
-
- delayed-type hypersensitivity
-
- ECS
-
- endocannabinoid system
-
- FAAH
-
- fatty acid amide hydrolase
-
- FLS
-
- fibroblast-like synoviocytes
-
- GPR55
-
- G protein-coupled receptor 55
-
- GTP
-
- guanosine triphosphate
-
- LOX
-
- lipoxygenases
-
- LPS
-
- lipopolysaccharides
-
- MAGL
-
- monoacylglycerol lipase
-
- MDSCs
-
- myeloid-derived suppressor cells
-
- MPO
-
- myeloperoxidase
-
- MS
-
- multiple sclerosis
-
- MZ
-
- marginal zone
-
- NAAA
-
- N-acylethanolamine-selective acid amidase
-
- NAPE
-
- N-acyl-phosphatidylethanolamine
-
- NAPE-PLD
-
- N-acyl-phosphatidylethanolamine phospholipase D
-
- OLDA
-
- N-Oleoyldopamine
-
- PAF
-
- platelet-activating factor
-
- PDC
-
- plasmacytoid dendritic cell
-
- PEA
-
- palmitoylethanolamide
-
- PGE-2
-
- prostaglandin-E2
-
- PPARγ
-
- peroxisome proliferator-activated receptor gamma
-
- PTPN22
-
- protein tyrosine phosphatase non-receptor type 22
-
- RA
-
- rheumatoid arthritis
-
- SLE
-
- systemic lupus erythematosus
-
- SRBC
-
- sheep red blood cells
-
- THC
-
- tetrahydrocannabinol
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- Tumour necrosis factor alpha
-
- TRPV1
-
- transient receptor potential cation channel subfamily V member 1
AN OVERVIEW OF THE ENDOCANNABINOID SYSTEM
The endocannabinoid system (ECS) modulates critical physiological functions such as pain, satiety, fear, memory and inflammation. The major targets of ECS include the brain, spinal cord, adipocytes, hepatocytes, endothelial cells and the immune system. Bioactive lipid molecules termed endocannabinoids (or endogenous cannabinoids), enzymes responsible for their biosynthesis and degradation, and the endocannabinoid receptors driving their downstream signalling essentially constitute the ECS [1].
The knowledge of ECS commenced with the elucidation of the structure, function and metabolism of the phytocannabinoid Δ9-tetrahydrocannabinol (THC), the major component of the marijuana plant [2]. THC specifically reduced cyclic-AMP accumulation with a concomitant decrease in membrane adenylate cyclase activity in resting and prostacyclin-treated neuroblastoma cells [3]. This enhanced in the presence of GTP but drastically dampened on treatment with a non-hydrolysable GTP analogue or pertussis toxin [4, 5]. Eventually, cannabinoid receptors—cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2)—were discovered, which are G protein-coupled receptors utilizing Gi/o proteins for downstream signalling [6, 7]. Subsequently, endogenous molecules with structural and functional similarity to THC were discovered and named ‘endocannabinoids’.
N-arachidonylethanolamine (AEA) or anandamide (named after the Sanskrit word ‘Ananda’ meaning bliss) was the first endocannabinoid discovered from porcine brain extracts [8]. The second endocannabinoid molecule 2-arachidonylglycerol (2-AG) was first isolated from the canine gut [9]. 2-AG is present at 170 times higher concentration than anandamide in the brain lysate [10] and displays higher binding affinities for both CB1 and CB2 compared with anandamide [11]. Other endocannabinoids were identified subsequently based on CB1/CB2 affinity, viz. noladin ether [12], virodhamine [13], N-arachidonoyl dopamine [14] and OLDA [15]. For instance, virodhamine shows high affinity to CB2 and acts as partial antagonist for CB1 [13]. This review will focus on the current knowledge of immunomodulation by the two major and best-characterized endocannabinoids—anandamide and 2-AG.
BIOSYNTHESIS, DEGRADATION AND INDUCTION OF ENDOCANNABINOIDS IN IMMUNE CELLS
Endocannabinoids are synthesized from membrane precursors in response to stimuli, unlike neurotransmitters that are pre-formed and stored in vesicles, although anandamide can also be stored inside cells [16, 17]. There are different pathways for anandamide biosynthesis, and N-acyl-phosphatidylethanolamine (NAPE) acts as the precursor for all of them [1]. The most studied pathway involves the action of phospholipase D (NAPE-PLD) on NAPE to produce anandamide and phosphatidic acid [18, 19]. NAPE-PLD is a member of the β-lactamase family of hydrolases, which is stimulated by calcium and highly expressed in the brain, spleen, kidney, liver, lung and heart [20, 21]. The second pathway is operative in immune cells, wherein NAPE-specific phospholipase C produces phospho-anandamide from NAPE, followed by dephosphorylation by PTPN22 [22]. Palmitoylethanolamide (PEA) is an endocannabinoid-like molecule with anti-inflammatory properties, synthesized along with anandamide. It potentiates anandamide action by increasing receptor affinity or reducing degradation of anandamide, known as ‘entourage effect’ [23]. Biosynthesis of 2-AG involves the membrane phospholipid 1-stearoyl-2-arachidonoyl-sn-glycerol being hydrolysed by PLCβ followed by further cleavage of the product 1,2-diacylglycerol by diacylglycerol lipase (DAGL) to yield 2-AG [24, 25]. There exist two isoforms of DAGL, viz. DAGLα in the central nervous system (CNS) [26] and DAGLβ in immune cells [27](Figure 1).
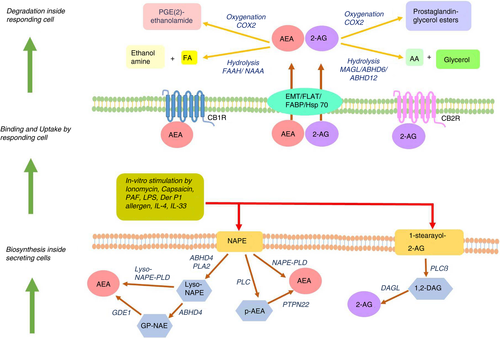
Lipopolysaccharides (LPS), platelet-activating factor (PAF), the TRPV1-agonist capsaicin, Δ9-THC and anandamide itself enhance anandamide synthesis in mouse macrophage cells RAW264·7 [28, 29]. Microglia polarized towards anti-inflammatory M2 phenotype with IL-4 and IL-33 produces 2-AG and anandamide, with elevated expression of DAGLα and CB2, while endocannabinoid signalling genes are downregulated in pro-inflammatory M1 macrophages [30]. A functional ECS is present in dendritic cells (DCs), in terms of expression of CB1, CB2 and fatty acid amide hydrolase (FAAH). Stimulation of immature monocyte-derived DCs with LPS or Derp1 allergen induces synthesis of 2-AG, which is further enhanced in mature DCs [31]. In murine models of delayed-type hypersensitivity (DTH) and strong antibody response, T cells and B cells produce significant levels of 2-AG [32] (Figure 1).
Circulating concentrations of anandamide and 2-AG have been reliably measured in human serum by high-pressure liquid chromatography–tandem mass spectrometry [33, 34]. Although systemic endocannabinoid concentration is extremely dynamic and depends on multiple factors [35], physiological levels of serum anandamide range from 1 to 5 nM, while serum 2-AG concentration varies broadly between 10 and 500 nM [34]. The physiological level of anandamide and 2-AG is controlled by endocannabinoid-degrading enzymes. Anandamide is primarily degraded by FAAH, a serine hydrolase active at alkaline pH [36]. N-acylethanolamine-selective acid amidase (NAAA) is another anandamide catabolic enzyme, a cysteine hydrolase working in acidic pH [37]. PEA serves as a specific substrate of NAAA, thereby interfering and reducing anandamide degradation [38]. Hydrolysis of 2-AG into arachidonic acid (AA) is carried out by three serine hydrolases, viz. monoacylglycerol lipase (MAGL), α/β-hydrolase domain-containing protein-6 (ABHD6) and α/β-hydrolase domain-containing protein-12 (ABHD12). MAGL is the major 2-AG-hydrolysing enzyme in the CNS [39]—it contributes to 85% of hydrolytic activity in rodent brains, while ABHD6 and ABHD12 account for the rest [40, 41]. Anandamide and 2-AG are also substrates for cyclooxygenase-2 (COX-2), lipoxygenases (LOX) and cytochrome P450 monooxygenases producing prostaglandin/prostaglandin esters, epoxy derivatives and hydroxyl derivatives respectively, which exerts varied physiological functions [42] (Figure 1).
ROLE OF ENDOCANNABINOIDS ON MAJOR IMMUNE CELLS
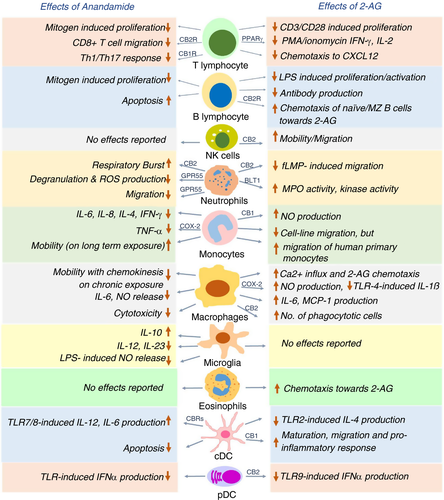
THC and cannabinoid receptors are long known to have immunomodulatory potential. CB1 is expressed at a modest level in human B cells, NK cells, monocytes, neutrophils, T cells and differentiated macrophages [43, 44], while being abundant in the CNS. CB2 is expressed predominantly in the immune cells, around 10–100 times higher than CB1 [43, 45]. Evidence for expression of the cannabinoid receptors on immune cells and reports on immunomodulatory effects of THC through these receptors (not reviewed here) led to studies on roles of endocannabinoids in the innate and adaptive immunity [46, 47]. Here, we curated the notable studies on immunomodulatory roles of anandamide and 2-AG on the major immune cells (Figure 2).
T cells and NK cells
Anandamide inhibits mitogen-induced proliferation of T cells at 10 μM dose [48] and hampers the chemokine SDF-1-induced migration of CD8+ T cells [49]. Anandamide directly suppresses cytokine release by CD4+ and CD8+ T cells via CB2 [50] and inhibits pro-inflammatory T-cell responses by blocking the production of Th1/Th17-polarizing cytokines by keratinocytes through CB1-mTOR axis [51]. 2-AG interferes with nuclear translocation of transcription factors NFAT1 and NFAT2 in mouse splenocytes, thus reducing IL-2 and IFN-γ expression [52]. IL-2 suppression by 2-AG is CB1/CB2-independent and involves a COX-2 metabolite that signals via PPARγ [53]. On exposure to super-antigens, human T cells upregulate expression of the otherwise negligible CB2 and increase phosphorylation and activation of ERK1/2 in response to 2-AG. 2-AG also abrogates T-cell chemotaxis towards CXCL12 [54]. Thus, both the endocannabinoids exert immunosuppressive effect on T-cell migration and function through different receptors. On the other hand, 2-AG, but not anandamide, induces CB2-dependent migration in NK cell line KHYG-1 and primary human NK cells [55].
B cells
Immunization of mice with either SRBC (sheep red blood cells) or cholera toxin and analysis of the migration of sorted B cells revealed that naïve B cells exhibit higher chemotaxis towards high 2-AG concentrations of 1 and 10 μM, in comparison with the B220+ class-switched B cells, which is CB2-dependent [56]. However, polyclonally activated murine B cells release 2-AG to a greater extent than naïve B cells, and the released 2-AG, in turn, reduces proliferation and antibody production in polyclonally activated or antigen-stimulated B cells [32]. Marginal zone (MZ) B cells display CB2-mediated chemotaxis towards 2-AG, and the retention of MZ B cells in the marginal zone compartment requires the presence of CB2 [57]. Thus, 2-AG serves dual role of chemo-attracting naïve B cells and MZ B cells and inhibiting function of activated B cells, with the latter being better 2-AG producers.
Neutrophils
Methanandamide, a stable synthetic analogue of anandamide [58], has been shown to stimulate respiratory burst in human neutrophils through CB2, while anandamide does not show any effect due to its rapid hydrolysis. 2-AG suppresses fMLP-induced migration of human neutrophils by CB2-dependent modulation of RhoA activity [59]. Anandamide also inhibits neutrophil migration, utilizing a receptor distinct from CB1 and CB2 [60]. The identity of this receptor was discovered to be GPR55, often considered the ‘third’ cannabinoid receptor and highly expressed in neutrophils. Activated GPR55 enhances the migration of human neutrophils towards 2-AG, while simultaneously inhibiting their degranulation and release of reactive oxygen species [61]. Human neutrophils get activated in terms of enhanced myeloperoxidase (MPO) release, increased kinase activity and calcium mobilization on 2-AG pretreatment. It was eventually revealed that 2-AG hydrolysis fuels the synthesis of leukotriene LTB4, which leads to the stimulation of neutrophils [62]. Accordingly, supernatants from 2-AG-treated neutrophils exhibit potent antibacterial and antiviral effects, which is completely abrogated on the blockade of LTB4 synthesis or antagonizing its cognate receptor BLT1 [63]. To summarize, 2-AG and anandamide exert mostly stimulatory effect on certain neutrophil functions while largely suppressing their migration.
Macrophages and monocytes
Anandamide regulates macrophage and monocyte function in both dose-dependent and temporal manner, while the effect of 2-AG relies on by its downstream products, which stimulate nitric oxide (NO) production and suppress pro-inflammatory function of macrophages. On assessing the effect of anandamide on cytokine production by stimulated human peripheral blood mononuclear cells (PBMCs), it was found that inhibition of IL-6 and IL-8 occurs only at nanomolar concentrations (3–30 nM), while inhibition of IL-4-, TNF-α-, IFN-γ- and p75 TNF-α-soluble receptors require micromolar concentrations (3–30 μM) of anandamide. The release of AA by unstimulated and fMLP-stimulated human monocytes is enhanced at 30 μM anandamide dose, with no effect on IL-10 release [64]. Interestingly, acute anandamide exposure renders macrophages round and immobile, while long-term anandamide exposure mobilizes macrophages and enhances its endothelial adherence, favouring transmembrane migration [65]. Anandamide suppresses NO and IL-6 release by LPS-activated J774 macrophages in a dose-dependent fashion, whereas 2-AG suppresses IL-6 but increases iNOS-mediated NO production. This puzzling effect of 2-AG is probably an outcome of 2-AG being hydrolysed and further oxygenated by COX-2 to yield AA and prostaglandin-E2 (PGE-2), both of which promote iNOS. Notably, PGE(2)-ethanolamide (oxygenated product of anandamide) does not have any effect on IL-6 or NO production [66]. Rather, PGE(2)-ethanolamide represses IL-12p40 promoter in LPS/IFN-γ-stimulated microglia. Consistently, anandamide itself blocks IL-12 and IL-23 production and enhances IL-10 production by activated microglia [67], through activation of JNK and ERK1/2, and NF-κB inhibition [68]. Anandamide causes neuroprotection by inhibiting NO release in LPS-activated microglia, signalling through cannabinoid receptors and upregulating MKP-1 expression, thereby repressing the MAP kinase pathway [69]. PGE(2)-ethanolamide also hinders TNF-α production by LPS-stimulated human monocytes, utilizing the EP2 receptor and downstream cAMP pathway [70].
Primary human monocytes show enhanced production of NO with increasing doses of 2-AG through CB1. Interestingly, 2-AG treatment of human primary monocytes renders them round and immobile, which appear immunosuppressive in terms of reduced cytokine production and adhesion [71].
Human promyelocytic leukaemic HL-60 cells differentiate into macrophage-like cells on exposure to vitamin D3 [72]. A lot of studies were done on HL-60 cells to understand the effect of 2-AG on human macrophages. The influx of Ca2+ influx increases transiently in HL-60 cells on activation of 2-AG-CB2-phospholipase C-axis[73]; migration towards 2-AG is enhanced in a dose-dependent manner (10 nM–10 μM range) [72]; and IL-6 and MCP-1 production is induced, which is further enhanced when treated with both 2-AG and LPS [74]. Furthermore, 2-AG increases the phagocytic ability of HL-60 cells based on β2 integrins, and signalling through CB2-Gi/o pathway [75]. A recent study revealed that elevated 2-AG inhibits TLR4-induced pro-inflammatory response in macrophages, mediated by oxygenation of 2-AG into prostaglandin-glycerol ester PGD2-G [76].
Dendritic cells
Exploratory work with murine bone marrow-derived DCs (BMDCs) revealed that anandamide induces their apoptosis by engaging both the cannabinoid receptors [77]. Anandamide inhibits TLR7/8-induced IL-12 and IL-6 production by human myeloid DCs (mDCs) and IFN-α production by human plasmacytoid DCs (pDCs) [78]. In a mouse model for DTH, 2-AG suppresses TLR2 agonist-mediated IL-4 production by mDCs and enhances chemotaxis of CD8+ CD11c+ DCs, causing stronger Th1-skewed DTH response—both these effects are CB2-dependent [79].
In a murine model of pancreatic ductal adenocarcinoma (PDAC), 2-AG exerts CB1-mediated anti-tumour effects by suppressing the proliferation of cancer cells and by favouring maturation of dendritic cells to produce pro-inflammatory mediators through p-STAT6 upregulation [80]. Genetic ablation of DAGLβ in BMDCs reduces the cellular level of 2-AG along with a direct reduction in LPS-stimulated TNF-α production. However, maturation of Daglb−/− DCs and their T-cell priming activity remains unhindered [81].
On assessing the effect on pDCs, it has been recently shown that 2-AG strongly suppresses TLR9-mediated type I interferon production by human primary pDCs. The effect is solely mediated by CB2, and abrogation of this 2-AG inhibition is found to be crucial in SLE pathogenesis [82].
ENDOCANNABINOIDS AND IMMUNE PATHOLOGIES
Majority of evidences support an anti-inflammatory role of anandamide, although 2-AG exerts both pro- and anti-inflammatory roles depending on cell type. Endocannabinoids might be beneficial in the suppression of inflammation, and their dysregulation may underlie certain instances of chronic inflammation. We next summarize our current understanding of the crosstalk of endocannabinoids with the immune system in three instances of chronic inflammatory diseases, viz. allergic disorders, autoimmune diseases and systemic inflammation associated with metabolic syndrome.
Inflammation and allergy
Both anandamide and 2-AG suppress neutrophil recruitment and TNF-α production in LPS-induced bronchopulmonary inflammation in mice [83, 84]. Endocannabinoids are increased in response to trinitrobenzene sulphonic acid (TNBS)-induced colitis in rodents. Elevated endocannabinoids appears anti-inflammatory as inhibiting their degradation reduces the severity of inflammation, while CB1 and CB2 deletion aggravates colitis. Anandamide level also correlated with disease severity in biopsies from patients with untreated ulcerative colitis [85, 86]. Capsaicin- and anandamide-induced activation of TRPV1 in gut mononuclear phagocytes enhances anandamide biosynthesis, and CB2–anandamide interaction expands the population of regulatory CX3CR1hi macrophages. This pathway increases anti-inflammatory Tr1 cells through IL-27, thus providing a mechanism of ECS-mediated gut immunotolerance [87]. In a murine model of cutaneous contact dermatitis, lack of cannabinoid receptors enhances hypersensitivity, while deficiency of endocannabinoid-hydrolysing FAAH increases anandamide level and dampens hypersensitivity [88]. Methylated BSA-stimulated Th17-mediated DTH gets diminished in mice exposed to anandamide, presumably through favoured IL-10 and reduced IL-17 and IFN-γ production [89]. A similar reduction in Th1- and Th17-related cytokines, and suppressed proliferation of lymphocytes are seen on 2-AG exposure to mice with DTH [32]. 2-AG suppresses acute neuroinflammation by reducing CNS-infiltrating immune cells and inducing immunosuppressive MDSCs (myeloid-derived suppressor cells) [90]. Likewise, enhanced anandamide levels due to systemic/central inhibition of FAAH attenuates neuroinflammation by inhibiting TLR4-induced pro-inflammatory cytokines in the frontal cortex, partially utilizing TRVP1 [91]. Endocannabinoids also suppress anti-tumour immunity by reducing Th1 response and inducing MDSCs [92, 93].
On the other hand, a pro-inflammatory role of endocannabinoids, especially 2-AG, has been reported in the context of allergic inflammation. This is mostly because 2-AG acts as a chemotactic agent for human eosinophils through CB2, probably by enhancing endothelial/leucocyte adhesion and transmigration [94-96]. A series of reports indicate that in murine models of contact dermatitis and allergic bronchitis, the 2-AG level is highly increased in the inflamed tissue, correlating strongly with disease severity and immune cell infiltration, which is effectively counteracted on CB2 blockade [97-100].
Autoimmune disorders
Endocannabinoids plays vital role in protecting against neuroinflammation in multiple sclerosis (MS) by engaging both CB1 and CB2, for indirect release of immunomodulatory molecules by nerves and direct effect on immune cells respectively [101]. The pathogenic cytokines of MS, that is IL-12p70 and IL-23, are effectively downregulated in the spinal cord and microglia by anandamide, in a virus-induced animal MS model [102]. In vivo administration of 2-AG to EAE (experimental autoimmune encephalitis) mice delays disease onset, severity and mortality, with a concomitant increase in anti-inflammatory macrophages [103]. Anandamide and 2-AG are also increased in cerebrospinal fluid and plasma of human MS patients with active disease and lesions [104-106]. Evaluating endocannabinoid levels showed that anandamide level is elevated in B cell, T cells and NK cells of MS patients, and declines to normal levels following 1 year of treatment with IFN-β [107]. Comparative analysis further revealed that monocytes from MS patients upregulate CB2 and downregulate FAAH, perhaps to increase the local anandamide level as a feedback mechanism to combat inflammation. Anandamide, however, only significantly suppresses TLR7/8-stimulated, but not TLR4 or TLR5-stimulated, cytokine release in MS monocytes, owing to high expression of TLR4 and TLR5 in MS monocytes compared with healthy monocytes [108]. Likewise, the inhibitory activity of anandamide on pDC function is compromised in MS patients, due to abundant FAAH expression and high anandamide-degrading capacity in these cells [78]. Understanding the local endocannabinoid tone in the microenvironment of the immune cells can be crucial when designing drugs to treat disorders.
Anandamide and 2-AG are detected at appreciable levels in the synovial fluid from patients with rheumatoid arthritis (RA) and osteoarthritis (OA) while being absent in healthy volunteers. Synovial biopsies from these patients display functional cannabinoid receptors and FAAH [109]. It was subsequently reported that low dose of glucocorticoid drives synovial fibroblasts to produce endocannabinoids [110]. Anandamide inhibits IL-6, IL-8, and MMP-3 induction in primary synoviocytes and synovial fibroblasts from RA patients by TRPA1/TRPV1 channel desensitization [111], thus exerting anti-inflammatory effects. Indeed, synthetic cannabinoids such as ajulemic acid and the CB2 agonist HU-320 hamper metalloproteinase and pro-inflammatory cytokine production by FLS (fibroblast-like synoviocytes), thereby reducing the severity of collagen-induced arthritis (CIA) [112, 113]. Moreover, a loss-of-function dinucleotide polymorphism in the CB2 gene has been linked with increased susceptibility of RA [114, 115].
A missense mutation in anandamide biosynthetic enzyme PTPN22 and a gain-of-function polymorphism in the 2-AG-hydrolysing enzyme ABHD6 have been highly associated with systemic lupus erythematosus (SLE)—these observations uncover a link between the ECS and lupus pathogenesis [116-118]. It was recently shown that enhanced expression of ABHD6 in a subset of SLE patients increases their 2-AG-hydrolysing ability, the latter being important in strong suppression of the pathogenic type I interferon response characterizing SLE [82].
Inflammation associated with metabolic syndrome
Chronic low-grade inflammation characterizes the visceral adipose tissue (VAT) in obesity [119]. Obese VAT shows altered expression of FAAH and CB1 under pro-inflammatory conditions in vitro [120]. High-fat diet-induced obesity in mice is also associated with enhanced plasma levels of endocannabinoids, presumably due to increased synthesis in VAT, evident from a strong correlation of endocannabinoid levels with endocannabinoid-synthesizing enzyme expression in VAT [121, 122]. Blockade of CB1 receptor reduces the expression of pro-inflammatory molecules MIP-1β and IL-7 in cultured adipocytes obtained from human adipose tissue explants [123]. A small-molecule inhibitor of CB1, rimonabant, has been used to reduce weight in humans, although currently discontinued due to psychiatric side-effects [124]. Chronic stimulation of CB1 in high-fat diet-fed mice promotes glucose intolerance and supports enhanced macrophage infiltration in muscles [125]. Interestingly, endocannabinoids favour type 2 diabetes by activating Nlrp3-inflammasome, which increases IL-1β in the infiltrating M1 macrophages of pancreatic islets, leading to inflammatory damage of the pancreatic β cells [126, 127].
CONCLUDING REMARKS
Research done over the past few decades has thoroughly proved the presence of extensive crosstalk between the ECS and the various facets of the immune system. The interaction is indeed complex, and the effects of endocannabinoids on the immune system can vary highly in a spatiotemporal and context-dependent manner. The levels of endocannabinoids can be significantly altered in the context of disease pathologies. The endocannabinoid tone affects and is also in turn affected by immune homeostasis and immune perturbations. The concentration of endocannabinoids in in vitro studies may not always reflect the physiological endocannabinoid levels in systemic and local environment; thus, heavily relying on these studies can further complicate the understanding of their immunomodulation. These studies have to be complemented with detailed exploration of physiological endocannabinoid levels in biological tissues, which is known to be dependent on multiple factors as reviewed elsewhere [35]. Filling up this knowledge gap can enable utilizing endocannabinoid levels as potential ‘biomarkers’ for disease course.
The ubiquitous nature of the ECS makes it appear difficult to target specific diseases without causing undesired side-effects. In this regard, one approach has been developing peripheral CB1 agonists, which cannot cross blood–brain barrier, thereby precluding undesirable effects on the CNS involving psychotropic manifestations. It is also beneficial to target CB2 due to its predominant expression in the immune cells. Quite encouragingly, a handful of peripheral CB1 and CB2 agonists such as drugs for example JBT-101, APD371 and NEO1940 are now in their phase II/phase III trials for use in inflammatory disorders and cancer [128]. Other likely drug target candidates such as GPR55, MAGL, ABHD6 and TRPV1 are only recently starting to be recognized. To sum up, the complex and multifactorial nature of ECS pathway opens up great opportunities for identifying therapeutic targets in multiple immunological aberrations, which can be harnessed optimally only with increased research and extremely detailed understanding of the ECS pathway.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
OR and DG conceptualized and wrote the manuscript. OR prepared the figures.




