Induction of the apoptosis, degranulation and IL-13 production of human basophils by butyrate and propionate via suppression of histone deacetylation
Yanbiao Shi, Meizhen Xu, Shuai Pan, Sijia Gao contribute equally and should be considered as joint first author.
Funding information
This work was supported by the National Natural Science Foundation of China (grant no. 31800750 and 81872114), Jiangsu Provincial Special Program of Medical Science (grant no. BE2019617), the Youth Innovation Team Grant and the Starting Grant by Xuzhou Medical University (grant no. D2018009).
Abstract
Allergic diseases are caused by dysregulated Th2 immune responses involving multiple effector cells including basophils. Short chain fatty acids (SCFAs), mainly acetate, propionate and butyrate, exert immunomodulatory functions via activation of its receptors GPR41 and GPR43, and inhibition of the histone deacetylases (HDACs) activity. In allergic diseases, SCFAs suppress the activity of mast cells, eosinophils and type 2 innate lymphoid cells (ILC2) but enhance the function of Th2 cells. Here, we aimed to elucidate the function of SCFAs on human basophils. Human basophils were purified from healthy donors by flow cytometric sorting. The surface proteins, apoptosis and degranulation of basophils were analyzed by flow cytometric analysis. The mRNA expression was assayed using real-time PCR. Interleukin 4 (IL-4) and IL-13 were measured by ELISA. Histone acetylation was examined by western blot. GPR41 was expressed by basophils and was enhanced by IL-3. Acetate induced intracellular calcium influx in basophils which was suppressed by blocking GPR41. Propionate and butyrate, but not acetate, induced the expression of CD69 and IL-13. In addition, propionate and butyrate enhanced IgE-mediated basophil degranulation but inhibited basophil survival and IL-4 secretion. Propionate and butyrate induced histone acetylation of basophils and suppression of HDACs activity mimicked the effects of propionate and butyrate on human basophils. Our findings demonstrate that propionate and butyrate may play a complex role in regulating basophil apoptosis, activation and degranulation via inhibiting HDACs activity. The in vivo effects of SCFAs on the regulation of basophil-associated allergic diseases need to be further explored.
Abbreviations
-
- CHC
-
- α-cyano-4-hydroxycinnamic acid
-
- EoE
-
- eosinophilic esophagitis
-
- HDACs
-
- histone deacetylases
-
- HB
-
- ±/-β-Hydroxybutyrate
-
- ILC2
-
- type 2 innate immune cells
-
- SCFAs
-
- Short chain fatty acids
-
- Th2
-
- T helper 2
-
- TSA
-
- Trichostatin A
INTRODUCTION
Allergic disorders are characterized by aberrant activation of T helper 2 (Th2)-associated immune responses involving multiple effector immune cells including Th2 cells, mast cells, basophils, eosinophils and type 2 innate immune cells (ILC2) [1-4]. Basophils, as the least abundant granulocytes in peripheral blood, are terminally differentiated with a short life span [1]. Despite this, basophils play important roles in regulating allergic immune responses by releasing pre-stored mediators in granules, e.g., histamine, following crosslinking of the surface FcεRI-bound IgE by allergen [1, 2]. In addition, basophil activation is regulated by interleukin-3 (IL-3), a master cytokine that regulates the survival and activation of basophils [1, 5, 6]. IL-3-mediated basophil activation is associated with surface expression of CD69 followed by secretion of IL-13 and IL-4, the latter of which is recognized as an early source of IL-4 for initiating Th2 cell differentiation [1, 7-10].
The gut microbiome plays an indispensable role in the development of multiple diseases [11, 12]. Short chain fatty acids (SCFAs), which mainly contain acetate, propionate and butyrate, are produced by commensal intestinal microbiota through fermentation of indigestible dietary fibers [13-15]. Therefore, SCFAs present at high concentrations in the gut but at a low level in the circulation [22, 23]. However, numerous studies indicate that SCFAs exert immunoregulatory functions in multiple organs and tissues, e.g., brain, lungs and skins, through two major mechanisms, i.e., activation of its receptors GPR41 and GPR43, and inhibition of histone deacetylases (HDACs) [13, 14, 16-18]. In allergic diseases, SCFAs suppress allergic immune responses by restraining the activity of multiple allergic immune cells, e.g., mast cells, eosinophils and ILC2 [14, 19, 20]. Propionate and butyrate, but not acetate, are capable of inhibiting eosinophil survival, migration, and adhesion, as well as suppressing mast cell degranulation via the inhibition of HDACs activity [14, 19]. For ILC2, butyrate, but not acetate or propionate, inhibits cell proliferation and IL-5 and IL-13 production without impacting ILC2 survival [20]. In contrast to these findings, a recent study reports that SCFAs enhance Th2 immune responses by promoting IL-4, IL-5 and IL-13 production by Th2 cells in eosinophilic esophagitis (EoE) [21]. However, the effect of SCFAs on basophils, another important player in allergic disorders, remains elusive.
Different from mast cells that reside in mucosal tissues, human basophils circulate in the peripheral blood and are rarely detectable in local tissues in healthy subjects [1]. However, multiple studies have revealed that basophils infiltrate in skin lesions of multiple Th2-biased skin diseases such as urticaria, bullous pemphigoid and eosinophilic pustular folliculitis, lung and sputum of patients with allergic asthma, as well as nasal mucosa and secretion of allergic rhinitis [8, 22-24]. Although the concentration of SCFAs in skin has not been reported, it has been shown that sputum SCFAs from patients with cystic fibrosis and SCFAs from human gingival crevicular fluid present in millimolar range [25, 26], indicating that the activity of local infiltrating basophils might be modulated by SCFAs.
Hence, we in this study examined the function of SCFAs in regulating the survival, IL-4 and IL-13 production by and degranulation of human basophils. We showed that propionate and butyrate, but not acetate, induced basophil apoptosis, IL-13 expression and IgE-mediated degranulation, and these effects were mimicked by inhibiting HDACs activity.
MATERIALS AND METHODS
Ethics statement
All the buffy coat used in this study were collected from healthy subjects. The study was approved by the ethics committee of Xuzhou Medical University and Xuzhou Red Cross Blood Center. Informed consent was obtained from all blood donors.
Cell purification and culture
Enrichment of PBMCs from buffy coat was performed as previously described [27]. Human basophils were purified by using Human CRTH2 microbead kit (Miltenyi Biotec) followed by flow cytometric sorting of SSClowCD4−CD127−HLA-DR−CRTH2+ [27, 28]. Purified basophils were cultured in 96-well plate using RPMI 1640 medium with 10% FBS as previously reported [28].
Expression analysis of SCFA receptors
The mRNA expression of GPR41 and GPR43 was examined using a public gene expression microarray data retrieved from Gene Expression Omnibus (accession number: GSE3982) and our in-house microarray data as previously described (accession number: GSE144096) [27, 29]. The microarray data was quantile normalized by R program as previously described [30].
Membrane expression of GPR41 and GPR109A was determined by flow cytometric analysis using rabbit anti-human GPR41 (MultiSciences), rabbit anti-human GPR109A (Sangon Biotech) and goat anti-rabbit IgG-Alexa488. For assaying surface GPR41 on activated basophils treated with or without different signaling inhibitors, purified basophils (1 × 105 cells/well) were stimulated as previously indicated [27].
Real-time PCR assay
Real-time PCR was performed using gene-specific primers including the forward primer 5’-ACTTTGAACAGCCTCACAGAG-3’ and the reverse primer 5’-TTGGAGGCAGCAAAGATGTC-3’ for human IL4, the forward primer 5’- TGAGGAGCTGGTCAACATCA-3’ and the reverse primer 5’-CAGGTTGATGCTCCATACCAT-3’ for human IL13, and the forward primer 5’-GAAGGTGAAGGTCGGAGTC-3’ and the reverse primer 5’-GAAGATGGTGATGGGATTTC-3’ for human GAPDH. The mRNA expression of IL4 and IL13 was determined relative to GAPDH using a delta-delta CT method [31].
Calcium influx
Calcium influx assay was performed using Fluo-8 (Abcam). Purified basophils pre-loaded with Fluo-8 (4 μM) were treated with or without ±/-β-Hydroxybutyrate(HB, 1 mM, Cayman)or rabbit anti-human GPR41 (10 μg/mL, MultiSciences) for 30 minutes prior to stimulation with sodium acetate (5 mM, Sigma), sodium propionate (5 mM, Sigma), or sodium butyrate (0·5 mM, Sigma). Calcium influx was measured by flow cytometric analysis of Fluo-8 in basophils for 30 seconds (baseline) prior to HB stimulation, 30 seconds prior to SCFA stimulation and then for indicated minutes post SCFA stimulation. Medium fluorescence intensity (MFI) was used to plot calcium influx changes by FlowJo software.
Analysis of apoptosis, membrane CD69 and Th2 cytokines
For the analysis of surface CD69, CRTH2-enriched basophils were stimulated with sodium acetate (0·2 mM, 1 mM, 5 mM), sodium propionate (0·2 mM, 1 mM, 5 mM) or sodium butyrate (0·02 mM, 0·1 mM, 0·5 mM) for indicated times. For assaying basophil apoptosis, purified basophils (2 × 105 cells/well) were cultured with sodium acetate (5 mM), sodium propionate (5 mM), sodium butyrate (0·5 mM), or trichostatin A (TSA, 50 nM) with or without IL-3 (20 ng/mL) for 36 hours. HB (1 mM) and α-cyano-4-hydroxycinnamic acid (CHC, 1 mM, Aladdin) was used to block GPR41 and monocarboxylate transportation, respectively. Annexin V-APC (Biolegend) was used for assaying basophils apoptosis. IL-4 and IL-13 in supernatant were analyzed by Human IL-4 and IL-13 ELISA kits (MultiSciences).
Degranulation analysis
For the degranulation analysis, CRTH2-enriched basophils were stimulated with diluent, HB (1mM) or CHC (1mM) for 30 minutes prior to stimulation with SCFAs at indicated concentrations or TSA (50 nM) for 2 hours followed by stimulation with mouse anti-human IgE (10 μg/mL, BD Biosciences, clone: G7-18) for 30 minutes. The degranulation surface marker CD63 was analyzed by flow cytometric analysis. For the measurement of basophil degranulation by direct analysis of exteriorized basophil granule matrix [32], surface staining of basophils with Avidin-FITC (Solarbio) was performed for subsequent flow cytometric analysis.
Western blot
Rabbit anti-Acetyl-Histone H3 (Lys9) (C5B11) and rabbit anti-Histone H3 (D1H2) antibodies (Cell Signaling Technology) were used for assaying the histone acetylation of human basophils (4 × 105 cells/well) pre-treated with or without CHC (1mM) for 30 minutes, followed by stimulation with or without IL-3 (20 ng/mL) in the presence of propionate (5 mM) or butyrate (0·5 mM) for 12 hours.
Statistical analysis
Data were shown as the mean ±SEM. Differences between two paired groups were analyzed by paired Student's t-test for normal distribution or Wilcoxon signed rank test for non-normal distribution. For multiple comparisons, one-way or two-way ANOVA followed by Bonferroni multiple comparison test was used. p < 0·05 was considered significant.
RESULTS
Human basophils express GPR41 which is enhanced by IL-3
GPR41 and GPR43 are receptors for SCFAs [33]. We initially analyzed GPR41 and GPR43 expression in human immune cells based on a publicly available set of gene expression microarray data [29]. GPR41 was relative highly detected in basophils while GPR43 was mainly detected in neutrophils and eosinophils rather than basophils (Figure 1a). To confirm this finding, we next analyzed GPR41 expression by flow cytometric analysis. In agreement with the mRNA data, human basophils expressed membrane GPR41, which was also detectable on eosinophils but not neutrophils (Figure 1b). To further understand if GPR41 and GPR43 were expressed in activated basophils, we analyzed our previous microarray data of IL-3-activated basophils, which showed that IL-3 significantly induced GPR41 but not GPR43 (Figure 1c) [27]. In addition to GPR41 and GPR43, GPR109A is another surface receptor recognizing SCFAs, although at low affinity [34, 35]. We analyzed GPR109A expression using aforementioned gene expression microarray data, which showed that GPR109A was not detectable in fresh or IL-3-activated basophils. Further analysis of surface GPR109A also showed that GPR109A was not detectable on human basophils (Data not shown).
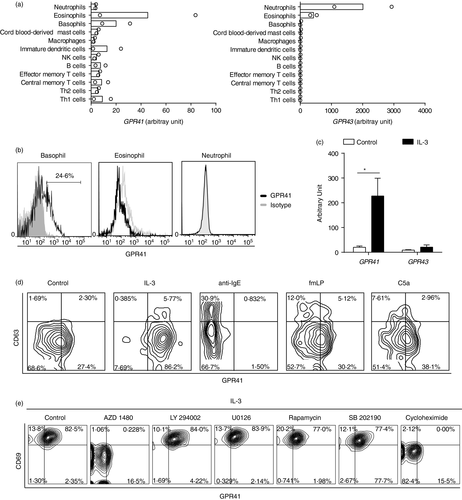
Basophils are activated by not only IL-3 but also multiple other modulators including anti-IgE, fMLP and C5a, which are known to induce expression of membrane CD63, a well-known degranulation marker of basophils [1, 36]. We therefore further examined expression levels of membrane GPR41 on basophils following activation by these modulators. In line with the mRNA data, GPR41 was preferentially enhanced by IL-3 (Figure 1d). While anti-IgE, fMLP and C5a could induce surface expression of CD63, GPR41 was greatly inhibited by anti-IgE. fMLP and C5a had a modest effect on inducing the expression of GPR41 (Figure 1d).
To clarify if GPR41 was induced via a pre-stored form or de novo protein synthesis, we analyzed membrane GPR41 on IL-3-stimulated basophils in the presence of the protein synthesis inhibitor cycloheximide. Cycloheximide inhibited IL-3-induced expression of GPR41 as well as basophil activation marker CD69, suggesting that the activation of basophils might be correlated to the surface de novo synthesis of GPR41 (Figure 1e). Next, we tried to confirm the induction of GPR41 was indeed a direct effect of IL-3 signaling by inhibiting multiple signaling proteins. In agreement with previous reports, AZD1480, which blocks the JAK2 protein that is known to be involved in IL-3 signaling, significantly diminished both GPR41 and CD69 expression (Figure 1e) [27, 37].
These data collectively indicate that GPR41 is expressed by human basophils and IL-3 is a potent inducer of the expression of this receptor.
Acetate induces intracellular calcium influx via activation of GPR41
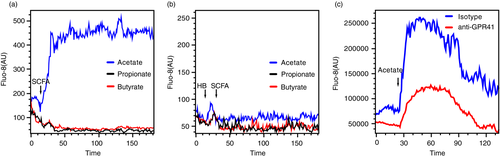
GPR41 is coupled to the Gi/o family and activation of GPR41 results in intracellular calcium influx [33]. To elucidate if GPR41 is active on human basophils, we examined intracellular calcium influx of basophils upon SCFA treatment. The concentration for SCFAs was selected based on previous reports [14, 19, 20]. As expected, acetate induced intracellular calcium influx (Figure 2a) and this effect was diminished by pre-treatment of basophils with ±/-β-hydroxybutyrate (HB), which is reported to block the activity of GPR41, or by rabbit anti-human GPR41 polyclonal antibody (Figure 2b-c) [14]. This indicates that GPR41 is active on human basophils and can be activated by acetate. However, no intracellular calcium influx was observed in basophils treated with propionate or butyrate (Figure 2a).
Propionate and butyrate induce the expression of CD69 and IL-13
To characterize if SCFAs play a role in regulating the activation of basophils, we set out to examine the expression of CD69, a basophil activation marker [8]. We treated purified basophils with sodium acetate (0·2 mM, 1 mM and 5 mM), sodium propionate (0·2 mM, 1 mM and 5 mM) or sodium butyrate (0·02 mM, 0·1 mM and 0·5 mM) for indicated time points. Interestingly, propionate or butyrate alone, but not acetate, induced CD69 expression at a time- and concentration-dependent manner (Figure 3a).
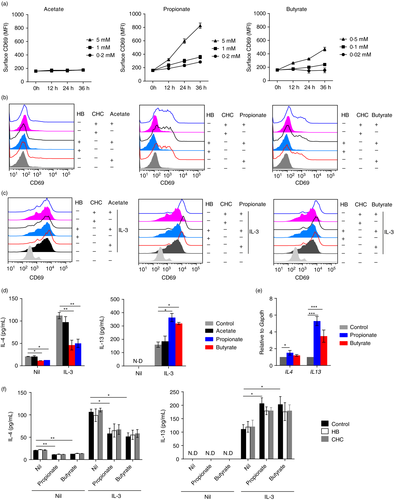
SCFAs exert stimulatory functions mainly through two mechanisms, namely activation of surface receptors and inhibition of histone deacetylases (HDACs). Therefore, we pre-treated basophils with HB (1 mM, 30 minutes) or α-cyano-4-hydroxycinnamic acid (1 mM, CHC, 30 minutes), a pan-inhibitor for the monocarboxylate transporter which is reported to prevent SCFAs from entering basophils [14], prior to addition of SCFAs. However, blocking of GPR41 by HB or inhibition of monocarboxylate transportation by CHC had no effect on propionate (5 mM)- and butyrate (0·5 mM)-induced CD69 expression (Figure 3b). We next analyzed if SCFAs had synergistic effects on IL-3-induced basophil activation. As shown in Figure 3c, propionate (5 mM) and butyrate (0·5 mM) promoted IL-3-induced CD69 expression while acetate (5 mM) still had no effect. Similarly, blocking of GPR41 by HB or inhibition of monocarboxylate transportation by CHC had no effect on propionate (5 mM)- and butyrate (0·5 mM)-enhanced CD69 expression by IL-3-stimulated basophils (Figure 3c).
To further confirm our findings, we examined the production of IL-4 and IL-13, key cytokines produced by activated basophils [7, 10, 38]. Without the addition of IL-3, propionate (5mM) and butyrate (0·5 mM), but not acetate (5 mM), suppressed IL-4 production (Figure 3d). In contrast, IL-13 was not detected in all the groups. In the presence of IL-3, which induced IL-4 and IL-13 expression, addition of propionate (5 mM) or butyrate (0·5 mM), but not acetate (5 mM), inhibited IL-4 production but promoted IL-13 expression (Figure 3d). Next, we confirmed the differential expression of IL-4 and IL-13 induced by propionate and butyrate by analyzing the mRNA expression of IL4 and IL13. We found that propionate (5 mM) and butyrate (0·5 mM) significantly induced IL13 expression while only a slight increase of IL4 was observed in propionate-stimulated basophils (Figure 3e).
The blocking assays revealed that no changes in the expression of IL-13 and IL-4 after pre-blocking of GPR41 by HB (1 mM, 30 minutes) or inhibition of monocarboxylate transportation by CHC (1 mM, 30 minutes) (Figure 3f). These data collectively implicate that propionate and butyrate promote basophil activation.
Propionate and butyrate induce basophil apoptosis
SCFAs are reported to induce eosinophil apoptosis [14]. To understand if SCFAs could regulate basophils apoptosis, purified basophils were treated with sodium acetate (5 mM), sodium propionate (5 mM) or sodium butyrate (0·5 mM). While a mild increase of basophil apoptosis was observed following acetate treatment, propionate and butyrate substantially induced basophil apoptosis (Figure 4a). Further analyses revealed that pretreatment with neither HB (1 mM, 30 minutes) nor CHC (1 mM, 30 minutes) could reverse SCFA-induced basophil apoptosis (Figure 4a), although treatment with HB or CHC alone slightly enhanced basophil apoptosis (Figure 4a).
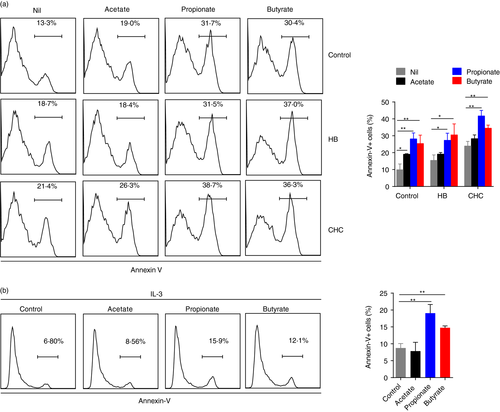
IL-3 is a master regulator for basophil survival and activation [1, 5]. Therefore, we next examined if SCFAs could induce basophil apoptosis upon activation by IL-3. Addition of IL-3 substantially suppressed basophil apoptosis (Figure 4b). However, even in the presence of IL-3, propionate and butyrate, but not acetate, induced basophil apoptosis (Figure 4b). These data indicate that propionate and butyrate have pro-apoptotic effects on basophils.
Propionate and butyrate promote IgE-mediated basophil degranulation
The key function of basophils in allergic immune responses is secretion of mediators, e.g, histamine and LTC4, via IgE-mediated degranulation [39]. We assayed if SCFAs could regulate basophil degranulation. Treatment of basophils with SCFAs alone for 2 hours had no effect on basophil degranulation revealed by flow cytometric analysis of surface CD63 expression (Figure 5a). Next, we examined if SCFAs regulate IgE-mediated basophil degranulation. CRTH2- enriched basophils were treated with sodium acetate (0·2 mM, 1 mM and 5 mM), sodium propionate (0·2 mM, 1 mM and 5 mM) or sodium butyrate (0·02 mM, 0·1 mM and 0·5 mM) for 2 hours followed by anti-IgE stimulation for another 30 minutes. Treatment with propionate and butyrate, but note acetate, increased CD63+ basophils induced by anti-IgE in a SCFA concentration-dependent manner (Figure 5b), indicating that propionate and butyrate could promote IgE-mediated basophil degranulation. In addition, pretreatment of basophils with CHC (1 mM, 30 minutes) or HB (1 mM, 30 minutes) for 30 minutes prior to addition of SCFAs treatment showed that CHC, but not HB, reversed propionate- and butyrate-enhanced basophil degranulation by anti-IgE (Figure 5b).
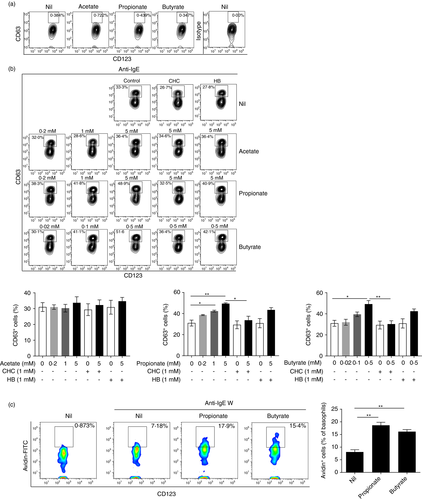
To further confirm the effect of propionate and butyrate on basophil degranulation, we examined basophil degranulation through direct measurement of exteriorized basophil granule matrix of propionate and butyrate-stimulated basophils followed by anti-IgE stimulation by flow cytometric analysis of Avidin-labelled basophils [32]. As expected, propionate (5 mM) and butyrate (0·5 mM) significantly increased Avidin+ basophils stimulated by anti-IgE (Figure 5c). These data consistently indicate that propionate and butyrate promote IgE-mediated basophils degranulation.
The HDAC inhibitor trichostatin A mimick the effects of propionate and butyrate on human basophils
Since CHC reversed the effects of propionate and butyrate on basophil degranulation, we speculated that propionate and butyrate might function as HDAC inhibitors in regulating the function of basophils. Hence, we examined the effects of propionate and butyrate on histone acetylation of human basophils. Western blot analysis showed that treatment of purified basophils with pan-HDACs inhibitor trichostatin A (TSA, 50 nM) for 12 hours enhanced histone acetylation (Figure 6a). Similarly, propionate (5 mM) and butyrate (0·5 mM) also promoted histone acetylation with or without IL-3 (Figure 6a).
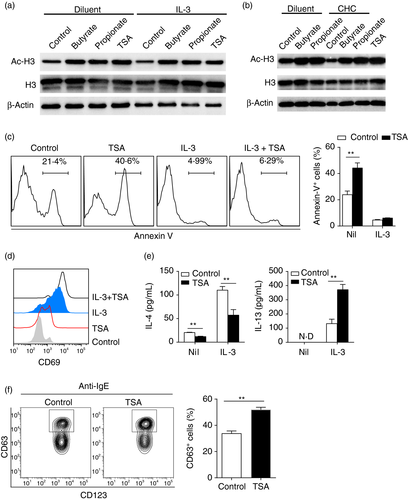
CHC showed no effect on reversing the expression of IL-13 and CD69 induced by propionate- or butyrate. This may be ascribed to that SCFAs can passively diffuse into cells [40]. We therefore examined the histone acetylation of propionate (5 mM) and butyrate (0·5 mM) stimulated basophils (12 hours after culture) that were pre-blocked with CHC (1 mM) for 30 minutes. As expected, propionate and butyrate still enhanced histone acetylation of basophils even after pre-blocking with CHC (Figure 6b).
To further substantiate our findings and confirm if propionate and butyrate acted through inhibiting HDACs in regulating the biology of basophils, we treated basophils with TSA (50 nM) with or without IL-3 (20 ng/mL). TSA alone significantly induced basophil apoptosis while this effect was reduced in the presence of IL-3 (Figure 6c). Similar as propionate and butyrate, TSA not only induced basal expression of CD69, but also augmented IL-3-mediated surface expression of CD69 (Figure 6d). The cytokine analysis also supported that TSA demonstrated a similar pattern as propionate and butyrate. TSA promoted IL-13 production but suppressed IL-4 secretion (Figure 6e). In addition, pre-treatment of basophils with TSA enhanced IgE-mediated basophil degranulation as evidenced by increased CD63+ cells following TSA treatment (Figure 6f). Collectively, the HDAC inhibitor TSA mimicked the effects of propionate and butyrate on human basophils.
DISCUSSION
SCFAs as the products of fermentation of dietary fibers by commensal intestinal microbiota have been implicated to play important roles in regulating allergic immune responses [14, 19-21]. Especially butyrate suppresses allergic immune responses by restraining the activity of ILC2, eosinophils and mast cells [14, 19, 20]. However, it has also been shown that SCFAs amplify Th2 cells-mediated immune responses in eosinophilic esophagitis (EoE) [21]. This discrepancy rendered us to examine the effects of SCFAs on basophils, another important effector cells in allergic diseases. In this study, we for the first time showed that propionate and butyrate induced the apoptosis, CD69 and IL-13 expression and degranulation of basophils and this effect was mimicked by the HDAC inhibitor TSA.
GPR41 and GPR43 are well known receptors for SCFAs and are expressed in various tissues and cells [14, 19-21, 41]. For human immune cells, GPR43 is expressed in eosinophils and neutrophils while GPR41 is reported to be expressed in Th2 cells [14, 21]. Here we showed that human basophils expressed GPR41 while GPR43 was not detected in basophils with or without IL-3 stimulation. It has been shown that activation of GPR41 and GPR43 by SCFAs induce intracellular calcium influx, although acetate has less potency on calcium influx induction [33]. In line with this, we also identified that acetate induced intracellular calcium influx of basophils and this effect was reversed by blocking GPR41 activity. However, we observed no changes by propionate and butyrate stimulation. This effect is similar to a report that butyrate has no effect on the induction of intracellular calcium influx of eosinophils that express GPR43 [14]. This may be explained by additional signaling molecules are needed for induction of intracellular calcium influx in different cells.
SCFAs are generally recognized to act via activation of GPR41 and GPR43, and inhibition of HDACs [14, 33, 41]. Although GPR41 was expressed on basophils, blocking of GPR41 had no effect on basophil survival, activation and degranulation. Instead, pretreatment of basophils with CHC reversed propionate and butyrate-induced basophil degranulation, while without effects on basophil survival and activation. Since SCFAs can passively diffuse into cells [40], we speculated that incubation of basophils with SCFAs for a long time may lead to passive diffusion of SCFAs into cells, even after pre-blocking with CHC. Our western blot analysis of histone acetylation of SCFAs-treated basophils in the presence of CHC confirmed this hypothesis. In addition, the HDAC inhibitor TSA mimicked the effects of propionate and butyrate on regulating basophil survival, activation and degranulation. Therefore, propionate and butyrate might act through inhibiting HDACs activity rather than activation of GPR41 in regulating basophil survival, activation and degranulation. In agreement with our findings, previous studies show that inhibition of HDACs activity induces Th2 cytokines production in human T cells and eosinophil apoptosis [14, 42, 43].
Basophil degranulation plays a critical role in regulating allergic immune reactions. In contrast to the effect of butyrate and HDACs inhibitor TSA on suppressing mast cell degranulation [19], we found that both propionate and butyrate enhanced IgE-mediated basophil degranulation as evidenced by that propionate and butyrate increased surface CD63+ basophils upon activation of IgE pathway. Further direct analysis of exteriorized basophil granule matrix by avidin staining also confirmed the effect of propionate and butyrate on basophils, although avidin staining showed a unimodal shift rather than a bimodal distribution during CD63 staining and avidin+ basophils were lower than those of CD63+ basophils, which is in agreement with a recent report [44]. The differential effect of SCFAs and TSA on the degranulation of human mast cells and basophils may be ascribed to different cells and experimental setups between two studies e.g. treatment with SCFAs for basophils for 2 hours and overnight for human mast cells, a higher dose of TSA (1000 nM) for human mast cells and TSA (50 nM) for basophils.
Propionate and butyrate inhibited basophil survival and IL-4 production but enhanced basophil degranulation and IL-13 expression, implicating a complex role of SCFAs in mediating basophil activity. Basophils are terminally differentiated with a short life span [1]. The impact on inhibiting basophil survival may have significant effects in regulating the activity of basophils in allergic diseases, despite that propionate and butyrate promoted basophil degranulation and IL-13 production. Previous studies on eosinophils, ILC2 and mast cells implicate that SCFAs suppress allergic immune responses [14, 19, 20]. However, a recent study reports that SCFAs may enhance Th2 immune response by promoting Th2 cytokines expression in vitro and in vivo. The differential effects of SCFAs in regulating the activity of different allergic effector cells may be explained by different roles of SCFAs in different cells. We believe that future in vivo studies are warranted to examine the in vivo effects of SCFAs in regulating basophil-associated allergic diseases.
In conclusion, propionate and butyrate may play a complex role in regulating basophils survival, activation and degranulation via inhibiting HDACs activity. The in vivo effects of SCFAs in mediating basophil-associated allergic diseases need to be further explored.
CONFLICT OF INTEREST
The authors have no conflict of interest in relation to this work.
AUTHOR CONTRIBUTIONS
Y.S., M.X., S.P., S.G., J.R., R.B., H.L., and C.H. performed the experiments. Y.S., J.R., R.B., and Z.S. collected buffy coat. Y.S., M.X. and H.W. performed data analysis and prepared the figures. S.Z., Y.F. and Z.X. participated in data interpretation and discussion. H.W. conceived and supervised the study and wrote the manuscript.




