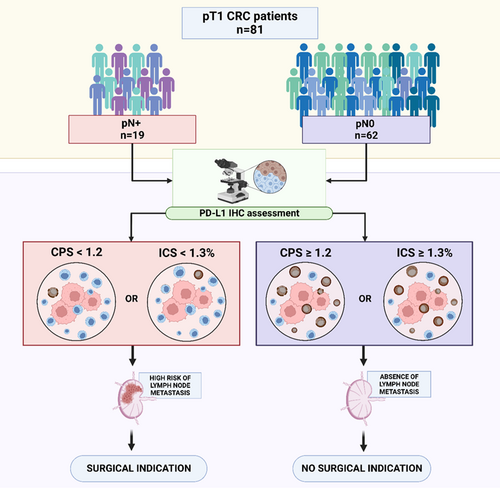
Low PD-L1 expression in immune cells predicts the presence of nodal metastasis in early invasive (pT1) colorectal cancer: a novel tool to tailor surgical treatment
Luca Bertero and Paola Cassoni contributed equally to the manuscript.
Abstract
Aims
The current criteria for surgical treatment after endoscopic resection of a pT1 colorectal carcinoma (CRC) are unsatisfactory because nodal involvement is rarely present. This study investigates the correlation between PD-L1 expression and nodal metastasis in pT1 CRCs to enable its use for tailoring surgical treatment after endoscopic removal.
Methods and results
Histopathological features of 81 surgically resected pT1 CRC, 19 metastatic and 62 non-metastatic cases were assessed. PD-L1 expression was evaluated by immunohistochemistry (clone 22C3) and independently assessed by two pathologists using tumour proportion score (TPS), combined positive score (CPS) and immune cell score (ICS). The correlation between PD-L1 expression and nodal metastasis, the optimal cut-off values, interobserver agreement and impact upon patients' surgical management were determined. PD-L1 expression in terms of CPS and ICS independently correlated with lymph node metastasis (PD-L1CPS: OR = –2.5, 95% CI = −4.11 to −0.97, P = 0.008 and PD-L1ICS: OR = −1.85, 95% CI = −2.90 to −0.79, P = 0.004) and < 1.2 CPS and <1.3% ICS were identified as the optimal cut-off values to discriminate between metastatic and non-metastatic patients. In our cohort, the implementation of these cut-off values would have avoided a significant rate of unnecessary surgeries in pN0 patients (PD-L1CPS = 43.2; PD-L1ICS = 51.9%). Ultimately, PD-L1 evaluation showed good interpathologist concordance in absolute terms [PD-L1CPS interclass correlation coefficient (ICC) = 0.91; PD-L1ICS ICC = 0.793] and using the identified cut-off values (PD-L1CPS ICC = 0.848; PD-L1ICS ICC = 0.756).
Conclusions
Our study shows that PD-L1 expression is an effective predictor of nodal status and could improve patient selection for surgery after endoscopic removal of pT1 CRCs.
Graphical Abstract
Low PD-L1 expression by immune cells predicts lymph node metastasis in pT1 colorectal carcinoma. PD-L1 CPS < 1.2 and PD-L1 ICS < 1.3% were determined to be the optimal cut-off values, allowing the identification of metastatic patients and thus to potentially avoid a significant rate of unnecessary surgeries.
Introduction
Colorectal cancer (CRC) represents the third most frequent cancer type1, 2 and the second most common cause of cancer-related death in the world.3 In developed countries, where nationwide screening programmes have been established and colonoscopies thoroughly increased, an overall decline in CRC incidence has been registered with a resultant increase in detection of non-invasive precursor lesions. Similarly, diagnoses of early invasive carcinomas (pT1 tumours according to the TNM classification4) have also increased.5
Although endoscopic removal has been demonstrated to be the gold standard treatment for non-invasive CRC precursors (i.e. less adverse events, lower mortality rate, better quality of life and cost-effectiveness),6 it is a matter of debate as to whether the same holds true for early invasive lesions.7 The risk of nodal metastases represents the primary driver for surgery following endoscopic resection according to European guidelines.8 Estimation of this risk is currently based upon the presence of specific histopathological hallmarks such as lymphovascular invasion, high tumour grade, high-grade tumour budding, micro-staging and positive resection margin status; even the presence of one single high-risk feature is currently sufficient for proposing surgery.7, 8 Nevertheless, the potential benefits of surgery ought to be carefully weighed against its morbidity, mortality and overall socioeconomic costs.9 Refining the current risk stratification schemes is critical to avoid the current high rate of unnecessary surgeries, as nodal metastases are ultimately detected in a limited subset (4.9–15.7%) of pT1 CRC only.9-14
In this context, some recent studies have suggested a possible relationship between programmed death ligand 1/CD274 (PD-L1) expression and prognosis in CRCs.15-18 Specifically, it has been demonstrated that higher expression of PD-L1 in tumour infiltrating immune cells imparts a better prognosis in different CRC stages,19 but this association has not been specifically tested in the subset of early invasive lesions.20
It should also be noted that the translation of these results into daily practice is hampered by the different scores used for PD-L1 evaluation [i.e. tumour proportion score (TPS),18 combined positive score (CPS)21 or immune cell score (ICS)19], contributing to partially conflicting results.22, 23
Based on this background, PD-L1 expression could represent a surrogate marker of tumour aggressiveness and therefore be associated with nodal metastasis in pT1 CRC. Confirmation of this hypothesis would provide a novel potential marker to tailor the surgical management of these patients, but differences between different PD-L1 evaluation scores should be specifically analysed to help translate this tool into routine diagnostic practice.
Thus, based on these considerations, the aims of our study were: (i) to investigate the relationship between the expression of PD-L1 and presence of lymph node (LN) metastasis in pT1 CRC, also comparing different PD-L1 scores; (ii) to identify the most effective PD-L1 expression thresholds for tailoring surgical treatment in patients with pT1 CRC; and (iii) to assess intra- and interobserver agreement in PD-L1 evaluation.
Methods
Study design and cohort composition
Surgically resected pT1 CRC diagnosed between January 2010 and May 2022 and not having undergone any neoadjuvant treatment were retrospectively retrieved at the Pathology Unit of ‘Città della Salute e della Scienza’ University Hospital in Turin, Italy. For each patient included in the study, a pre-operative colonoscopy followed by a total body computed tomography scan with contrast medium were obtained prior to surgical resection as part of the standard pre-operative assessment protocol. Similarly, each sample was managed according to standard protocols within the same CRC unit. From the overall series of 242 patients, we collected all cases with nodal metastases (pT1N+ CRC) (n = 19) and selected 62 samples of pT1N0 CRC, which served as controls, to achieve an approximately 1:3 cases to controls ratio similar to other studies.24, 25 These control samples were consecutively selected from more recent to older cases. Clinical and histopathological data were collected and pseudonymised.
Histological and immunohistochemical analysis
Haematoxylin and eosin (H&E) slides of all selected samples (cases and controls) were reviewed and reclassified according to the 2019 World Health Organisation (WHO) Classification of Digestive System Tumours.26 Histological features, including lymphatic and blood vessel invasion, tumour grading, tumour budding (i.e. defined according to the binary system reported in literature)27, 28 and micro-staging, were independently assessed by three pathologists (G.T., G.M.B., L.B.); in case of disagreement, a consensus was reached by joint review and discussion. A representative tumour invasion area was then selected and a 3-μm-thick section was prepared from the corresponding paraffin block and immunohistochemistry for PD-L1 (22C3 pharmDx; Agilent Technologies, Inc., Santa Clara, CA, USA) was performed on the Ventana BenchMark ULTRA AutoStainer (Ventana Medical Systems, Tucson, AZ, USA). PD-L1 was independently assessed by two pathologists (G.T. and G.M.B.) using the following scoring systems: (i) tumour proportion score (TPS), (ii) combined positive score (CPS) and (iii) ICS. Briefly, TPS was defined as the percentage of viable tumour cells showing partial or complete membrane staining at any intensity. CPS was defined as the [number of positive tumoral and tumour-associated mononuclear inflammatory cells (lymphocytes and macrophages) with respect to total viable tumour cells] × 100. Tumour cells showing partial or complete membrane staining at any intensity were considered positive, while both membrane and cytoplasm positivities were considered for immune cells. Lastly, ICS was defined as the ratio between the area occupied by PD-L1-positive immune cells (lymphocytes, dendritic cells, macrophages and granulocytes) and the whole tumour area.
Statistical analysis
Statistical analysis was performed in October 2022 using R software (R version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 2021.09.0 + 351 ‘Ghost Orcid’; RStudio, Boston, MA, USA). Continuous data were reported as mean ± standard deviation (SD) and compared using Student's t-test. Categorical data were reported with frequencies and relative percentages and compared using the chi-squared test or Fisher's exact test.
To assess intrapathologist correlation between different methods of PD-L1 expression assessment, R2 was used. Interpathologist concordance was assessed using intraclass correlation coefficient.
Multivariable generalised logistic regression models were used to evaluate the effects of lymphatic and blood vessel invasion, tumour grade, tumour budding and PD-L1 expression on the probability of LN metastasis. In a second analysis, the covariates found to be significantly different among cases and controls were evaluated. The Akaike information criterion (AIC) was used to select the best explanatory model.
To dissect the role of PD-L1 expression, we fitted a receiver operator characteristic (ROC) curve, and to identify the most balanced cut-off for ICD implementation we calculated the area under the curve (AUC), sensitivity, specificity and positive and negative predictive values. True positive rate was defined as the proportion of patients who would have undergone surgery based on PD-L1 assessment and had a LN metastasis. The false-positive rate was defined as the proportion of patients with a surgery indication but without nodal metastasis. To calculate the most balanced PD-L1 expression cut-off to guide indication for surgery, we calculated the Youden's index.29 Additionally, different PD-L1 cut-offs were compared. The number needed to treat (NNT) with 95% confidence interval (95% CI)30 values were also calculated for each cut-off. A two-sided P < 0.05 was considered significant.
The study protocol was approved by the IRB of University of Turin, Italy (DSM-CHBU 6/2019). The study was conducted in accordance with the ethical standards of the University of Turin IRB and with the Code of Ethics of the World Medical Association (Declaration of Helsinki and following amendments). Informed consent to surgical procedure and data collection was obtained from all subjects involved in the study. Dedicated written informed consent was deemed to be unnecessary because of the retrospective nature of the study. To protect patients' privacy and comply with ethical requirements, the collected/analysed data are not publicly available; aggregated data supporting the study findings are available from the corresponding author upon reasonable request.
Results
Baseline clinicopathological characteristics
The study cohort included 81 patients with pT1 CRC: 19 cases (patients with pN+ pT1CRC; 47% female, age at diagnosis = 65.0 ± 11.6 years) and 62 controls (patients with pN0 pT1CRC; 52% female, age at diagnosis = 72.2 ± 10.5 years). Only a minority of patients (six of 19 cases and four of 62 controls) were referred to surgery within the regional CRC screening programme, while most of the patients presented because of spontaneous screening or, more rarely, because of signs/symptoms.
Table 1 summarises the baseline characteristics of the study population. Analysed lesions were mostly non-pedunculated in both cases [14 of 19 (74%)] and controls [55 of 62 (89%), P = 0.214] and were predominantly localised in sigmoid colon or rectum (Table 1). The main tumour characteristics, such as macroscopic maximum diameter, presence of a mucinous component and histological grade, were comparable between cases and controls. Vascular invasion documented was intramural, and was more frequently observed in cases [lymphatic vessel invasion: six of 19 (32%) versus two of 62 (3%), P < 0.001; blood vessel invasion: one of 19 (5%) versus none of 62 (0%), P < 0.001], whereas perineural invasion was not [one of 19 (5%) versus none of 62 (0%), P = 0.529]. Of interest, considering the study aim, controls showed a higher lymphocytic infiltrate than cases (Table 1, P = 0.012).
| N = 81 patients | LN metastasis (n = 19) | No LN metastasis (n = 62) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 65.0 ± 11.6 | 72.2 ± 10.5 | 0.013 |
| Gender: female, n (%) | 9 (47.4) | 32 (51.6) | 0.951 |
| Polyp type, n (%) | |||
| Pedunculated | 5 (26.3) | 7 (11.3) | 0.214 |
| Non-pedunculated | 14 (73.7) | 55 (88.7) | |
| Site (%) | |||
| Right/Cecum | 7 (36.8) | 20 (32.3) | 0.898 |
| Left | 1 (5.3) | 5 (8.1) | |
| Transverse | 1 (5.3) | 6 (9.7) | |
| Sigma/rectum | 10 (52.6) | 31 (50.0) | |
| Maximum diameter (mm), mean ± SD | 25.7 ± 14.0 | 26.6 ± 11.6 | 0.768 |
| Mucinous component, n (%) | 3 (15.7) | 18 (29.0) | 0.394 |
| Grade, n (%) | |||
| G1 | 1 (5.3) | 1 (1.6) | 0.915 |
| G2 | 17 (89.6) | 57 (91.9) | |
| G3 | 1 (5.3) | 2 (3.2) | |
| Infiltrating type, n (%) | 15 (78.9) | 48 (77.4) | 1 |
| Invasion depth (mm), mean ± SD | 4.3 ± 1.7 | 3.8 ± 2.6 | 0.441 |
| Invasion width (mm), mean ± SD | 8.2 ± 4.7 | 9.8 ± 4.7 | 0.187 |
| Vascular invasion, n (%) | |||
| Lymphatic | 6 (31.6) | 2 (3.2) | < 0.001 |
| Blood | 1 (5.3) | 0 | |
| Perineural invasion, n (%) | 1 (5.3) | 0 (0) | 0.529 |
| Lymphocyte infiltration, n (%) | |||
| None | 5 (26.3) | 4 (6.5) | 0.012 |
| Scarce | 11 (57.9) | 26 (41.9) | |
| Moderate | 3 (15.8) | 21 (33.9) | |
| Conspicuous | 0 (0) | 11 (17.7) | |
| High tumour budding, n (%) | 6 (31.6) | 16 (25.8) | 0.841 |
| Distant polyps, n (%) | 3 (15.7) | 10 (16.1) | 1 |
| Number of LN examined, n (%) | 12.8 ± 5.4 | 16.1 ± 4.9 | 0.015 |
- LN, lymph node; SD, standard deviation.
- Statistically significant values (P < 0.05) are in italics.
A greater number of LN were dissected in controls (16.1 ± 4.9 LN examined) compared to cases (12.8 ± 5.4 LN, P = 0.015). Within pN+ cases, 14 of 19 (74%) cases were classified as pN1 (n = 10 pN1a, n = 3 pN1b, n = 1 pN1c) and the remaining five of 19 (26%) cases as pN2 (n = 4 pN2a and n = 1 pN2b).
Concerning patients' outcome, all pN0 patients remained free of nodal metastasis and tumour recurrence after a median clinical follow-up of 3.4 years [interquartile range (IQR = 1.6–5.2) years] at our institution.
Evaluation of PD-L1 expression
PD-L1 expression varied according to the score system used. TPS, assessed independently by two pathologists, was negative in all cases. Conversely, PD-L1 expression as evaluated by CPS and ICS revealed marked differences between cases and controls. Specifically, PD-L1 expression was significantly lower among cases compared to controls both in terms of CPS (0.34 ± 0.47 in cases versus 8.34 ± 13.59 in controls, P = 0.013) and ICS (0.42 ± 0.51% in cases versus 8.88 ± 13.26%, P = 0.007). Two example cases are shown in Figure 1. Intrapathologist correlation between PD-L1CPS and PD-L1ICS was good (R2 = 0.932 for G.T., R2 = 0.835 for G.M.B.). Concerning the pT1 CRC type, no difference was observed between pedunculated and non-pedunculated lesions: median PD-L1CPS was 1% in both pedunculated and non-pedunculated samples (P = 0.238), while median PD-L1ICS values were 1.5 and 2%, respectively (P = 0.397).
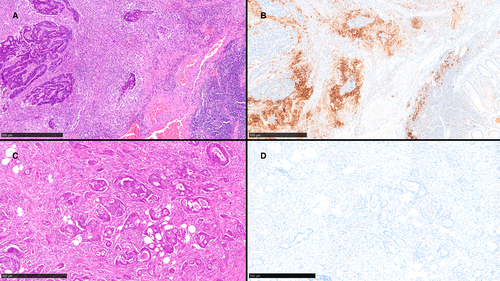
Low PD-L1 expression is a predictor of LN metastasis in pT1 CRC
We first tested the classical predictors of LN metastasis: lymphovascular invasion, tumour grade and tumour budding. Lymphatic vessel invasion was associated with a threefold probability of LN metastasis [odds ratio (OR) = 3.23, 95% CI = 1.11–5.36, P = 0.012], while tumour grade and high-grade tumour budding were not significantly associated with LN metastasis in our cohort (Table 2).
| n | OR (95% CI) | P-value | |
|---|---|---|---|
| PD-L1CPS | * | −2.52 (−4.10 to −0.95) | 0.008 |
| High tumour budding | |||
| No | 59 | Ref. | |
| Yes | 22 | 0.42 (−1.06 to 1.89) | 0.641 |
| Vascular invasion | |||
| No | 72 | Ref. | |
| Lymphatic vessel | 8 | 3.23 (1.11 to 5.36) | 0.012 |
| Blood vessel | 1 | 16.1 (NA) | – |
| Tumour Grade | |||
| G1 | 2 | Ref. | |
| G2 | 74 | −0.05 (−2.28 to 2.19) | 0.974 |
| G3 | 3 | 4.73 (−0.51 to 9.99) | 0.137 |
| n | OR (95% CI) | P | |
| PD-L1ICS | * | −1.84 (−2.90 to −0.78) | 0.004 |
| High tumour budding | |||
| No | 59 | Ref. | |
| Yes | 22 | −0.88 (−2.52 to 0.75) | 0.374 |
| Vascular invasion | |||
| No | 72 | Ref. | |
| Lymphatic vessel | 8 | 6.05 (0.55–11.56) | 0.070 |
| Blood vessel | 1 | 17.0 (NA) | – |
| Tumour grade | |||
| G1 | 2 | Ref. | |
| G2 | 74 | 0.48 (−1.98 to 2.95) | 0.747 |
| G3 | 3 | 5.40 (−2.52 to 13.32) | 0.263 |
- * Assessed as a linear parameter. OR, odds ratio; CI, confidence interval; NA, not available; PD-L1, programmed death ligand 1.
- Statistically significant values (P < 0.05) are in italics.
Concerning PD-L1 expression, multivariable logistic regression demonstrated that low expression of PD-L1, as assessed by both CPS (OR = –2.5, 95% CI = −4.10 to −0.95, P = 0.008; Table 2) and ICS (OR = −1.84, 95% CI = −2.90 to −0.78, P = 0.004; Table 2), was significantly associated with LN metastasis.
In a second analysis, which considered variables shown to be significantly different among cases and controls at univariate analysis (i.e. age and lymphocyte infiltration; see Table 1), PD-L1 expression and lymphatic vessel invasion were confirmed to be independently associated with LN metastasis (Table 3), achieving a similar performance compared with the previous analysis (AICmodel 1: = 57.3 versus AICmodel 2 = 57.2).
| n | OR (95% CI) | P-value | |
|---|---|---|---|
| PD-L1CPS | * | −1.79 (−3.17 to −0.41) | 0.033 |
| Age | * | −0.02 (−0.08 to 0.04) | 0.649 |
| Lymphocyte infiltration | |||
| No | 9 | Ref. | |
| Yes | 72 | −1.45 (−3.05 to 0.15) | 0.137 |
| Vascular invasion | |||
| No | 72 | Ref. | |
| Lymphatic vessel | 8 | 3.15 (1.07 to 5.22) | 0.013 |
| Blood vessel | 1 | 16.7 (NA) | – |
| n | OR (95% CI) | P-value | |
| PD-L1ICS | * | −1.62 (−2.54 to −0.69) | 0.004 |
| Age | * | −0.60 (−0.12 to 0.00) | 0.109 |
| Lymphocyte infiltration | |||
| No | 9 | Ref. | |
| Yes | 72 | −0.88 (−2.49 to 0.73) | 0.368 |
| Vascular invasion | |||
| No | 72 | Ref. | |
| Lymphatic vessel | 8 | 5.18 (1.05 to 9.32) | 0.039 |
| Blood vessel | 1 | 16.1 (NA) | – |
- * Assessed as a linear parameter.
- OR, odds ratio; CI, confidence interval; NA, not available; PD-L1, programmed death ligand 1.
- Statistically significant values (P < 0.05) are in italics.
Identification of the optimal cut-off for LN metastasis prediction
After demonstrating the significant association between low PD-L1 expression (assessed as CPS and ICS) and LN metastasis, we examined its potential use as a predictive marker for tailoring the surgical indication. To this end, we examined the relationship between PD-L1 expression and LN metastasis in the entire cohort of 81 pT1 CRC patients to identify the cut-off value achieving the optimal balance between true positive (patients with a surgical indication and nodal metastasis) and false positive (patients with a surgical indication and no nodal metastasis) rates. PD-L1 CPS expression (AUC = 0.85, sensitivity = 0.56, specificity = 1.00) and PD-L1 ICS expression (AUC = 0.87, sensitivity = 0.68, specificity = 1.00) were confirmed to be good predictors of LN metastasis. PD-L1 CPS ≥ 1.2 and PD-L1 ICS ≥ 1.3% were determined to be the optimal cut-off values by ROC curve analysis using the Youden method.
Potential impact of PD-L1 assessment on surgical management
The effect in clinical practice of different thresholds of PD-L1 expression to detect LN metastasis was simulated both for CPS (Table 4 and Figure 2) and ICS (Table 5 and Figure 3).
| PD-L1 cut-off for surgery | LN metastasis (n = 19) | No LN metastasis (n = 62) | NNT (95% CI) | ||
|---|---|---|---|---|---|
| Surgery | No surgery | Surgery | No surgery | ||
| > 0 PD-L1 | 12 | 7 | 5 | 57 | 2 (1.3–3.1) |
| ≥ 1.2 PD-L1 | 19 | 0 | 27 | 35 | 2 (1.5–2.3) |
| ≥ 2 PD-L1 | 19 | 0 | 31 | 31 | 2 (1.6–2.7) |
- NNT, number needed to treat; CI, confidence interval; PD-L1, programmed death ligand 1.
| PD-L1 cut-off for surgery | LN metastasis (n = 19) | No LN metastasis (n = 62) | NNT (95% CI) | ||
|---|---|---|---|---|---|
| Surgery | No surgery | Surgery | No surgery | ||
| > 0% PD-L1 | 12 | 7 | 5 | 57 | 2 (1.3–3.1) |
| ≥ 1.3% PD-L1 | 19 | 0 | 27 | 35 | 2 (1.5–2.3) |
| ≥ 2% PD-L1 | 19 | 0 | 31 | 31 | 2 (1.6–2.7) |
- NNT, number needed to treat; CI, confidence interval; PD-L1, programmed death ligand 1.
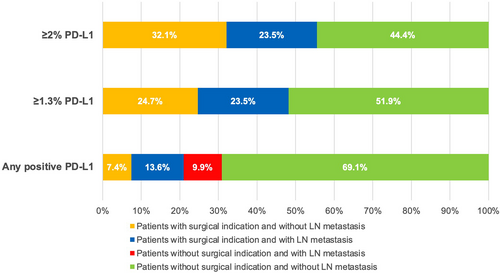
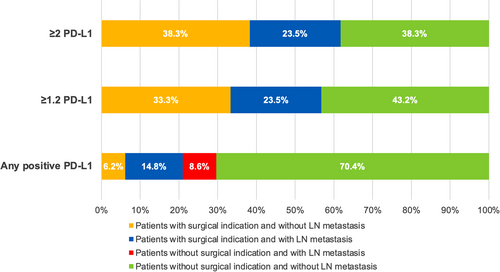
Overall, the implementation of <1.2 as PD-L1 CPS expression cut-off for surgery (i.e. surgical indication for patients with PD-L1 CPS expression <1.2) would result in all patients with LN metastasis (19 of 19, 100%) being given a surgical indication and avoidance of 43.2% of unnecessary surgeries in patients without LN metastasis (pN0), with an overall NNT (the number of patients undergoing surgery necessary to resect LN metastasis) of two NNT, 95% CI = 1.5–2.3; Table 4 and Figure 2. Similar results would be achieved using the PD-L1 ICS 1.3% cut-off (Table 5 and Figure 3).
To help improve the practical relevance of PD-L1 assessment in this setting, we simulated the effect of a rounded cut-off of two PD-L1 CPS. Also in this case, all patients with LN metastasis (19 of 19, 100%) would have been given a surgical indication, with avoidance of 38.3% of unnecessary surgeries in patients without LN metastasis (pN0) (Table 4 and Figure 2). Similar results would have been achieved using 2% PD-L1 ICS (Table 5 and Figure 3).
Interobserver agreement of PD-L1 expression assessment
Overall, absolute interpathologist concordance was good [PD-L1CPS interclass correlation coefficient (ICC) = 0.91, 95% CI = 0.854–0.943; PD-L1ICS ICC = 0.793, 95% CI = 0.677–0.867]. We also analysed the interpathologist concordance using the identified cut-off values. Overall, adopting the ≥1.2 PD-L1 CPS cut-off value, concordance was good (PD-L1CPS ICC = 0.848, 95% CI = 0.767–0.901). Specifically, discordance was identified in six of 81 cases (7.4%), none of which showed LN metastasis, and in all six cases the second pathologist (G.M.B.) assessed PD-L1 expression more conservatively (ΔPD-L1 = –2.6 ± 1.2, range = − 1.5 to −4) than the first pathologist (G.T.).
In cases of PD-L1 estimated with ICS, and adopting the cut-off value of ≥1.3%, concordance was also good (PD-L1ICS ICC = 0.756, 95% CI = 0.644–0.837), with 10 of 91 (11.0%) discordant cases (none of 10 LN metastases). Specifically, in eight of 10 cases the second pathologist (G.M.B.) assessed PD-L1 expression more conservatively (ΔPD-L1 = –2.1 ± 1.1, range = − 1.5 to −4.5) than the first pathologist (G.T.). In the remaining two cases, the second pathologist was less conservative (ΔPD-L1 = 2.75 ± 0.35) than the first pathologist (G.T.).
Discussion
This study demonstrates that PD-L1 expression in immune cells of early invasive (pT1) CRCs is significantly associated with lymph node metastasis and could be used effectively to reduce the number of unnecessary surgeries performed after endoscopic removal of these lesions.
To date, surgical indication after endoscopic removal of a pT1 CRC is based on several histopathological criteria,6, 31 but nodal metastases are ultimately present in 4.9–15.7% of pT1 CRC only.9-14, 32 Similarly, in our series of surgically resected specimens only a minority of patients (19 of 81; 23.4%) had nodal metastasis, suggesting a large degree of overtreatment.
In this context, the role of PD-L1 as a novel marker for risk stratification is worthy of consideration. PD-L1 expression has already been shown to be an effective predictor of poor prognosis in several solid tumours (e.g. renal cell carcinoma, non-small-cell lung cancer, breast cancer, ovarian cancer, pancreatic cancer, bladder urothelial carcinoma and head and neck squamous cell carcinoma).33 However, only a few studies have been performed focusing upon its prognostic role in CRC: in a series of advanced CRC, PD-L1 expression in tumour cells (observed in 54.4% of cases) was nearly significantly associated with poor survival (P = 0.051).16 Recently, a meta-analysis showed that increased PD-L1 expression in tumour cells correlates with poor prognosis (OR = 2.87, 95% CI = 2.18–3.78, P < 0.00001) and is significantly associated with the presence of nodal metastasis (OR = 2.69, 95% CI = 2.07–3.48, P < 0.00001).15 Interestingly, in our pT1 CRCs cohort, PD-L1 expression was restricted to immune cells, whereas neoplastic cells were consistently negative. This is not surprising, as these data suggest that PD-L1 expression could be longitudinally modulated across different CRC stages17, 22, 33 specifically, PD-L1 seems to be acquired by tumour cells in advanced tumour stages only, while in early stages it could be mainly involved in the modulation of tumour immune microenvironment.
In the field of immuno-oncology, a variety of PD-L1 immunohistochemical score systems have been proposed to predict response to immunotherapy:23 in this study, we have evaluated two of them (i.e. CPS and ICS) which are frequently used to determine PD-L1 expression in immune cells, finding comparable results. More importantly, concerning the main aim of our study, we found that low PD-L1 expression in immune cells (according to both CPS and ICS) significantly correlated with the presence of nodal metastasis.
Our findings are in line with mounting evidence supporting a relationship between PD-L1 expression in immune cells and more favourable clinical outcomes.17, 18
In order to confirm this biomarker as an independent predictor of nodal status, we also investigated its relationship with variables recognised to be classical independent predictors of nodal metastasis (e.g. lymphatic and blood vessel invasion, tumour grade and high-grade tumour budding).12, 34-36 The performed multivariate analyses confirmed that low PD-L1 expression, assessed by CPS and ICS, is an independent predictor of nodal metastasis. As expected, lymphovascular invasion, and specifically lymphatic vessel invasion, was one of the strongest predictors of nodal involvement, with a threefold increase in risk (P = 0.012). Conversely, neither tumour grade nor high-grade tumour budding were significantly associated with nodal status (Table 2). Of note, we did not include depth of submucosal invasion among the analysed variables given the recent evidence supporting the absence of a significant independent correlation between this feature and nodal involvement.37, 38
We then tried to establish the optimal cut-off value to discriminate between pN+ and pN0 patients: in our series, this value was very similar and extremely low for both CPS and ICS (PD-L1 CPS < 1.2 and PD-L1 ICS < 1.3%). These values showed good sensitivity and specificity according to ROC analysis (AUC = 0.85 for CPS and AUC = 0.87 for ICS).
Although very low PD-L1 cut-off values are routinely used to select patients for immunotherapy, interobserver agreement using such specific values could be challenging, but in our study it proved to be good for both CPS (ICC = 0.848) and ICS (ICC = 0.756).
As a workflow based on conventional glass slide-based microscopy is still the mainstay at most pathology laboratories worldwide, in this study we decided to verify the reproducibility by means of interpathologist agreement. Nevertheless, in future years approaches based on computational pathology could improve both prognostic stratification and reproducibility, as recently shown in other tumour types.39
Ultimately, the value of a novel potential biomarker relies upon its clinical impact on the patient's course. By adopting the proposed PD-L1 CPS and ICS cut-offs, all patients with nodal metastases within our series would have received surgical indication, and more than 40% of unnecessary surgeries (pN0 patients) would have been avoided. If we compare these results with the high rate (even more than 85%) of merely stadiative surgeries observed using the current criteria,9-14, 32 it is evident how evaluation of this marker could help to achieve a significant improvement.
To ease the translation into everyday diagnostic work-up, we simulated the clinical impact of rounded cut-off values of PD-L1CPS 2 and PD-L1ICS 2%. We found a comparably good performance adopting these values, confirming the surgical indication in all the metastatic patients without missing any pN+ case and avoiding approximately 40% of unnecessary surgeries in patients without nodal metastasis (pN0).
This study presents the limitations inherent in a retrospective, single-centre analysis addressing a rare condition. Validation of the results in a geographically diverse, larger and multicentre cohort is necessary to confirm these findings and enable subgroup analyses.
Analysis of a larger sample size would allow exploration of the correlations between PD-L1 expression and other tumour characteristics, such as immune cell infiltration patterns, and molecular features such as microsatellite instability (MSI) status and consensus molecular subgroups (CMS). For instance, based upon recent data showing a correlation between CMS4 and the presence of lymph node metastases in a non-pedunculated series of pT1 CRC, molecular stratification would enable investigattion of the role played by PD-L1 expression in shaping this prognostic association.40
In conclusion, our work suggests that PD-L1 is an important biological marker in early-stage invasive CRC and it could be translated, pending further validation, into a reproducible, informative tool for tailoring surgical indication in endoscopic-removed pT1 CRC.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
Open Access Funding provided by Universita degli Studi di Torino within the CRUI-CARE Agreement.